ABSTRACT
Objectives:
In clinical practice, the majority of α-thalassaemia cases arise from deletions of the α-globin genes. However, a subset of cases is attributed to rare haemoglobin variants, which can manifest with borderline or normal screening results, potentially leading to missed diagnoses in clinical practice.
Methods:
Blood samples were collected from family members and underwent haematological, DNA and RNA analysis.
Results:
The five-month-old proband presented a haematological phenotype consistent with Hb H disease. The mother’s haematology profile was consistent with an α-thalassaemia carrier, while the father exhibited a borderline reduction in MCV and MCH. MALDI-TOF identified an abnormal α-chain in the proband. DNA analysis revealed a novel α-globin variant (HBA2:c.175C>A, α58His>Asn, Hb DG-Nancheng) affecting the distal histidine in the family. The father and the mother had α-genotype of --SEA/αα and αDG-Nanchengα/αα, respectively; while the proband inherited both mutant alleles (--SEA/αDG-Nanchengα). Sequencing of cDNA from HBA2 gene identified an equal ratio of normal and mutant alleles.
Conclusion:
This rare case highlighted the importance of identifying rare haemoglobin variant during prenatal screening. The clinical and genetic data provides useful information on the pathogenicity of this variant and further insight into the role of distal histidine residue of α-globin.
Introduction
Alpha-thalassaemia (α-thal) is the most prevalent autosomal recessive disorder with geographical prevalence in Southeast Asia, the Mediterranean region and parts of Africa. It arises from mutations in the α-globin genes, impacting α-globin production and leading to a relative β-globin excess. This excess β-globin can tetramerize into HbH (β4), a form with poor oxygen affinity and decreased membrane stability [Citation1]. This imbalance disrupts erythrocyte development and manifests in varying clinical severity, ranging from asymptomatic carriers to individuals with severe anemia and hemolysis. The spectrum depends on the number and type of mutant alleles inherited. Carriers typically remain asymptomatic. Conversely, individuals with α-thal major (Hb Bart’s hydrops fetalis), characterized by the complete absence of α-globin production, often exhibit severe anemia and hydrops fetalis prenatally. The intermediate phenotypes, Hb H disease, manifest with varying degrees of hemolysis and anemia, influenced by the specific mutations.
Over 1000 α-thal variants have been documented to date in databases of HbVar (https://globin.bx.psu.edu/globin/hbvar/) and ITHANET, (https://www.ithanet.eu/db/ithagenes). Deletions involving one or both α-globin genes are the most common α-thal variants, leading to complete transcript loss from the affected genes. However, single nucleotide variations (SNVs) also contribute significantly. These SNVs can impact α-globin production through diverse mechanisms [Citation2]. One such mechanism involves the production of highly unstable mutant α-globin chains. These unstable chains undergo rapid post-synthetic degradation and are likely unable to form dimers or tetramers. Consequently, there is no associated loss of normal β-globin chains, which remain in excess within the red blood cell [Citation3,Citation4]. The hematological findings in patients with unstable variants are complex, influenced by factors such as whether the HBA1 or HBA2 gene is involved, the degree of instability of the mutant chain, and the presence of interacting alleles [Citation5,Citation6]. In this study, we identified a novel α-chain variant (HBA2:c.175C > A) on the distal histidine residue in a Chinese family, this variant probably cause unstable haemoglobin by disrupting haeme – globin interactions.
Materials and methods
Subjects
The family originated from Guangdong Province of Southern China. The parents were healthy and exhibited no signs of anaemia or cyanosis. During pregnancy, the mother was screened as mild α-thal, consistent with her --SEA/αα genotype, the father had borderline low MCV and MCH values, but normal Hb typing. He tested negative for common α/β-thal mutations prevalent in the Chinese population. They were informed of the risk of a fetus with Hb H or Bart’s hydrops due to rare α-thal mutations. However, after genetic counselling, the couple opted not to perform further testing Their decision was based on the exclusion of Bart’s hydrops fetalis by foetal ultrasound and their acceptance of a baby with Hb H disease, along with the ability to afford the associated treatment costs. The proband was born without any exaggerated or prolonged neonatal jaundice. However, he was suspected to have Hb H disease due to the detection of a 17.4% Hb Bart’s level during newborn screening. He was subsequently referred to us for a genetic diagnosis at the age of five months. Upon examination, he displayed no jaundice, splenomegaly, hepatomegaly and cyanosis. With informed consent obtained, blood samples were collected from proband and his parents for this study. Study ethics approval was granted by the institutional review board of Dongguan Maternal and Children Health Hospital (Ethical approval No.2021-35).
Haematological and Hb analysis
The haematological parameters were determined with a Sysmex XT-2000i automated blood cell counter (Sysmex Co Ltd, Kobe, Japan). Hemoglobin was studied by capillary zone electrophoresis (CE) (Capillarys, Sebia, Montpellier, France).
The matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (MS) technology was also used for abnormal Hb detection as described by Zhang Q, et al [Citation7]. In brief, 200 µl whole blood was diluted with 1800 µl deionized water. Then, the hemolysate and matrix solution (10 g/L sinapinic acid [Sigma-Aldrich, St. Louis, USA], 34% Acetonitrile [Sigma-Aldrich, St. Louis, USA], 0.1% Trifluoroacetic Acid [Sigma-Aldrich, St. Louis, USA]) was mixed a ratio of 1:9, and 2.5 µl of the mixture was spotted onto a stainless steel target and was dried at 39 °C. Finally, MS analysis was performed on the MALDI-TOF mass spectrometer (Intelligent Biosystems, Guangzhou, China) and mass spectra data was processed with m/z: 5000–20000 and Focus mass: 15000.
Genetic analysis
Genomic DNA was extracted from all peripheral blood samples using an automatic nucleic acid extraction system (LabAid820; Xiamen Zhishan Biotechnology, Xiamen, China). The most common α-thal mutations [--SEA (Southeast Asian), –THAI (Thai), -α3.7 (rightward), -α4.2 (leftward), Hb Constant Spring (HBA2:c.427T > C), Hb Quong Sze (HBA2:c.377T > C) Hb Westmead (HBA2:c.369C > G)] in Chinese were genotyped using a commercial thalassaemia genotyping kit (Yaneng BioSciences Co., Ltd, Shenzhen, China). To identify an unknown α-thal allele, the HBA2 and HBA1 genes were amplified and directly sequenced [Citation8].
In order to evaluate the effect of the novel mutation at the mRNA level, total cellular RNA was extracted from whole blood using TRIzol reagent (GIBCO BRL, Gaithersburg, MD, USA), as recommended by the manufacturer. The cDNA synthesis was performed using the PrimeScriptTM RT reagent kit (Takara Co. Ltd., Dalian, China). cDNA form the α2-mRNA was selectively amplified and sequenced with a forward primer (5’-CTGCCGACAAGACCAACGTC-3’) and a reverse primer (5’-AACGGGCAGGAGGAATGGCT-3’).
Results
The haematological and genotypic results are summarised in . The proband presented with microcytic hypochromic anemia (Hb 95 g/L, MCV 67.4 fL, MCH 16 pg) and a haemoglobin pattern of Hb A2 2.4%, Hb F 2.2% and Bart’s 1.9%, consistent with Hb H disease. The mother’s haematological profile was consistent with an α-thal carrier, while the father exhibited borderline reductions in both MCV and MCH (). Routine α-thal genotyping identified that the proband and mother were heterozygous for --SEA type deletion. Direct sequencing of HBA2(1) genes revealed that the proband was homozygous for a novel transversion at codon 58 (HBA2:c.175C > A, see ). This mutation changes the protein residue histidine to asparagine (). The father was heterozygous for this mutation. We have named the novel variant Hb DG-Nancheng for the region from where the family originated. Therefore, the father’s α-genotype was αDG-Nanchengα/αα, and the proband was a compound heterozygote for --SEA and Hb DG-Nancheng.
Figure 2. DNA and RNA analysis results of each family member. DNA (A-C) and RNA (D-F) results of the α2-globin gene indicated a homozygous and heterozygous variant of HBA2:c.175C > A (red arrow) in proband (A and D) and father (B and E), respectively, which was not identified in mother (C and F). Further, an equal ratio of normal and mutant alleles could be observed from cDNA sequencing result in the heterozygote father (E).
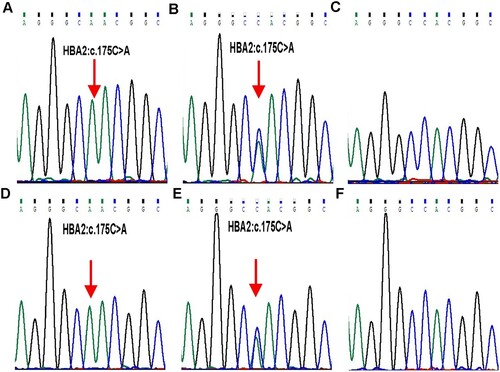
Figure 3. Stick models of α native (A) and (H58N) (B) distal pockets in adult human haemoglobin. The structure of Hb DG-Nancheng-O2 was generated from native HbA-O2 (PDB code 2DN1) by mutating the distal E7 residue His58 to Asn in the program Pymol. Red sphere: oxygen. The native His (E7) side chain forms a hydrogen bond with bound O2, in Hb DG-Nancheng-O2, the distal E7 residue Asn58 changes its side chain orientation out of the heme pocket and does not form a hydrogen bond with bound ligands. Nascent globin chains rapidly incorporate heme, which stabilizes their native folding into Hb subunits composed of seven or eight a helices named A–H, which fold together into a globular structure. The alteration by Asn58 possibly caused the inability of mutant α-chains bind to haeme, ultimately resulting in proteolytic degradation.
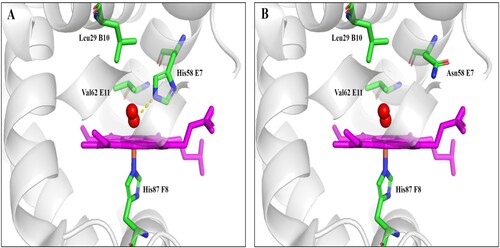
Table 1. Hematological data and α- and β- genotypes of the family members.
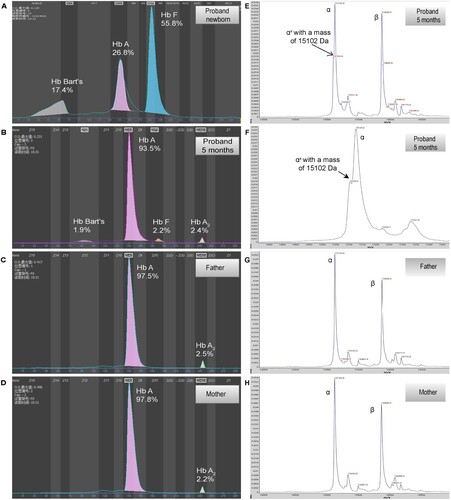
Since CE did not identify any abnormal haemoglobin in the proband and the father, MALDI-TOF was employed for further haemoglobin analysis. An abnormal α-globin chain at 15103 m/z with a 23 Da mass decrease compared with the normal α-chain (15,127 Da) was identified in the proband, which was agreed with the molecular weight change caused by amino acid replacement (histidine > asparagine) (B). However, MALDI-TOF did not identify any abnormal α-globin chain in the father. Sequencing analysis of cDNA derived from the proband and father’s α2-mRNA revealed a homozygote and heterozygous C > A change, respectively. This finding indicates the presence of mRNA transcripts originating from the mutant allele.
Figure 1. Hemoglobin patterns from capillary electrophoresis (CE) and MALDI-TOF. By CE, a Bart’s level of 17.5% and 1.9% was identified in proband at newborn (A) and 5 months (B), respectively. No abnormal peak was identified in father (C) and mother (D). By MALDI-TOF, a normal α chain at 15,127 m/z and an abnormal α chain (αX) at 15,103 m/z were identified in proband (E and F, the peak of abnormal α chain is zoomed at F), the abnormal α chain was not identified in father (G) and mother (H).
Discussion
In this study, the proband could be diagnosed as Hb H disease based on the haematological phenotypes observed at newborn and 5-month-old (). No other pathogenic variant was identified in HBA2/1 genes by sequencing and MLPA analysis. Therefore, it is likely that the αDG-Nanchengα/αα and --SEA/αDG-Nanchengα genotypes are responsible for the α+-thal and the Hb H phenotype observed in the father and proband, respectively. Consequently, the HBA2:c.175C > A variant probably results in a decrease in α-chain production.
The distal histidine (E7 helical position) plays a critical role in distal haem binding through hydrogen bonding. This interaction is essential for preventing auto-oxidation and regulating the ligand affinity [Citation9]. Experimental evidence demonstrates mutations affecting amino acids within the ligand pocket frequently produce strong functional effects, including destabilization, altered affinity for O2, and increased rates of methaemoglobinemia (metHb) formation and haeme loss [Citation10,Citation11].
Besides, naturally occurring mutations at codon α58 have also been described in clinical cases. These include Hb M-Boston (HBA2 or HBA1:c.175C > T, α58His > Tyr) [Citation12], Hb Flurlingen (HBA2:c.175C > G, α58His > Gln), