Abstract
The purpose of the study was to develop physically cross-linked novel pH-responsive gelatin – Wells–Dawson-type polyoxometalate (POM)-based self-assembled hydrogels using acrylic acid as a pH-responsive monomer. Cross-linking was achieved through electrostatic interactions between the cationic polymer and anionic Wells–Dawson POM [P2W15O56]12−. Ammonium persulfate and sodium hydrogen sulfite were used as initiators. The hydrogels were yellowish in color and exhibited low mechanical strength. Swelling, drug release, and pH sensitivity studies were conducted at pH 1.2 and 7.4. pH-dependent swelling and release of [P2W15O56]12− from the prepared hydrogels were observed, with a maximum at pH 7.4. The hydrogels were characterized by thermogravimetric analysis, differential scanning calorimetry, scanning electron microscopy, and Fourier transform infrared spectroscopy for evaluation of the surface morphology, hydrogel confirmation, and thermal properties. The results obtained confirmed the development of a gelatin–POM-based self-assembled hydrogel. It can be concluded that as a result of successful physical cross linking, the prepared hydrogels possess desired characteristics of a drug delivery system and can hence be used for a controlled delivery of the encapsulated polyanions. .
1. Introduction
Hydrogels are three-dimensional hydrophilic cross-linked polymeric chain networks having high water content holding capacity [Citation1,2] possessing aqueous solution as a solvent inside their structure.[Citation3] Hydrogels retain their own structure and do not get dissolve because of formed chemical or physical linkages in between polymer chains.[Citation4] Because of such exceptional soft and wet structure they are used as compatible materials for multiple applications like tissue engineering scaffolds, biosensors, and drug delivery. Hydrogels classification is dependent on constituting polymer source whether it is synthetic, hybrid, or purely natural. Cross-linking in hydrogels is either physical cross-linking mediated through non-covalent interactions, chemical cross-linking involving covalent interactions, or a combination of both. Water sorption inside the hydrophilic structure of hydrogels is through the osmosis, capillary action and hydration force balanced by the resisting expansion force offered by the cross-linked polymer chains.[Citation5]
Controlled drug delivery systems offers an alternative way to regulate the therapeutic agents bioavailability in which an active therapeutic moiety is merged or placed in a polymeric structure in such a way that the material releases the drug in a predetermined desirable manner.[Citation4] The drug released from a polymeric network structure depends on the formulation and the purpose of its application which may be anywhere either for few hours, to a month or for several years using various natural and synthetic polymers as drug delivery vehicles.[Citation6]
Oftenly the uniform dispersal of the hydrophobic drug molecules in a high water content hydrogel is thermodynamically unstable. The chemical potential associated with concentration of drug promotes the drug redistribution and recrystallization outside or inside of the gel network. In order to cope with this stability issue one approach is the incorporation of the hydrophobic moieties into the hydrophilic nanoscale structures having reduced drug surface energy,[Citation7] involving the use of certain functional groups which are chemically linked to the functional groups present in the polymer backbone leading to the formation of self-assembled structures attaining thermodynamic equilibrium state.[Citation8] Forces involved in self-assembling gelation process are the physical interactions like electrostatic interactions, hydrogen bonding, π–π stacking, van der wall forces, and hydrophobic interactions.[Citation9] The gel networks in self-assembled structures is through physical interactions having direct effect on integrity as well as gel properties.[Citation10]
Polyoxometalates (POMs) are macro anionic metal oxide clusters of early d block metal ions in high oxidation states like molybdenum, tungsten, and vanadium linked by shared oxygen atoms revealing prominent molecular diversity and having potential applications in various fields like catalysis, medicine, and analytical chemistry.[Citation11] POMs have been reported with significant biomedical activities against pathogenic organisms like viruses (human immunodeficiency virus, respiratory syncytial virus, and influenza virus), bacteria (Streptococcus pneumonia, methicillin resistant Staphylococcus aureus), anticancer, antidiabetic [Citation12], and currently anti-Alzheimer as well.[Citation13] In mammals, the persistent use of POMs is not recommended because of appearance of toxicity as a result of lack of selectivity in action. For better cellular uptake and reduced toxicity there is a need to encapsulate POMs in biocompatible and biodegradable polymer networks with the aim of controlled and targeted delivery.[Citation14]
Gelatin is the natural polymer obtained from collagen hydrolysis. Gelatin is biodegradable and biocompatible at physiological environment having the ability to load charged biomolecules and is used as plasma expander and drug formulation ingredient. Biopolymer like gelatin are extensively investigated for their structure, mechanical properties, and mechanism of gel networks formation and is one of the attractive starting material option because of its exclusive gelling properties and the presence of a large number of functional groups due to which it rapidly undergoes cross-linking making it suitable option to be used as biomaterial either for drug delivery or wound dressing.[Citation15] In spite of extensive research in the field of hydrogel formulations using various cross-linking methods with different cross-linkers, practical applications are still awaiting to be available as commercial products. Gels made as a result of chemical reactions should be pure enough in order to meet the FDA specifications as some of the cross-linkers are highly toxic, carcinogenic, and teratogenic making the situation difficult to win FDA approval.[Citation16]
The objective of the present study was to develop the pH-responsive self-assembled hydrogel formulation of the trilacunary Wells–Dawson-type tungstophosphate and gelatin using acrylic acid as pH-responsive monomer through a cost-effective method exploiting electrostatic interactions between the cationic polymer and polyanion in order to get rid of the toxicities associated with the various cross-linkers.
2. Materials and methods
2.1. Chemicals
Gelatin, acrylic acid, sodium hydrogen sulfite, ammonium persulfate, sodium hydroxide pellets, potassium dihydrogen phosphate were purchased from DAEJUNG Company (Korea), and hydrochloric acid was purchased from Scharlau, (Spain). The sodium salt of the trilacunary Wells–Dawson-type tungstophoshate Na12[α-P2W15O56]·24H2O was prepared following a published procedure.[Citation17] Deionized distilled water was freshly prepared in our laboratory at the COMSATS institute of Information Technology, Abbottabad.
2.2. Synthesis of pH-responsive gelatin–POM complexes
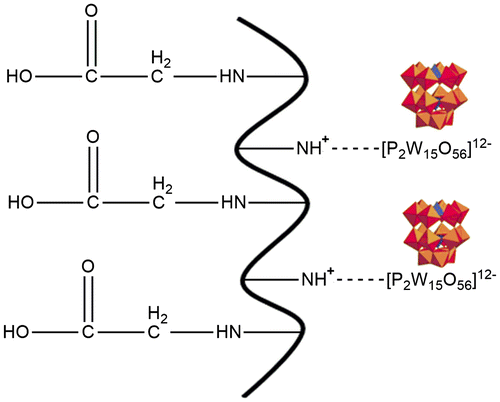
Gelatin–POM hydrogels were prepared using self-assembling phenomenon through electrostatic interactions in polymer, monomer, and POM in various ratios as shown in Table . Specific amount of gelatin was dissolved in weighed amount of warm water at 50 °C. Nitrogen gas was purged through polymer solution in order to remove the dissolved oxygen. Weighed amount of initiators, sodium hydrogen sulfite and ammonium persulfate were dissolved in specific amount of water. POM was dissolved in water using vortexer. Acrylic acid and initiators solution were added gradually to the polymer solution while continuous stirring. At the end, respective amount of POM sodium salt was added with constant stirring to small amount of the final solution sufficient to make hydrogel disk and placed in water bath. Temperature schedule was 55 °C for 4 h and 60 °C for 4 h. After successful formulation development it was air dried and placed in oven at 35 °C (Figure ).
2.3. Swelling studies
Swelling studies of the developed gelatin–POM hydrogels were conducted to evaluate their pH-sensitive behavior. For the provision of the gastric simulated pH conditions swelling studies were performed in pH 1.2 (0.1 M HCl buffer) and 7.4 (0.2 M phosphate buffer) buffer solutions. Hydrogel disks (dried) were accurately weighed and placed at room temperature in respective 100-ml buffer solution of each pH 1.2 and 7.4. Swollen hydrogel disks after regular time intervals were removed from respective buffer solutions and weighed using analytical balance (SHIMADZU, model ATY224) after blotting the excess water present on the surface of hydrogel disks. Disks were again placed in respective buffer solutions after weighing and the process was carried out until the constant weight was achieved by hydrogel disks. Following formula was used in order to calculate the percentage swelling ratio (SR)
where ‘Ws’ is the weight of disk in swollen state at specific time while ‘Wd’ represents the dry weight of hydrogel disk.
2.4. In vitro drug release
To confirm the drug release behavior and controlled delivery characteristics from the developed hydrogels, in vitro drug release studies were performed both at low pH (1.2) as well as high pH (7.4) conditions in 900-ml dissolution medium at 37 ± 0.5 °C using USP dissolution apparatus-II (Galvano Pak/GDT_7TV3). After specific time intervals 5-ml dissolution medium sample was collected and analyzed by UV–spectrophotometer (IRMECO U2020) at 210 nm detection wavelengths. In order to maintain the sink condition, same amount of fresh dissolution medium was added to the basket. Following mathematical equations were used for the determination of release mechanisms (Peppas and Higuchi models) and release kinetics (zero and first order).
