ABSTRACT
Objective:
Post-transplantation cyclophosphamide (PTCy) can reduce the incidence of graft versus host disease (GVHD) and this intervention is often applied on adults with hematologic malignancy. However, the high relapse rate hinders the development of the intervention and data of PTCy used on children with hematologic malignancy remains limited. In order to overcome issue of high relapse rate in PTCy treatment, we used fludarabine (Flu), enhanced dose of cytarabine (Ara-C, 9 g/m2), busulfan (Bu), Cy, anti-thymocyte globulin (ATG) combined with PTCy for an intensified conditioning regimen.
Methods:
A total of 22 children with acute leukemia received intensified PTCy conditioning regimen (PTCy intensified group). We matched with 18 children who received modified Bu-Cy and ATG conditioning regimen in the same period (ATG group).
Results:
The two-year cumulative incidences of grade II–IV acute GVHD was significantly lower in PTCy intensified group (13.6 ± 7.7% vs 38.9 ± 11.5%, P = 0.048). Two-year GVHD-free relapse free survival (GRFS) in PTCy seems to be better among the increment group despite not being significant (63.3 ± 10.3% vs 35.4 ± 11.9%, P = 0.092). The positive rate of minimal residual disease after transplantation was significantly lower than that before transplantation (20.0% vs 2.5%, P = 0.029).
Conclusion:
In conclusion, ATG and PTCy combined with Flu-based increased intensity conditioning regimen is effective for acute leukemia in children. It could reduce GVHD rate significantly and potentially improve GRFS.
Introduction
The prognosis of children with high-risk (HR) acute leukemia (AL) is poor, characterized by high incidence of relapse. It is difficult to achieve good survival time by chemotherapy alone. The long-term survival rate of patients with HR AL is less than 20% [Citation1]. Allogeneic hematopoietic stem-cell transplantation (allo-HSCT) brings good prognosis for those children [Citation2,Citation3]. There are various sources and methods of allo-HSCT, which include haploidentical transplantation, matched unrelated donor, and cord blood transplantation [Citation4,Citation5]. Haploidentical allo-HSCT (haplo-HSCT), which has been widely used in the recent decades in China, is a viable option for leukemia children who lack a matched donor. Relapse and graft-versus-host disease (GVHD) are the main complications influencing mortality for children with HR AL. A mass of clinical studies have confirmed that post-transplantation cyclophosphamide (PTCy) regimen can significantly reduce the incidence of GVHD after transplantation [Citation6], but weakness of this regimen is high relapse rate [Citation7]. In order to overcome this weakness, a new intensified conditioning regimen was brought in. FLAG protocol composed of fludarabine (Flu), cytarabine (Ara-C), and granulocyte colony-stimulating factor (G-CSF), with encouraging therapeutic efficacy and low relapse rate, is widely used in the conditioning regimen for refractory relapsed AL in adults and children [Citation8,Citation9].
Therefore, in order to combine the advantages of PTCy and FLAG, we added Flu and increased dose of cytarabine to control the relapse, and combined with PTCy to control GVHD based on modified Bu-Cy with anti-thymocyte globulin (ATG). This modified conditioning regimen, including Flu and increased dosage of Ara-C, busulfan (Bu), ATG, and PTCy, is called PTCy intensified conditioning regimen. We performed a retrospective analysis of children with HR AL who received haplo-HSCT with PTCy intensified conditioning regimen to assess the safety and efficacy of the regimen.
Materials and methods
Patients
A total of 22 patients with HR AL treated at The Affiliated People’s Hospital of Ningbo University from January 2015 to December 2021 were admitted into this study. They had PTCy intensified conditioning regimen (PTCy intensified group). We used an institutional database to retrospectively identify 18 children with HR AL who received modified Bu-Cy combined with ATG (ATG group) in the same period. All patients received a combination of bone marrow and peripheral blood stem cells. The eligibility criteria were HR AL patient younger than 18 years old and received PTCy intensified conditioning regimen. HR AL was defined according to previous definitions [Citation10,Citation11]. HR AL included induction treatment failure, adverse cytogenetic, minimal residual disease (MRD) persistent positive during chemotherapy, relapse more than twice, hyperleukocyte at diagnosis, as well as myelodysplastic syndrome (MDS) to acute myeloid leukemia (AML). Patients with severe infection and major organ dysfunction were excluded. All patients were fully informed of their disease status and treatment options. The informed consent was obtained from all patients and donors in accordance with the Declaration of Helsinki and the transplant protocol was approved by the institutional review board of our center.
