ABSTRACT
Objectives
Compared with the 3 + 7 regimen, the cladribine-containing regimen has led to improvements in the rate of complete remission (CR) in the treatment of newly diagnosed acute myeloid leukemia (AML) patients. We conducted a systematic review and meta-analysis to investigate the overall efficacy and safety of cladribine-containing regimens in the induction treatment of newly diagnosed AML patients.
Methods
Eligible studies were identified from the PubMed, EMBASE, and Cochrane Library databases. Efficacy was assessed by CR rate, disease-free survival (DFS), and overall survival (OS). Safety was evaluated based on the early death (ED) rate, days for neutrophils<0.5 × 109/L, days for platelets<50 × 109/L, and duration of hospital stay after treatment.
Results
A total of 14 clinical trials were included in this meta-analysis, enrolling a total of 1058 newly diagnosed AML patients. The pooled estimate with a 95% confidence interval (CI) for CR was 64% (95% CI: 58–70%). Compared with the control group, the CR rate of the cladribine-containing regimen was higher (OR was 1.92 (95% CI: 1.55–2.38)). The combined ED rate was estimated to be 10% (95% CI: 5–14%). Compared with the control group, the ED rate of the cladribine-containing regimen was not increased (OR was 1.09 (95% CI: 0.78–1.53)).
Conclusion
This meta-analysis suggests that cladribine-containing regimens are likely to be effective and safe for induction treatment of newly diagnosed AML patients. However, large sample size and prospective controlled studies are needed to confirm our findings.
Introduction
Acute myeloid leukemia (AML) is the most common type of leukemia in adults. Successful treatment for AML relies on the ability to induce long-term remission. Since 1982, the standard regimen of AML induction therapy is still the 3 + 7 regimen, that is, 3 days of anthracycline combined with 7 days of cytarabine (Ara-C). The complete remission (CR) rate after first induction for untreated AML patients ranges from 60 to 80%, while elderly patients and patients with high-risk adverse prognostic factors experience a significantly lower CR rate[Citation1,Citation2]. Researchers have tried to improve the CR rate by changing the dose of anthracycline and/or Ara-C or adding a third drug, but the current efficacy of these approaches are still uncertain. Recent studies have explored the addition of cladribine to several different induction chemotherapy regimens, and some have shown promising results.
Cladribine is a purine analogue that is converted to its active triphosphate form in cells with high levels of deoxycytidine kinase and low 5’-nucleotidase activity. The triphosphate form resists degradation and accumulates to cytotoxic levels inside cells, which inhibits DNA synthesis, prevents DNA repair, and induces programmed cell death. Previous studies have shown that cladribine not only has a direct anti-leukemia effect, but can also be combined with Ara-C and has a synergistic effect, thereby increasing the absorption of Ara-C by cells and the accumulation of Ara-CTP in leukemia cells[Citation3]. Cladribine-containing induction chemotherapy regimens include the following: cladribine combined with daunorubicin and Ara-C (DAC) regimen, cladribine combined with idarubicin and Ara-C (IAC) regimen, cladribine combined with Ara-C, granulocyte-colony stimulating factor (G-CSF), and/or mitoxantrone (CLAG ± M) regimen, and so on.
During 1999–2000, the Polish Adult Leukemia Working Group (PALG) carried out a pilot study of the DAC regimen for the first-line induction therapy of 45 adult AML patients. The conclusion was that 58% of AML patients achieved CR after one course of treatment[Citation4]. Based on this, PALG published the results of a multicenter, phase III clinical trial in 2004. The study included 400 AML patients and concluded that the addition of cladribine increased the anti-leukemia efficacy of the DA regimen, resulting in a higher CR rate after one induction cycle without additional toxicity[Citation5]. The 2013 National Comprehensive Cancer Network (NCCN) guidelines refer to the DAC regimen as an option for induction therapy for AML patients younger than 60 years. However, because of the shortcomings of the clinical trial, the 2016 NCCN guidelines changed the DAC regimen from a category 1 recommendation to a category 2B recommendation, and changed it to a category 2A recommendation in the latest guidelines. A series of recent studies have shown that in AML patients with abnormal karyotypes, leukocytosis, or age between 50 and 60 years, the DAC regimen had a higher benefit[Citation6,Citation7]. Since 2009, the IAC regimen has been applied in induction therapy for newly diagnosed AML patients in a series of clinical studies, and most of them have achieved promising efficacy[Citation8–10]. The CR rate of the CLAG ± M regimen for refractory AML patients was 49%, suggesting that this regimen had a good anti-leukemia effect[Citation11]. Several studies conducted induction chemotherapy based on the CLAG ± M regimen for untreated AML patients, and the results also indicated that it was effective[Citation12,Citation13]. Among them, the CLAG ± M regimen used to treat young patients or patients with poor prognostic factors could improve the efficacy. In addition, due to the demethylation effect, cladribine combined with a low-dose Ara-C sequential decitabine regimen for the treatment of elderly AML patients also achieved a good therapeutic effect[Citation14].