Equation used for zero order:(1)
Q0 represents the amount of POM released at time ‘t0’, Qt is the amount of POM released at specific time ‘t’ while K0 represents rate constant for zero order.
Equation used for first order:(2)
Mt represents the POM remaining amount at time ‘t’, M0 represents initial POM amount, k1 is the rate constant for first order.
Higuchi equation:(3)
M represents cumulative POM amount released at time ‘t’, kH is the Higuchi constant and t is the time in hours.
Peppas model:(4)
(Mt/M∞) shows the released fraction of drug at time ‘t’ and n represents slope determining the polymer matrix diffusion process type.
2.5. Fourier transform infrared spectroscopy
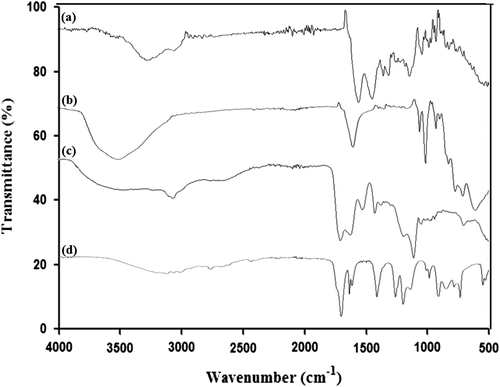
To confirm the formation of the pH-responsive gelatin–POM self-assembled hydrogels Fourier transform infrared spectroscopy (FT-IR) was used. FT-IR analysis was performed for all components like gelatin, acrylic acid, POM, and developed hydrogel formulations. All solid samples were properly crushed before analysis. For FT-IR analysis TENSOR II, Bruker, USA, instrument was used obtaining spectra in the range of 4000–500 cm−1.
2.6. Thermogravimetric analysis
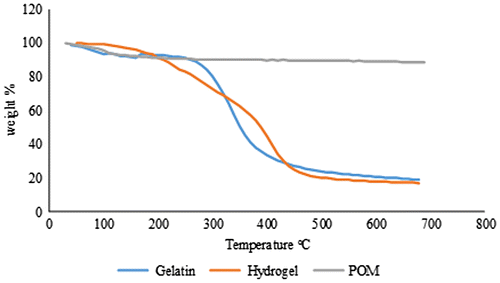
Thermogravimetric analyses (TGA) for POM, gelatin and developed hydrogel were performed using TA instrument SDT Q600 series thermal analysis system. Powdered samples of 0.5–5 mg were used for analysis in the sample pan at a temperature ranging from 0 to 800 °C. Analysis was performed under nitrogen gas purging at a flow rate of 100 ml/min with the adjusted heating rate at 20 °C/min. Measurements for all samples were recorded in triplicate.
2.7. Differential scanning calorimetry
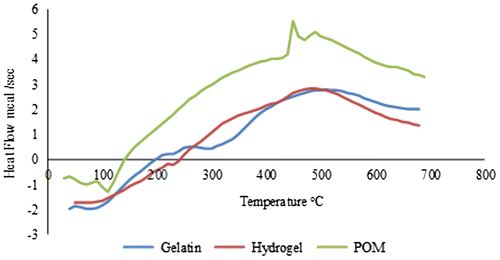
In order to determine samples’ glass transition temperature, TA instrument SDT Q600 series thermal analysis system was used. Calibration was performed with indium 99% at 156.6 °C followed by confirmation with zinc standard at 419.5 °C. In standard aluminum pan, samples were sealed keeping the temperature range from 0 to 800 °C and purging the nitrogen at 100 ml/min, maintaining the heating rate at 20 °C/min.
2.8. Scanning electron microscopy
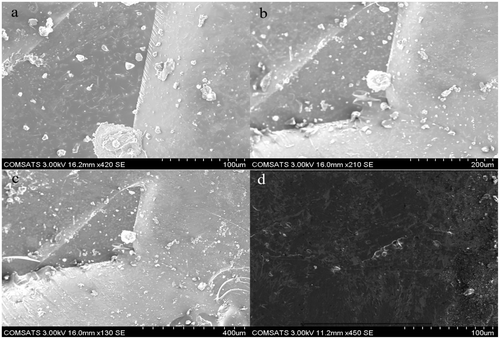
To evaluate the surface morphology of the hydrogel samples scanning electron microscopy (SEM) was used. On aluminum stub with double adhesive tape powdered hydrogel samples having appropriate particle size were fixed. Gold coating was done on stubs with gold sputter coater under argon atmosphere. Random photomicrographs were taken to analyze the surface morphology.