Conditioning regimens and GVHD prophylaxis
Patients were given Flu-based intensified conditioning regimen, which consisted of Flu (35 mg/m2/day) on day −9 to −7, Ara-C (3 g/m2/day) on day −9 to −7, Bu (3.2 mg/kg/day) on day −6 to −4, Cy (1.8 g/m2/day), i.v. on day −5 to −4; semustine (250 mg/m2) on day −3 and ATG 7.5 mg/kg on day −5 to −2. Patients were given cyclosporine A (CsA) and mycophenolate mofetil (MMF) for GVHD prophylaxis. The dose of CsA was 2.5 mg/kg/day i.v. from day −1 (target serum concentration, 150–250 ng/ml), which was later switched to oral CsA when gut function returned normal. MMF was administered at 0.5 g orally twice a day from day −1 to day +30; then MMF dose was tapered to half until day 90 and discontinued thereafter. PTCy was administered at a dose of 50 mg/kg i.v. on day +3.
Supportive treatment
All children with HR AL received a comprehensive physical examination before haplo-HSCT to remove the potential infection foci in the respiratory tract, oral cavity, perianal, and other parts. Compound sulfamethoxazole, fluconazole, and intestinal disinfectant were taken orally one week before transplantation. Ganciclovir was used to prevent cytomegalovirus (CMV) infection. Routine hydration and alkalization of urine during pretreatment were performed to prevent hemorrhagic cystitis. Prostaglandin E was used to prevent hepatic venous occlusive disease. Component blood transfusion and other supportive treatment were performed according to the blood routine results of children during transplantation. Sensitive antibiotic treatment was done in case of fever under agranulocytosis.
Laboratory monitoring post-transplantation
At +30, +60, +90, +180, +270 days and 1 year after transplantation, bone marrow puncture, specific gene, Minimal residue disease (MRD), chromosome (donor recipient sex incompatibility), donor T, B, NK cell chimerism by short tandem repeat, immunoglobulin, and cellular immune function were performed to evaluate the efficacy of the therapy. In case of increased MRD, immunosuppressants or donor lymphocyte infusion (DLI) were given in time. The concentration of CsA and CMV DNA was monitored every week.
Study endpoints and definitions
The primary endpoints of the study were the incidence of GVHD and relapse. Secondary endpoints were composed of the infection incidence, overall survival (OS), leukemia-free survival (LFS), and non-relapse mortality (NRM). OS was defined as the time from the date of diagnosis to death or last follow-up date, and LFS was computed from the transplanted date to relapse or the last disease-free follow-up. NRM was defined as death that not related to primary disease progression. GVHD-free and relapse-free survival (GRFS) time meant the time from HSCT to one of the following endpoints: grade III–IV acute GVHD (aGVHD), severe chronic GVHD (cGVHD), relapse, or death.
Statistical analysis
Chi-square test and Mann–Whitney U test were used for categorical variables and continuous variables, respectively. The survival rate was calculated using the Kaplan-Meier method. GVHD analyses were performed as competing risk analyses (death without GVHD as competing risk). P-value of less than 0.05 was considered as statistically significant. Statistical analyses were conducted using SPSS 22.0 (SPSS Inc./IBM, Amonk, NY, USA).
Results
Patient characteristics
Specific clinical characteristics of the study population (n = 40) were shown in . There was no significant difference between the two groups in terms of gender, donor type, disease type, and age.
Table 1. Patient characteristics.
Engraftment and chimerism
The engraftment rates of the two groups were similar. The median time of white blood cell engraftment was 11 days for PTCy intensified group (range, 9–13 days) and 12 days for ATG group (range, 10–17 days), respectively (P = 0.150). Both PTCy intensified group and ATG group had the same median time of platelet engraftment of 12 days (range, 8–19 days and 9–22 days, respectively; P = 0.793). All 40 patients successfully obtained neutrophil and platelet engraftment, and achieved 100% donor chimerism. None of the patients presented donor-specific antibody.