In regard to the therapeutic effect of the cladribine-containing regimen for newly diagnosed AML patients and whether the increased intensity of the treatment regimen is accompanied by increased toxicity and side effects, the answer is still unclear. Based on the above clinical studies, we conducted a systematic review and meta-analysis to assess the efficacy and safety of adding cladribine to induction treatment of newly diagnosed AML patients.
Materials and methods
Data source
We searched the following databases in December 2020: (1) PubMed, (2) EMBASE, and (3) Cochrane Library. The search strategy was ‘acute myeloid leukemia’ and ‘cladribine’. Two review authors screened the titles ± abstracts of the studies identified from the above sources, and duplicate reports were excluded. Full texts were retrieved if the screening process could not be done satisfactorily from titles ± abstracts alone. In addition, the authors reviewed references to the articles to identify additional eligible studies.
Study selection
The inclusion criteria were as follows: (1) clinical trials included untreated patients with a diagnosis of AML, consistent with the WHO classification for AML; (2) random assignment of participants to treatment with cladribine in combination with other chemotherapy drugs; and (3) investigated the efficacy ± safety for patients treated with cladribine combination strategies. The authors independently screened reports that included the key terms by their titles ± abstracts for relevance. Then, the full texts of relevant articles were retrieved to assess eligibility.
Definition of outcomes
The primary outcomes of these studies were the CR rate and early death (ED) rate. CR referred to bone marrow (BM) blast counts<5%, neutrophil counts>1 × 109/L, platelet counts>100 × 109/L, and no extramedullary lesions. ED is the mortality rate during or shortly after treatment (most studies have a threshold of approximately 28–30 days between the start of treatment and the assessment of remission). Secondary outcomes included overall survival (OS), disease-free survival (DFS), days for neutrophils<0.5 × 109/L, days for platelets<50 × 109/L, and duration of hospital stay after treatment for all evaluable patients. We also analyzed hematologic and nonhematologic toxicity data if they were available.
Data extraction
Two review authors performed data extraction independently using a standard form containing a set of required information. The following data were extracted: general information (first author, year of publication, country), trial characteristics (study design, sample size, treatment plan), patient characteristics (age, sex), and outcome measures (CR rate, ED rate, OS, DFS, days for neutrophils<0.5 × 109/L, days for platelets<50 × 109/L, duration of hospital stay). Two review authors resolved any disagreement on the eligibility of the included studies and discrepancies in the extracted data by consensus. If disagreements could not be resolved, the third review author made a final decision.
Quality assessment
Randomized studies were evaluated by the Cochrane Bias Risk Assessment Tool, while nonrandomized studies were evaluated by the Newcastle-Ottawa (NOS) Literature Quality Assessment Scale. Two review authors assessed the quality of a study independently. If the primary or secondary endpoints involved 10 or more studies, publication bias was assessed by Begg’s test, and P < 0.05 was considered statistically significant.
Statistical analysis
The presence of heterogeneity was quantified using the I2 statistic, which calculated values between 0 and 100%. A higher value indicates a greater degree of heterogeneity. As a cut-off P > 0.05 or I2 < 50%, a fixed effects model was applied. Otherwise, the random effects model was applied. Subgroup analysis for response rate was performed if the heterogeneity was too great for a summary estimate. Estimated proportions with 95% confidence intervals (CIs) were calculated for all ratio outcomes. Sensitivity analysis was conducted by deleting each study, one at a time, to assess the stability of the results. Statistical analysis was performed with meta-analysis software STATA version 12 (STATA Corp, College Station, TX, USA).