3. Results and discussion
3.1. Physical appearance
Stable acrylic acid–gelatin–POM hydrogels were prepared after successful physical cross-linking through electrostatic interactions. Generally hydrogels were light yellowish in color when freshly prepared but turns into yellow color on drying and become transparent on swelling. Freshly prepared hydrogels color was largely dependent on gelatin content ratio. Hydrogels having high gelatin ratio were creamy in color (Figure ) which turned also transparent on swelling. All formulations were having almost uniform structure with little bit low mechanical strength.
3.2. In vitro [P2W15O56]12− release
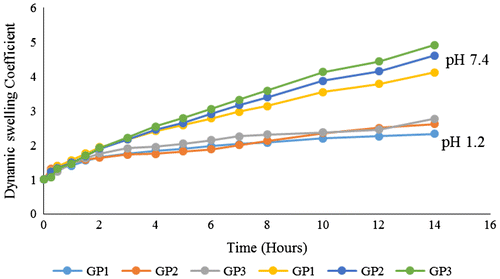
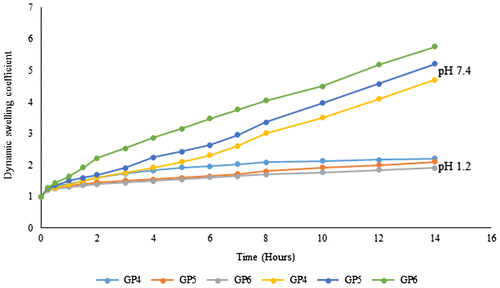
To evaluate the release characteristics of the hydrogels, tungstophosphate sodium salt, Na12[α-P2W15O56]·24H2O was analyzed for its release. As a function of time total percentage of [P2W15O56]12− release from developed hydrogels is shown in Figures , and . For the analysis of released [P2W15O56]12− after specific time intervals UV–spectrophotometer was used. pH of the surrounding media, composition, and nature of the polymeric system, ratios of the contents used and interaction of the drug with the polymer are the main factors affecting the release of a drug from hydrogels.[Citation4] In the present study, the developed hydrogels releases the [P2W15O56]12− in a pH-dependent manner and there was a significant difference in the amount of POM released at pH 1.2 and pH 7.4 values. Results were similar to the study of Anirudhan et al. [Citation18] while studying the controlled delivery of 5-flourouracil via pH-responsive gelatin-based hydrogels. Maximum percent [P2W15O56]12− release was observed at pH 7.4 for formulation (GP6) having highest concentration of acrylic acid. Maximum percent [P2W15O56]12− released at pH 1.2 was 19 and 84% at pH 7.4 as shown in Figures , and . The release kinetics from self-assembled gelatin–POM hydrogels showed that the hydrogels releases the POM in zero-order kinetic fashion. The regression coefficient values (R2) are shown in Table . Peppas and Higuchi models were used for determination of [P2W15O56]12− release from hydrogels. Diffusion mechanism was primarily involved in [P2W15O56]12− release from polymeric network. As n ˃ 0.89, the drug release occurs through super case transport-II which also refers to polymer erosion process.
Table 1. Mechanism of encapsulated POM release in dissolution experiments.