aGVHD and cGVHD
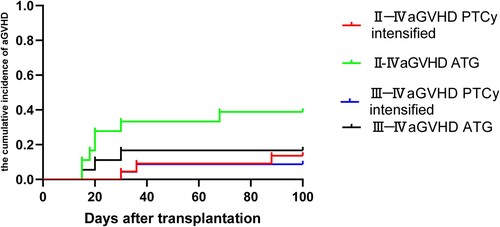
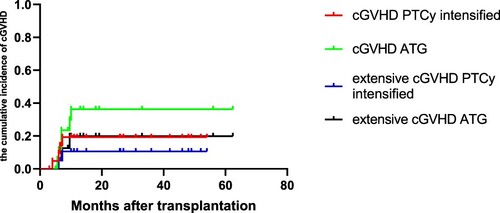
As shown in , the cumulative incidence of grade II–IV aGVHD was significantly lower in PTCy intensified group compared to the ATG group (13.6 ± 7.7% vs 38.9 ± 11.5%, P = 0.048). The cumulative incidence of III–IV aGVHD in PTCy increment and ATG groups were similar (4.5 ± 4.4% vs 16.7 ± 8.8%, P = 0.193). As shown in , the cumulative incidence of two-year cGVHD (18.4 ± 8.6% vs 34.2 ± 11.8%, P = 0.318) and extensive cGVHD (10.1 ± 7.0% vs 19.8 ± 10.7%, P = 0.440) did not significantly differ between the PTCy intensified and ATG group.
Survival analysis
With a median follow-up time of 22 months (range, 3–62 months) after transplantation, there were five and six deaths in PTCy intensified and ATG groups, respectively. In PTCy intensified group, there were one death due to GVHD, two deaths due to infection, and two due to relapse. ATG group had two deaths due to GVHD, one death due to infection, two due to relapse, and one death due to bleeding.
The two-year OS and two-year LFS in PTCy intensified and ATG groups were not significantly different (74.6 ± 10.0% vs 60.1 ± 13.1%, P = 0.446; 72.4 ± 9.6% vs 49.4 ± 11.9%, P = 0.168) (). PTCy intensified group had higher two-year GRFS rate compared to ATG group (63.3 ± 10.3% vs 35.4 ± 11.9%, P = 0.092) ().
MRD, relapse, days of in-hospital stay, and infection complication
(d) revealed no significant difference in the two-year cumulative relapse rate between PTCy intensified and ATG group (13.6 ± 7.3% vs 33.2 ± 12.5%, P = 0.274). Eight patients (20.0%) were MRD positive before transplantation. Bone marrow and blood biochemical tests were screened again three months after haplo-HSCT. Only one patient (2.5%) remained positive MRD. The MRD positive rate after transplantation was significantly lower than that before transplantation (P = 0.029). There was no significant difference in days of in-hospital stay between PTCy intensified and ATG group (40.6 ± 5.2 vs 42.3 ± 6.5, P = 0.368). showed no significant difference in infection rates between two groups within 100 days after transplantation.
Figure 3. Comparison of two-year OS (a), LFS (b), GRFS (c), and RI (d) in PTCy intensified group vs ATG group.
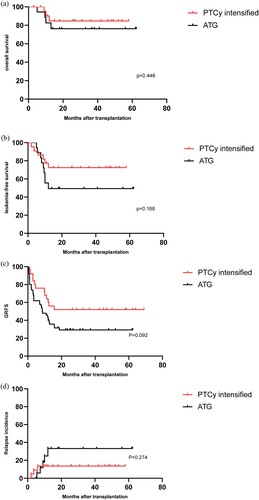
Table 2. Infection complication.
Discussion
In recent years, due to the progress of hematopoietic stem cell transplantation technology, haplo-HSCT has become a conventional means of transplantation. As a common conditioning regimen, FLAG has been widely used in children with HR AL [Citation8]. GVHD and relapse were the main problems that hinder the long-term survival and quality of life for patients after transplantation. How to reduce GVHD while ensuring a low relapse rate has always been a research hotspot in this field [Citation12,Citation13]. PTCy after haploid transplantation could significantly reduce the incidence of GVHD in patients after transplantation, without affecting the graft anti-leukemia effect, obtaining engraftment smoothly without any immune reconstruction delay [Citation14,Citation15]. However, PTCy intervention still brings new problems such as high relapse rate, infection, and engraftment failure rate after transplantation [Citation16,Citation17]. P. Vo’donnell found that adding immunosuppressants like cyclophosphamide (CTX) before transplantation could increase the engraftment rate during PTCy treatment [Citation18]. Therefore, by using ATG before transplantation to enhance immunosuppression, we hope to improve the engraftment rate and reduce the incidence of GVHD. PTCy regimen has achieved positive efficacy in the prevention of GVHD after adult HSCT [Citation19–21]. However, there are few reports of PTCy regimen used in HSCT in children. In addition, we used ATG in this regimen to enhance immunosuppression.