Results
Search results
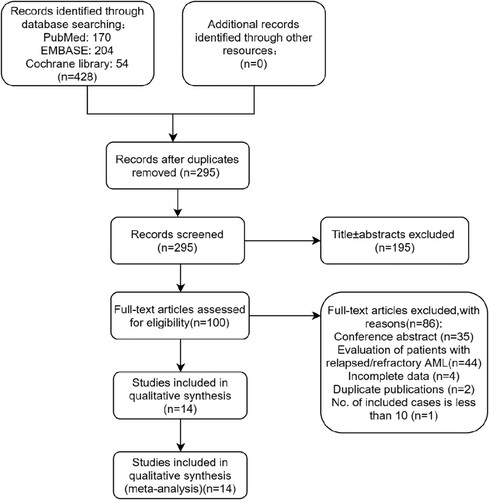
Our initial electronic search of the PubMed, EMBASE, and Cochrane Library databases retrieved a total of 428 publications. shows the PRISMA flow diagram of study selection. After the removal of duplicates, 295 articles were screened for eligibility. After the screening of titles ± abstracts, 100 articles were identified for full-text review. Finally, the reviewers identified 14 publications for qualitative and quantitative analysis. Studies were excluded for the following reasons: retrieval of abstracts from the literature (n = 35), incomplete data (n = 4), duplicated literature content (n = 2), fewer than 10 included cases (n = 1), and only involving the treatment of recurrent and/or refractory AML patients (n = 44).
Study characteristics
A total of 1058 patients in the 14 included studies were identified in this meta-analysis. The characteristics of the included studies are summarized in and . Among the included studies, 6 were prospective studies[Citation5,Citation6,Citation13–16], 8 were respective studies[Citation8–10,Citation12,Citation17–20], and 4 were randomized clinical trials[Citation5,Citation6,Citation15,Citation16].
Table 1 Characteristics of the included studies
Table 2 Characteristics of the included studies
The number of AML patients included in these studies ranged from 12 to 222, with a median age of 43–70 years. The CR rate was reported by 14 studies, ED rate by 12 studies, OS by 8 studies, and DFS by 4 studies. After treatment, days for neutrophils<0.5 × 109/L, days for platelets<50 × 109/L, and duration of hospital stay were provided by 10, 8, and 7 studies, respectively. Among them, the DAC regimen was used in 3 studies, the IAC regimen in 5 studies, the CLAG ± M regimen in 3 studies, and the treatment of newly diagnosed elderly AML patients (≥60 years old) was involved in 3 studies.
Efficacy
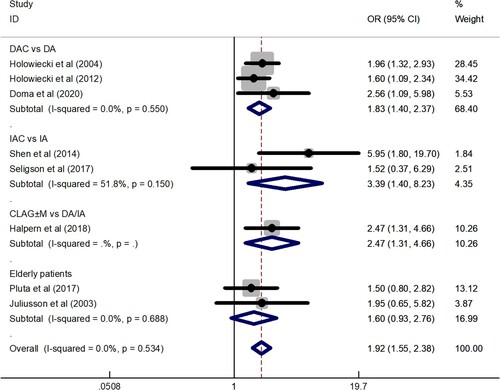
Data for the CR rate were available for analysis from a total of 14 trials including 1058 patients. The CR rate fluctuated between 42 and 79.2%, and the total CR rate was 64% (95% CI: 58–70%), which was determined by the random effects model since the CR rates of 14 trials were highly heterogeneous (I2 = 72.8%, P = 0.000). To explore the heterogeneity of the included studies, subgroup analysis according to different treatment protocols was constructed. The results showed that CR rate of DAC regimen was 67% (95% CI: 59–76%), CR rate of IAC regimen was 68% (95% CI: 58–77%), CR rate of CLAG ± M regimen was 69% (95% CI: 54–84%), and CR rate of elderly patients group was 52% (95% CI: 42–61%) (). Subgroup analysis showed that the CLAG ± M regimen achieved a relatively high CR rate, while the CR rate of the elderly patients group was relatively low. A total of 736 patients in 8 studies provided data on CR rate comparison between the cladribine-containing regimen and the control group. The meta-analysis results showed that the OR was 1.92 (95% CI: 1.55–2.38). The results were analyzed using a fixed effects model (I2 = 0.0%, P = 0.534) ().
Figure 2. A forest graph showing the overall and subgroup CR rates achieved in the meta-analysis. Abbreviations: DAC, daunorubicin, Ara-C, and cladribine; IAC, idarubicin, Ara-C, and cladribine; CLAG ± M, cladribine, Ara-C, G-CSF ± mitoxantrone.
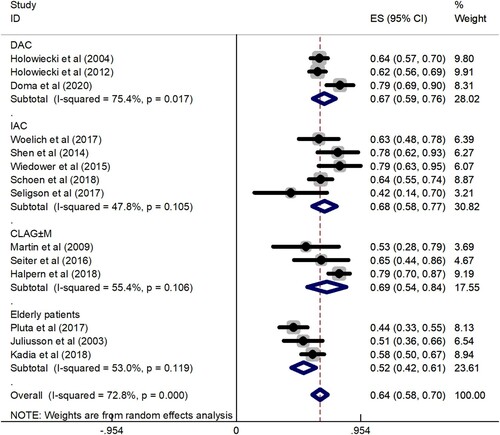
Figure 3. A forest graph showing the comparison of the CR rate between the cladribine-containing regimen and the control group. Abbreviations: DAC, daunorubicin, Ara-C, and cladribine; IAC, idarubicin, Ara-C, and cladribine; CLAG ± M, cladribine, Ara-C, G-CSF ± mitoxantrone; DA, daunorubicin and Ara-C; IA, idarubicin and Ara-C.
In terms of OS, data from 865 patients were provided from 8 studies. Among them, 3 studies reported that the 3-year OS fluctuated between 34 and 56%, 1 study reported that the 1-year OS was 69%, and 4 studies reported that the median OS was 8.6, 13.8, 17.3, and 29.0 m. In terms of DFS, data from 564 patients were provided from 4 studies. Among them, 3 studies reported that the 3-year DFS fluctuated between 36 and 45 ± 5%, and 1 study reported that the median DFS was 10.8 m.
Safety
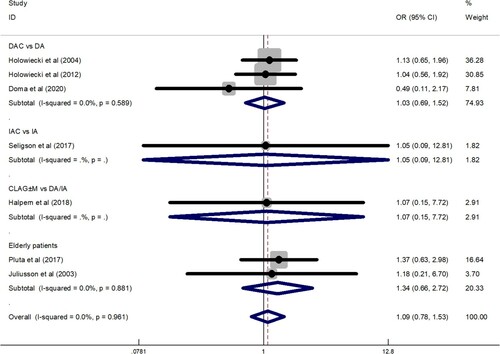
Data for the ED rate were obtained from 1011 patients enrolled in 12 trials. The overall ED rate was 10% (95% CI: 5–14%), as determined by the random effects model (I2 = 85.4%, P = 0.000). To explore the heterogeneity of the included studies, a subgroup analysis was constructed according to different treatment regimens. The results showed that ED rate of DAC regimen was 11% (95% CI: 5–16%), ED rate of IAC regimen was 10% (95% CI: 6–15%), ED rate of CLAG ± M regimen was 8% (95% CI: −8–25%), and ED rate of elderly patients group was 11% (95% CI: −3–25%) (). Subgroup analysis showed that the ED rate of the CLAG ± M regimen was relatively low. A total of 709 patients in 7 studies provided data on ED rate comparison between cladribine-containing regimens and the control group. The meta-analysis results showed that the OR was 1.09 (95% CI: 0.78–1.53). The results were analyzed using a fixed effects model (I2 = 0.0%, P = 0.961) ().
Figure 4. A forest graph showing the overall and subgroup ED rates achieved in the meta-analysis. Abbreviations: DAC, daunorubicin, Ara-C, and cladribine; IAC, idarubicin, Ara-C, and cladribine; CLAG ± M, cladribine, Ara-C, G-CSF ± mitoxantrone.