3.3. Effect of pH on swelling and [P2W15O56]12− release
Dynamic swelling and effect of pH on gelatin–POM hydrogels release properties are shown in Figures . There was a swelling difference at both pH values with the maximum swelling index at pH 7.4 and less at pH 1.2. Swelling and release studies were conducted up to 14 h because after that hydrogel then get broken as the mechanical strength of the prepared hydrogels was little bit low. pH strongly effects the swelling capacity and release of the drug because of the presence of ionizable pendant carboxylic groups in the hydrogel structure. Acidic groups are undissociated at low pH, hence there is less drug release. At high pH, drug release is high because of the ionization of carboxylic group resulting in high swelling due to the repulsion between negatively charged carboxylate groups. To get the efficient swelling index of the gelatin and acrylic acid-based hydrogels, the pKa value of the buffer solution should be higher than the pKa value of the carboxylic group as in such phenomenon the buffer solution will accept the proton released as a result of ionization of carboxylic group. Ionization of the carboxylic group changes with the respective variations in pH of the surrounding medium. As pH increases above the polymer acidic component pKa, swelling started because of the ionization of the carboxylic group and subsequently the encapsulated drug release rate also increases. At high pH (7.4), swelling and release were high due to anion–anion electrostatic repulsive forces creating voids while at low pH (1.2) there is hydrogel shrinking phenomenon,[Citation19] and less release was due to un-protonated carboxylic group effect as it is neutralized to COOH from the ionized COO− form supporting the results of other conducted studies as well.[Citation20]
3.4. Effect of gelatin content on swelling and [P2W15O56]12− release
Three formulations with different concentrations of gelatin (GP1, GP2, and GP3) were prepared keeping ratios of acrylic acid, POM and initiator constant as shown in Table . Swelling and release studies were conducted at pH 1.2 and pH 7.4 using 0.1M HCl buffer and 0.2 M phosphate buffers, respectively. Effect of gelatin ratio on release and swelling of the prepared formulations is shown in Figures and . Effect of gelatin ratio on swelling behavior of formulations is opposite of the acrylic acid ratio. Dynamic swelling coefficient of formulations having increased gelatin ratio decreases at pH 7.4 while it increases at pH 1.2. Isoelectric point of gelatin plays important roles both in swelling as well as in release of drug from gelatin-based polymeric formulations. Gelatin chains stays protonated at pH below isoelectric point possessing ions responsible for cationic repulsions and creation of intermolecular spaces leading to high swelling, same results were also revealed by other hydrogels.[Citation20] Extent of swelling has direct impact on percent release of drug. Increased concentration of gelatin increases the release of [P2W15O56]12− at low pH because of the increased swelling and decreases at high pH because of decreased swelling at this pH, corresponding with the results obtained by Burugapalli et al. [Citation21], while studying the thermal and swelling behaviors of the gelatin and polyacrylic acid interpenetrating polymeric networks.
Table 2. Composition of the various hydrogel formulations.
3.5. Effect of acrylic acid content on swelling and [P2W15O56]12− release
To check the effect of acrylic acid ratio on swelling and release of the encapsulated POM, three formulations GP4, GP5, and GP6 were prepared as shown in Table . Figures and show the effect of acrylic acid content on [P2W15O56]12− release and swelling of the hydrogels. The sensitive response of hydrogels to pH variation is likely because of the carboxylic group hydrophilicity present in the hydrogel structure.[Citation22] When pH increases, carboxylic group is deprotonated to carboxylate ion bearing negative charge,[Citation23] resulting in electrostatic repulsions causing the swelling of hydrogels.[Citation24] At higher pH, the restricted interaction of the acid molecules with water molecules and carboxylic group non-ionized condition results in less SRs.[Citation25] Cumulative percent release of [P2W15O56]12− was less at pH 1.2 than the pH 7.4 which was due to the less swelling of hydrogel. Formulation GP6 having highest amount of acrylic acid was showing highest swelling and highest [P2W15O56]12− release at pH 7.4 showing greater pH sensitivity. In a study designed by Elliot et al. [Citation26], it is reported in the same way that as we increase the concentration of acrylic acid in polymerization reaction subsequently swelling also increases and same results were also produced by Changez et al. [Citation27]. Highest swelling and [P2W15O56]12− release at pH 7.4 can be attributed to the deprotonation phenomenon of the carboxyl group (COOH) to (COO)− resulting in the generation of repulsive forces creating spaces. Voids produced then result in high swelling as well as high [P2W15O56]12− release from the hydrogel.
3.6. Effect of POM ratio on swelling and [P2W15O56]12− release
In a series of developed formulations, three formulations (GP7, GP8, and GP9) were having different concentrations of [P2W15O56]12−. Encapsulated [P2W15O56]12− here acts as a cross-linker because of the electrostatic interaction between the polycation () of polymer and the polyanion [P2W15O56]12−. Similar mechanism of POMs hydrogel formation is also reported.[Citation14] The encapsulation of other biological molecules like SiRNA is also mediated through the formation of complexes between cationic polymer and SiRNA.[Citation28] The formulation got optimized at specific concentration of the [P2W15O56]12− (Table ) which was safe from the toxicity point of view, as the dose administered to the rats while studying the pharmacokinetics of antiviral POMs.[Citation29] In last formulation (GP9) there is the highest concentration of [P2W15O56]12− so consequently there was minimum swelling (Figure ) and release from the formulation. From the GP9 formulation, 57% of the drug was released, 59% from the GP8 and 63% from the GP7 as shown in Figure .
3.7. Scanning electron microscopy
One of the important property of the hydrogel is to consider its morphological characteristics in intact form as well as in microstructure. To evaluate the morphological characteristics of the hydrogels, scanning electron microscope (Hitachi S-2460N) was used. Morphological characteristics of gelatin–POM self-assembled hydrogels were studied both in powdered form as well as intact hydrogel disk form for cross-sectional surface morphology. Photomicrographs obtained are shown in Figure showing smooth surface morphology of the prepared hydrogels.
3.8. FT-IR spectroscopy
FT-IR spectra of gelatin, trilacunary tungstophosphate sodium salt, acrylic acid, and hydrogel are shown in Figure . In gelatin spectra, wide band at 3279 cm−1 is due to stretching of N–H bond connected to O–H bond.[Citation30] Absorption Peaks at 1626 and 1520 cm−1 are because of amide-I and amide-II of gelatin, respectively.[Citation31] Peak present at 1230 cm−1 is due to stretching vibration of C–N bond while peaks at 3066 and 2865 cm−1 are due to stretching of C–H bond in CH3 and CH2 groups. In FT-IR spectra of acrylic acid the absorption peak at 2988 cm−1 refers to O–H stretching and absorption band recorded at 1696 cm−1 is attributed to C=O stretching.[Citation32] Peak recorded at 1568 cm−1 represents bending of C=O of COOH group. Peak observed at 1294 cm−1 shows C–C stretching while C–O stretching is shown by peak present at 1183 cm−1. Appearance of new modified absorption peak in hydrogel FT-IR spectra at 2930 cm−1 corresponds to the successful development of interaction between gelatin and acrylic acid. In tungstophosphate FT-IR spectra, the characteristic region (1000–500 cm−1) is called as ‘finger print’ because the absorptions occur here due to oxygen-metal stretching vibrations. As compared to the simple FT-IR spectra of the tungstophosphate, there are some characteristic bands appeared in the region of 1000–500 cm−1 showing the successful development of the hydrogel between gelatin and polyanion [P2W15O56]12−.[Citation33]
3.9. Thermogravimetric analysis
To check the physical properties of the polymer, trilacunary tungstophosphate sodium salt and hydrogel, thermal analysis was performed which shows the respective mass change and decomposition of the material with the increasing temperature. Thermogravimetric analysis plots of tungstophosphate sodium salt, hydrogel, and gelatin are shown in Figure . In gelatin TGA plot, normally, there are three stages, i.e., water loss at 25–100 °C, decomposition of gelatin from 250 to 450 °C and combustion of the residual material at 450–700 °C.[Citation34] TGA plot of gelatin is compared with the one of cross-linked gelatin (hydrogel) in a temperature range starting from 0 to 700 °C. Both the samples exhibited the thermal phenomenon like loss of water and material decomposition. Gelatin TGA plot shows the 8% water loss up to 80 °C while in case of hydrogel the water loss of 6% occurred at 170 °C showing that the hydrogel system impedes the water content removal. In TGA plot of the tungstophosphate sodium salt 4% water loss occurs up to 100 °C and after 100 to 300 °C the crystallized water is lost gradually by the tungstophosphate sodium salt which contributes to 6% of the total weight. After 300 °C there may be the process of phase change from α to β as there is heat change at 450 °C. In case of the hydrogel spectra it can be seen that the water loss occurs up to 170 °C and the degradation of the hydrogel occurs up to 600 °C showing the thermal stability of hydrogel.
3.10. Differential scanning calorimetry
Differential scanning calorimetry (DSC) analysis was also performed for the purpose of identification of thermal transitions in hydrogel network. In comparison with the other polymers, the biopolymers including gelatin thermal characterization are not always straightforward and depend largely on the polymer source and thermal history of the material. Therefore, no sole value is reported in the literature,[Citation35] but the non-cross-linked gelatin DSC spectrum displays generally an endothermic peak which is associated with the helix-coil transition. Figure shows the DSC curves of tungstophosphate sodium salt, gelatin, and hydrogel. Two endothermic peaks in gelatin DSC spectra are present in the range of 50–90 and 250–300 °C indicating water loss and decomposition, respectively. From tungstophosphate sodium salt DSC spectra, it is evident that the first endothermic peak at 100 °C shows water loss while second at 410 °C shows the decomposition or the phase inversion phenomena from α to β. In case of hydrogel the first endothermic peak at 220 °C shows water loss and second at 420 °C dictates decomposition showing that thermal stability of the hydrogel get increased as a result of cross-linking between gelatin–[P2W15O56]12− complex.
4. Conclusion
Self-assembled, pH-responsive hydrogels of gelatin, and the trilacunary Wells-Dawson ion [P2W15O56]12− were developed using acrylic acid as pH-responsive monomer. The developed formulations are light yellowish in color and have low mechanical strength, but can act as a drug delivery system due to efficient release of the encapsulated POMs. Several analytical tools such as FTIR, TGA, DSC, and SEM demonstrated the successful development of the self-assembled gelatin-acrylic acid-based [P2W15O56]12− hydrogels. Both the swelling and POM release were pH-dependent, and we identified one formulation (GP6) with optimal swelling and maximum release (84%) of the POM at physiological pH 7.4.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
J.I. is thankful to the Organization for the Prohibition of Chemical Weapons (OPCW), The Hague, The Netherlands and the Higher Education Commission of Pakistan for the financial support through Project No. 20-3733/NRPU/R&D/14/520. U.K thanks Jacobs University and the German Science Foundation (DFG) for research support over the years. A.H. thanks Deutscher Akademischer Austauschdienst (DAAD) for a doctoral fellowship. W.L. thanks the China Scholarship Council (CSC) for a doctoral fellowship. S.A.J. acknowledges Department of Science and Technology (DST), Government of India (GoI) for sanctioning leave to carry out research in Jacobs University, Bremen, Germany.
References
- Annabi N, Tamayol A, Uquillas JA, et al. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26:85–124.10.1002/adma.201303233
- Ganguly K, Chaturvedi K, More UA, et al. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J. Controlled Release. 2014;193:162–173.10.1016/j.jconrel.2014.05.014
- Faghihi S, Gheysour M, Karimi A, et al. Fabrication and mechanical characterization of graphene oxide-reinforced poly (acrylic acid)/gelatin composite hydrogels. J. Appl. Phys. 2014;115:083513–083513.10.1063/1.4864156
- Sohail M, Ahmad M, Minhas MU, et al. Controlled delivery of valsartan by cross-linked polymeric matrices: synthesis, in vitro and in vivo evaluation. Int. J. Pharm. 2015;487:110–119.10.1016/j.ijpharm.2015.04.013
- Buwalda SJ, Boere KW, Dijkstra PJ, et al. Hydrogels in a historical perspective: From simple networks to smart materials. J. Controlled Release. 2014;190:254–273.10.1016/j.jconrel.2014.03.052
- Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Delivery Rev. 2010;62:83–99.10.1016/j.addr.2009.07.019
- Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007.10.1016/j.polymer.2008.01.027
- Branco MC, Schneider JP. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5:817–831.10.1016/j.actbio.2008.09.018
- Chung HJ, Park TG. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today. 2009;4:429–437.10.1016/j.nantod.2009.08.008
- Nagarajan R. Solubilization of “guest” molecules into polymeric aggregates. Polym. Adv. Technol. 2001;12:23–43.10.1002/(ISSN)1099-1581
- Yang H-K, Cheng Y-X, Su M-M, et al. Polyoxometalate–biomolecule conjugates: a new approach to create hybrid drugs for cancer therapeutics. Bioorg. Med. Chem. Lett. 2013;23:1462–1466.
- Ilyas Z, Shah HS, Al-Oweini R, et al. Antidiabetic potential of polyoxotungstates: in vitro and in vivo studies. Metallomics. 2014;6:1521–1526.10.1039/C4MT00106K
- Gao N, Sun H, Dong K, et al. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer’s disease. Nat Commun. 2014;5:1–9.
- Pandya VM, Kortz U, Joshi SA. Encapsulation and stabilization of polyoxometalates in self-assembled supramolecular hydrogels. Dalton Trans. 2015;44:58–61.10.1039/C4DT01372G
- Santoro M, Tatara AM, Mikos AG. Gelatin carriers for drug and cell delivery in tissue engineering. J. Controlled Release. 2014;190:210–218.10.1016/j.jconrel.2014.04.014
- Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug Delivery Rev. 1998;31:197–221.10.1016/S0169-409X(97)00121-X
- Ginsberg AP. Inorganic syntheses. New Jersey: Wiley; 1990.10.1002/SERIES2146
- Anirudhan T, Mohan AM. Novel pH switchable gelatin based hydrogel for the controlled delivery of the anti cancer drug 5-fluorouracil. RSC Adv. 2014;4:12109–12118.10.1039/c3ra47991a
- Rathna G. Gelatin hydrogels: enhanced biocompatibility, drug release and cell viability. J. Mater. Sci.: Mater. Med. 2008;19:2351–2358.