The combination of Flu with high-dose Ara-C increases the intracellular Ara-C content in leukemic cells, which has shown a synergistic effect. Thus, administration of Flu prior to Ara-C may enhance the clinical efficacy of Ara-C. Several studies have added mitoxantrone, amsacrine, or idamycin in combination with FLAG scheme, to achieve better prognosis for refractory and recurrent AML patients [Citation22–24]. Flu and Ara-C have synergistic effect. The application of Flu in the first 4 h of Ara-C could increase the concentration of Ara-c in tumor cells, and the chance of cross-resistance is low; The FLAG intervention composed of Flu, Ara-C, and G-CSF has been widely used in the treatment of refractory and recurrent leukemia or pretreatment of allo-HSCT, and achieved good results [Citation25,Citation26]. Some studies tried to incorporate drugs such as mitoxantrone or idarubicin to the FLAG pretreatment to intensify the pretreatment. However, drug-related heart damage limited the application of this protocol. Considering the safety and efficacy of pretreatment, we increased the dose of Ara-C in FLAG conditioning regimen, in order to enhance the intensity of the pretreatment regimen and reduce the later recurrence.
ATG-based and PTCy-based T cell removal in vivo were commonly used after haplo-HSCT for GVHD prevention. Wang et al. [Citation6] added GVHD prevention program based on G-CSF/ATG, low-dose CTX (14.5 mg/kg/d on day +3, +4) after transplantation. One hundred fourteen patients who used ATG + PTCy and 125 patients who used ATG alone as GVHD prevention were compared, results showed that III–IV aGVHD and NRM in ATG + PTCy group were significantly lower than those in ATG group (5% vs 18%, P = 0.003 and 6% vs 15%, P = 0.045, respectively). There was no significant difference between the two-year cumulative recurrence rate (13% vs 14%, P = 0.62) and OS (83% vs 77%, P = 0.18). GRFS in ATG PTCy group was significantly better than that in ATG group (63% vs 48%, P = 0.039). Law et al. [Citation27] reported that the addition of ATG (total 4.5 mg/kg, −3 to −1 day) on the basis of PTCy prevention program also achieved good results. The cumulative incidence of total and III–IV aGVHD 100 days after transplantation was 38.3% and 5.2%, respectively, the incidence of chronic GVHD was 15.5%.
In this study, we used appropriate ATG (7.5 mg/kg/d on day −5 to day −2) before transplantation and appropriate cyclophosphamide (CTX, 50 mg/kg on day +3) after transplantation to prevent GVHD, and intensified the pretreatment at the same time. The pretreatment achieved satisfactory results. The results showed that II–IV aGVHD and in ATG + PTCy group were significantly lower than ATG group (13.6% vs 38.9%, P = 0.048), and GRFS had an improvement trend (63.3% vs 35.4%, P = 0.092). There was no significant difference between the two-year LFS and OS (72.4% vs 49.4%, P = 0.168; 74.6% vs 60.1%, P = 0.446). We adopted Flu intensified conditioning regimens combined with PTCy and ATG for GVHD prophylaxis, achieving satisfactory results of two-year OS (74.6%) and low relapse rate (13.6%), which was better than the results reported in the literature. When compared with other studies using PTCy regimen, the incidence of GVHD was not high [Citation20,Citation28,Citation29], and most of aGVHD was grade I–II, and cGVHD was mainly limited. The above results showed that this regimen had good GVHD prevention effect.
Relapse is a main cause of death after transplantation. The two-year cumulative relapse rate of patients in this study was 13.6%, which is lower than that other using PTCy regimen studies [Citation20,Citation28,Citation29]. The lower relapse rate of this study may be attributed to the following factors: all patients achieved CR before transplantation; most patients MRD were negative before transplantation.
Notably, we increased the cytarabine dose from 8 to 9 g/m2 based on conventional FLAG conditioning regimen, which is effective to eliminate the residual leukemia cells. Seven patients died, three of them died to relapse, indicating that relapse was the main cause of death for children with AL after allo-HSCT, which was consistent with previous reports [Citation30,Citation31]. Previous studies have proved that FLAG can reduce relapse rate and improve survival for patients with HR or refractory hematological diseases [Citation32,Citation33]. However, how to effectively combine different chemo drug and find the optimal dosage still require further studies.