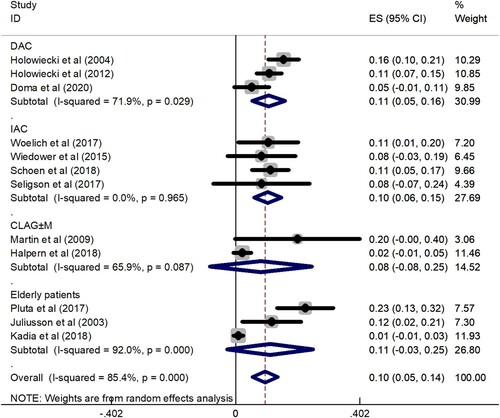
Figure 5. A forest graph showing the comparison of the ED rate between the cladribine-containing regimen and the control group. Abbreviations: DAC, daunorubicin, Ara-C, and cladribine; IAC, idarubicin, Ara-C, and cladribine; CLAG ± M, cladribine, Ara-C, G-CSF ± mitoxantrone; DA, daunorubicin and Ara-C; IA, idarubicin and Ara-C.
In the included studies, almost all patients had grade 3–4 myelosuppression. Ten studies showed that the median time of days for neutrophils<0.5 × 109/L fluctuated between 16 and 30.5 days. Eight studies showed that the average duration of days for platelets<50 × 109/L fluctuated between 15 and 27 days. Seven studies showed that patients’ average duration of hospital stay ranged from 26.6–33 days. For nonhematological toxicity, infection was present in nearly all patients after chemotherapy, which was a major cause of death for patients after chemotherapy.
The risk of bias within studies and sensitivity analysis
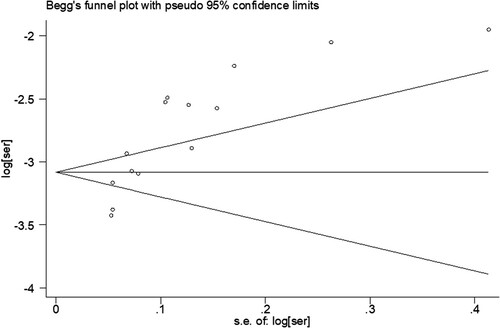
Four randomized studies were evaluated by the Cochrane Bias Risk Assessment Tool and 10 nonrandomized studies were evaluated by the Newcastle-Ottawa (NOS) Literature Quality Assessment Scale, as shown in and , respectively. Publication bias was detected by Begg’s funnel plot method, and P < 0.05 indicated the existence of publication bias (). Sensitivity analysis indicated that excluding any single study did not significantly affect the pooled outcomes, suggesting that the results of our meta-analysis were stable.
Table 3 Risk of bias in the prospective randomized controlled studies
Table 4 Risk of bias in retrospective studies using a modified Newcastle-Ottawa scale
Discussion
In this study, we found that the CR rate of the cladribine-containing regimen for induction therapy of untreated AML patients was 64% (95% CI: 58–70%), and when compared with the control group, the OR was 1.92 (95% CI: 1.55–2.38). The ED rate of the cladribine-containing regimen for induction therapy of untreated AML patients was 10% (95% CI: 5–14%), and when compared with the control group, the OR was 1.09 (95% CI: 0.78–1.53). Despite significant heterogeneity in these included clinical trials, the results confirmed the efficacy and safety of cladribine-containing regimens in untreated AML patients. In this study, the diversity of treatment options might be the main reason for the significant heterogeneity. Therefore, a random effects model was used to minimize bias, and subgroup analysis according to treatment regimens was used to reduce heterogeneity and improve the reliability of the results.
Cladribine has shown unique anti-leukemic activity in AML patients. It has inhibitory effects on both dividing cells and quiescent cells. Some of the most important features are inhibition of ribonucleic acid reductase and DNA polymerase, induction of DNA fragmentation, and inhibition of DNA methylation. In addition, cladribine and Ara-C have synergistic effects. It was reported that pretreatment with cladribine increased the rate of Ara-CTP accumulation in leukemic blasts by 50–65% in in vitro and in vivo pharmacological studies[Citation21]. A recent meta-analysis reported that the CR rate of cladribine used in refractory AML patients was 42.2% (95% CI: 31.0–54.3%), and the ED rate was 6.8% (95% CI: 4.3–10.6%)[Citation22]. To date, there have been reviews report on cladribine-containing regimens used for induction therapy of newly diagnosed AML patients, but these studies only listed the results of clinical trials without statistical analysis and no comparison between different regimens was conducted[Citation3,Citation21]. Our study showed that the CR rate of the cladribine-containing regimen for newly diagnosed AML patients was 64%, which was better than that of refractory AML patients. The ED rate of newly diagnosed AML patients treated with the cladribine-containing regimen was 10%, slightly higher than that of refractory AML patients, which might be related to the inclusion of the elderly patients in our study. Additionally, our meta-analysis concluded that the inclusion of cladribine in the standard induction regimen was superior to the standard induction regimen alone, such as the DA or IA regimen, without an increase in the ED rate.
In the subgroup analysis, our study found that the CLAG ± M regimen was more effective than other cladribine-containing regimens in newly diagnosed AML patients, which might be related to the fact that this regimen contained drugs with different mechanisms of action. The addition of G-CSF might further improve the efficacy of cladribine combined with Ara-C. The CLAG ± M regimen has been successfully used in refractory or recurrent AML patients[Citation23,Citation24]. However, the experience of applying this regimen in induction therapy for newly diagnosed AML patients was not sufficient. The CLAG ± M regimen has shown to improve the CR rate without increasing the ED rate, so it could be considered more widely used for first-line and lifesaving treatment of AML patients. Although the original PALG clinical trial excluded elderly patients, some studies have been conducted only in the elderly population. Our results showed that in the elderly patients group, the CR rate was relatively low and the ED rate was relatively high. This is due to the poor physical condition of elderly patients and the characteristics of easy comorbidities. The conclusion is consistent with the actual situation. These results suggest that cladribine does not appear to be a significant benefit in induction therapy in elderly patients, but these studies have not yet taken factors such as age stratification and disease risk stratification into account.
Bone marrow suppression and infection are the main limiting factors for the use of cladribine. In this meta-analysis, the overall evaluation of post-chemotherapeutic toxicity was somewhat difficult because some clinical trials only briefly summarized hematologic and nonhematologic adverse events (AEs) without listing specific data. The most common toxicities included neutropenia and thrombocytopenia, and the most common non-toxicities included nausea, diarrhea, mucositis, and hemorrhage. In this study, the median time of days for neutrophils<0.5 × 109/L, days for platelets<50 × 109/L, and duration of hospital stay after treatment were analyzed. The results showed that neutropenia lasted for more than 2 weeks on average. Given that cladribine can reduce lymphocytes for a long time, it is recommended to routinely use antibacterial and antifungal drugs to prevent infection after cladribine treatment. Similarly, thrombocytopenia lasted for more than 2 weeks, suggesting that more supportive treatment was needed after chemotherapy. The length of hospital stay was not significantly longer than that of conventional treatment, which reflected the tolerability of cladribine. In terms of OS and DFS assessment after chemotherapy, the included clinical studies provided few data and inconsistent standards, which need to be further analyzed by more clinical studies.
In addition, this study has several limitations. First, few randomized controlled trials were included in this study. Most of the studies were retrospective analysis or phase I/II clinical trials, which might be in the range of relatively low confidence. Second, another limitation is significant heterogeneity. The possible causes for heterogeneity were possibly mainly because of the different schedules and the various average daily doses applied in these trials. Therefore, we used a random effects model to reduce heterogeneity. Additionally, publication bias might exist since unpublished articles were not included in our meta-analysis, and the number of patients and trials enrolled in this study was relatively small. To determine whether our study fully evaluated the cladribine-containing regimen as an effective and safe treatment for newly diagnosed AML patients, we need more large-sample and high-quality clinical trials to increase the reliability of our findings.
Conclusion
Our meta-analysis observed that the cladribine-containing regimen might be an effective and well-tolerated treatment option for newly diagnosed AML patients, with high efficacy and acceptable side effects. To improve the overall response and maintain durable remission, further studies should focus on determining the best administration schedule and developing the optimal combination with cladribine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Sigal DS, Miller HJ, Schram ED, et al. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood. 2010;116(16):2884–2896.
- Hołowiecki J, Robak T, Kyrcz-Krzemień S, et al. Daunorubicin, cytarabine and 2-CdA (DAC-7) for remission induction in “de novo” adult acute myeloid leukaemia patients. Evaluation of safety, tolerance and antileukemic activity. Acta Haematol Pol. 2002;33(2):239–247.