- Curcio M, Altimari I, Spizzirri UG, et al. Biodegradable gelatin-based nanospheres as pH-responsive drug delivery systems. J. Nanopart. Res. 2013;15:1–11.
- Burugapalli K, Bhatia D, Koul V, et al. Interpenetrating polymer networks based on poly(acrylic acid) and gelatin. I: Swelling and thermal behavior. J. Appl. Polym. Sci. 2001;82:217–227.10.1002/(ISSN)1097-4628
- Amin MCIM, Ahmad N, Halib N, et al. Synthesis and characterization of thermo-and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012;88:465–473.10.1016/j.carbpol.2011.12.022
- Da Silva R, De Oliveira MG. Effect of the cross-linking degree on the morphology of poly(NIPAAm-co-AAc) hydrogels. Polymer. 2007;48:4114–4122.10.1016/j.polymer.2007.05.010
- Chaturvedi K, Ganguly K, Nadagouda MN, et al. Polymeric hydrogels for oral insulin delivery. J. Controlled Release. 2013;165:129–138.10.1016/j.jconrel.2012.11.005
- Halib N, Amin MCIM, Ahmad I. Unique stimuli responsive characteristics of electron beam synthesized bacterial cellulose/acrylic acid composite. J. Appl. Polym. Sci. 2010;116:2920–2929.
- Elliott JE, Macdonald M, Nie J, et al. Structure and swelling of poly(acrylic acid) hydrogels: effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer. 2004;45:1503–1510.10.1016/j.polymer.2003.12.040
- Changez M, Burugapalli K, Koul V, et al. The effect of composition of poly(acrylic acid)–gelatin hydrogel on gentamicin sulphate release: in vitro. Biomaterials. 2003;24:527–536.10.1016/S0142-9612(02)00364-2
- Chaturvedi K, Ganguly K, Kulkarni AR, et al. Cyclodextrin-based siRNA delivery nanocarriers: a state-of-the-art review. Expert Opin. Drug Delivery. 2011;8:1455–1468.10.1517/17425247.2011.610790
- Ni L, Henson GW, Darkes MC, et al. Pharmacokinetics of polyoxometalates after multiple-dose administration in rats. Pharm. Pharmacol. Commun. 1996;2:137–140.
- Yu H, Xiao C. Synthesis and properties of novel hydrogels from oxidized konjac glucomannan crosslinked gelatin for in vitro drug delivery. Carbohydr. Polym. 2008;72:479–489.10.1016/j.carbpol.2007.09.023
- Dong Z, Wang Q, Du Y. Alginate/gelatin blend films and their properties for drug controlled release. J. Membr. Sci. 2006;280:37–44.10.1016/j.memsci.2006.01.002
- Tang Q, Wu J, Lin J, et al. A multifunctional poly(acrylic acid)/gelatin hydrogel. J. Mater. Res. 2009;24:1653–1661.10.1557/jmr.2009.0210
- Shah HS, Al-Oweini R, Haider A, et al. Cytotoxicity and enzyme inhibition studies of polyoxometalates and their chitosan nanoassemblies. Toxicol. Rep. 2014;1:341–352.10.1016/j.toxrep.2014.06.001
- Panzavolta S, Fini M, Nicoletti A, et al. Porous composite scaffolds based on gelatin and partially hydrolyzed α-tricalcium phosphate. Acta Biomater. 2009;5:636–643.10.1016/j.actbio.2008.08.017
- Chiou B-S, Avena-Bustillos RJ, Bechtel PJ, et al. Cold water fish gelatin films: effects of cross-linking on thermal, mechanical, barrier, and biodegradation properties. Eur. Polym. J. 2008;44:3748–3753.10.1016/j.eurpolymj.2008.08.011



![Figure 3. Effect of gelatin concentration on [P2W15O56]12- release.](/cms/asset/bc28c4ec-3446-49c0-8393-d301263b729e/tdmp_a_1209629_f0003_oc.gif)
![Figure 5. Effect of acrylic acid concentration on [P2W15O56]12- release.](/cms/asset/0404b6fa-f92f-4a80-a390-c73b32739a01/tdmp_a_1209629_f0005_oc.gif)
![Figure 7. Effect of [P2W15O56]12- concentration on cumulative % release.](/cms/asset/f3e41871-925b-4c30-bbf7-b23c6ddbad83/tdmp_a_1209629_f0007_oc.gif)
![Figure 8. Effect of [P2W15O56]12- concentration on swelling of hydrogel.](/cms/asset/0353fee8-ab97-4995-af2a-c535f0b05880/tdmp_a_1209629_f0008_oc.gif)