Actually, pretreatment could kill most leukemia cell and reduce them below the MRD detection level. Therefore, it causes lower proportion of MRD. In this study, eight patients were MRD positive before transplantation. After transplantation, only one patient remained MRD positive. Transplantation significantly increased the proportion of negative MRD in this study, which was consistent with the results reported by Zhou [Citation34].
In conclusion, it’s safe and feasible to adopt Flu intensified pretreatment combined with PTCy as the conditioning regimen in childhood hematological malignancy treatment. This therapy achieved satisfactory results in long-term OS, relapse rate, and GVHD incidence. Compared with modified Bu-Cy and ATG conditioning regimen, ATG and PTCy combined with Flu-based increased intensity conditioning regimen could reduce GVHD rate significantly and potentially improve GRFS. However, it is worth noting that due to the small sample size and the short follow-up time in some cases, it is still necessary to validate finding and making adjustment on the dosage, drug compatibility, and strengthening anti-infection treatment with larger sample size. The theoretical basis needs further investigation.
Ethical statement
The study was approved by the institutional ethics committee.
Supplemental Material
Download MS Word (54.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shen S, Cai J, Chen J, et al. Long-term results of the risk-stratified treatment of childhood acute lymphoblastic leukemia in China. Hematol Oncol. 2018;36(4):679–688.
- Ibrahimova A, Pommert L, Breese EH. Acute leukemia in infants. Curr Oncol Rep. 2021;23(3):27–36. doi:10.1007/s11912-021-01021-1
- Takachi T, Watanabe T, Miyamura T, et al. Hematopoietic stem cell transplantation for infants with high-risk KMT2A gene rearranged acute lymphoblastic leukemia. Blood Adv. 2021;5(19):3891–3899.
- Xu L, Chen H, Chen J, et al. Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. J Hematol Oncol. 2017;10(1):33–49. doi:10.1186/s13045-017-0390-6
- Hyery K. Treatments for children and adolescents with AML. Blood Res. 2020;55(S1):S5–S13.
- Wang Y, Wu DP, Liu QF, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. 2019;12(1):88–96. doi:10.1186/s13045-019-0781-y
- Khan MA, Bashir Q, Chaudhry QU, et al. Review of haploidentical hematopoietic cell transplantation. J Glob Oncol. 2018;4:1–13.
- Kato K, Nao Y, Kimikazu M, et al. Fludarabine, cytarabine, granulocyte colony-stimulating factor and melphalan (FALG with L-PAM) as a reduced toxicity conditioning regimen in children with acute leukemia. Pediatr Blood Cancer. 2014;61(4):712–716. doi:10.1002/pbc.24922
- Shargian-Alon L, Wolach O, Rozovski U, et al. Sequential treatment with FLAG-IDA/treosulfan conditioning regimen for patients with active acute myeloid leukemia. Ann Hematol 2020;99(12):2939–2945. doi:10.1007/s00277-020-04232-x
- Warrick K, Althouse SK, Rahrig A, et al. Factors associated with a prolonged hospital stay during induction chemotherapy in newly diagnosed high risk pediatric acute lymphoblastic leukemia. Leuk Res 2018;71:36–42. doi:10.1016/j.leukres.2018.06.013
- Huschart E, Miller H, Salzberg D, et al. Azacitidine and prophylactic donor lymphocyte infusions after hematopoietic stem cell transplantation for pediatric high-risk acute myeloid leukemia. Pediatr Hematol Oncol. 2021;38(2):154–160.
- Shargian-Alon L, Wolach O, Rozovski U, et al. Sequential treatment with FLAG-IDA/treosulfan conditioning regimen for patients with active acute myeloid leukemia. Ann Hematol 2020;99(12):2939–2945.
- Kazemi MH, Dehaghi BK, Roshandel E, et al. Oncolytic virotherapy in hematopoietic stem cell transplantation. Hum Immunol 2021;88(9):640–648.
- Shah RM. Contemporary haploidentical stem cell transplant strategies in children with hematological malignancies. Bone Marrow Transplant. 2021;56(7):1518–1534. doi:10.1038/s41409-021-01246-5
- Yadav SP, Sharma A, Kapoor R, et al. Thiotepa based conditioning for haploidentical stem cell transplantation with post transplant cyclophosphamide for pediatric acute leukemia Is highly effective. Biol Blood Marr Transplant. 2020;26(3):S163–S164.
- Katsanis E, Sapp LN, Varner N, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide/bendamustine in pediatric and young adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2018;24(10):2034–2039. doi:10.1016/j.bbmt.2018.06.007
- Uygun V, Karasu G, Dalolu H, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant high-dose cyclophosphamide in high-risk children: a single-center study. Pediatr Transplant. 2019;23(7):e13546–e13555.
- O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood and Marrow Transplant. 2002;8(7):377–386. doi:10.1053/bbmt.2002.v8.pm12171484
- Ahmed S, Kanakry JA, Ahn KW, et al. Lower graft-versus-host disease and relapse risk in post-transplant cyclophosphamide–based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol Blood and Marrow Transplant. 2019;25(9):1859–1868. doi:10.1016/j.bbmt.2019.05.025
- Symons HJ, Zahurak M, Cao Y, et al. Myeloablative haploidentical BMT with posttransplant cyclophosphamide for hematologic malignancies in children and adults. Blood Adv. 2020;4(16):3913–3925.
- Dreger P, Sureda A, Ahn KW, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3(3):360–369. doi:10.1182/bloodadvances.2018027748
- Quarello P, Berger M, Rivetti E, et al. FLAG-liposomal doxorubicin (myocet) regimen for refractory or relapsed acute leukemia pediatric patients. J Pediatr Hematol Oncol. 2012;34(3):208–216. doi:10.1097/MPH.0b013e3182427593
- Fong CY, Grigoriadis G, Hocking J, et al. Fludarabine, cytarabine, granulocyte-colony stimulating factor and amsacrine: an effective salvage therapy option for acute myeloid leukemia at first relapse. Leuk Lymphoma. 2013;54(2):336–341. doi:10.3109/10428194.2012.713479
- Yilmaz BS, Ataseven E, Kizmazoglu D, et al. Nüks/refrakter akut lösemili Çocuklarda İdarubisin eklenerek veya eklenmeden FLAG tedavisi: Bir türk pediatrik hematoloji merkezi deneyimi. Turkish J Hematol. 2017;34(1):46–51. doi:10.4274/Tjh.2015.0411
- Shargian-Alon L, Wolach O, Rozovski U, et al. Sequential treatment with FLAG-IDA/treosulfan conditioning regimen for patients with active acute myeloid leukemia. Ann Hematol 2020;99(12):2939–2945. doi:10.1007/s00277-020-04232-x
- Wang L, Devillier R, Wan M, et al. Clinical outcome of FLAG-IDA chemotherapy sequential with Flu-Bu3 conditioning regimen in patients with refractory AML: a parallel study from Shanghai Institute of Hematology and Institut Paoli-Calmettes. Bone Marrow Transplant. 2019;54(3):458–464. doi:10.1038/s41409-018-0283-5
- Law AD, Salas MQ, Lam W, et al. Reduced intensity conditioning and dual T-lymphocyte suppression with anti-thymocyte globulin and post-transplant cyclophosphamide as graft versus host disease prophylaxis In haploidentical stem cell transplants For hematological malignancies. biology of blood & marrow transplantation. J Am Soc Blood Marrow Transpl. 2018;24(11):2259–2264.
- Mccurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. doi:10.1182/blood-2015-01-623991
- Klein OR, Buddenbaum J, Tucker N, et al. Nonmyeloablative haploidentical bone marrow transplantation with post-transplantation cyclophosphamide for pediatric and young adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transpl. 2017;23(2):325–332. doi:10.1016/j.bbmt.2016.11.016
- Maher OM, Silva JG, Wu J, et al. Outcomes of children, adolescents, and young adults following allogeneic stem cell transplantation for secondary acute myeloid leukemia and myelodysplastic syndromes-The MD Anderson Cancer Center experience. Pediatr Transplant. 2017;21(3):e12890–e12898. doi:10.1111/petr.12890
- Molina B, Gonzalez VM, Herrero B, et al. Kinetics and risk factors of relapse after allogeneic stem cell transplantation in children with leukemia: A long-term follow-Up single-center study. Biol Blood Marrow Transplant. 2018;25(1):100–106.
- Farooq MU, Mushtaq F, Farooq A, et al. FLAG vs FLAG-IDA: outcomes in relapsed/refractory acute leukemia. Cancer Chemot Pharm. 2019;83(6):1191–1193.
- Westhus J, Noppeney R, Schmitz C, et al. Etoposide combined with FLAG salvage therapy is effective in multiple relapsed/refractory acute myeloid leukemia. Acta Haematol 2019;143(5):1–8.
- Zhou Y, Othus M, Araki D, et al. Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016;30(7):1456–1464. doi:10.1038/leu.2016.46