- Holowiecki J, Grosicki S, Robak T, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia. 2004;18(5):989–997.
- Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30(20):2441–2448.
- Libura M, Giebel S, Piatkowska-Jakubas B, et al. Cladribine added to daunorubicin-cytarabine induction prolongs survival of FLT3-ITD+ normal karyotype AML patients. Blood. 2016;127(3):360–362.
- Wiedower E, Jamy O, Martin MG. Induction of acute myeloid leukemia with idarubicin, cytarabine and cladribine. Anticancer Res. 2015;35(11):6287–6290.
- Shen Y, Chen J, Liu Y, et al. Addition of cladribine to idarubicin and cytarabine during induction increases the overall efficacy rate in adult patients with acute myeloid leukemia: A matched-pair retrospective comparison. Chemotherapy. 2015;60(5–6):368–374.
- Seligson ND, Hobbs ALV, Leonard JM, et al. Evaluating the impact of the addition of cladribine to standard acute myeloid leukemia induction therapy. Ann Pharmacother. 2018;52(5):439–445.
- Wrzesien-Kus A, Robak T, Wierzbowska A, et al. A multicenter, open, noncomparative, phase II study of the combination of cladribine (2-chlorodeoxyadenosine), cytarabine, granulocyte colony-stimulating factor and mitoxantrone as induction therapy in refractory acute myeloid leukemia: a report of the Polish Adult Leukemia Group. Ann Hematol. 2005;84(9):557–564.
- Martin MG, Welch JS, Augustin K, et al. Cladribine in the treatment of acute myeloid leukemia: a single-institution experience. Clin Lymphoma Myeloma. 2009;9(4):298–301.
- Halpern AB, Othus M, Huebner EM, et al. Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms. Leukemia. 2018;32(11):2352–2362.
- Kadia TM, Cortes J, Ravandi F, et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: a phase 2 single-arm trial. The Lancet Haematology. 2018;5(9):e411–e421.
- Pluta A, Robak T, Wrzesien-Kus A, et al. Addition of cladribine to the standard induction treatment improves outcomes in a subset of elderly acute myeloid leukemia patients. Results of a randomized Polish Adult Leukemia Group (PALG) phase II trial. Am J Hematol. 2017;92(4):359–366.
- Juliusson G, Höglund M, Karlsson K, et al. Increased remissions from one course for intermediate-dose cytosine arabinoside and idarubicin in elderly acute myeloid leukaemia when combined with cladribine. A randomized population-based phase II study. Br J Haematol. 2003;123(5):810–818.
- Anzej Doma S, Skerget M, Pajic T, et al. Improved survival of AML patients by addition of cladribine to standard induction chemotherapy. Ann Hematol. 2020;99(3):519–525.
- Woelich SK, Braun JT, Schoen MW, et al. Efficacy and toxicity of induction therapy with cladribine, idarubicin, and cytarabine (IAC) for acute myeloid leukemia. Anticancer Res. 2017;37(2):713–717.
- Schoen MW, Woelich SK, Braun JT, et al. Acute myeloid leukemia induction with cladribine: outcomes by age and leukemia risk. Leuk Res. 2018;68:72–78.
- Seiter K, Ahmed N, Shaikh A, et al. CLAG-based induction therapy in previously untreated high risk acute myeloid leukemia patients. Leuk Res. 2016;46:74–78.
- Qasrawi A, Bahaj W, Qasrawi L, et al. Cladribine in the remission induction of adult acute myeloid leukemia: where do we stand? Ann Hematol. 2019;98(3):561–579.
- Zhou A, Han Q, Song H, et al. Efficacy and toxicity of cladribine for the treatment of refractory acute myeloid leukemia: a meta-analysis. Drug Des Devel Ther. 2019;13:1867–1878.
- Robak T, Wrzesien-Kus A, Lech-Maranda E, et al. Combination regimen of cladribine (2-chlorodeoxyadenosine), cytarabine and G-CSF (CLAG) as induction therapy for patients with relapsed or refractory acute myeloid leukemia. Leuk Lymphoma. 2000;39(1-2):121–129.
- Wierzbowska A, Robak T, Pluta A, et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: A final report of the Polish Adult Leukemia Group. Eur J Haematol. 2008;80(2):115–126.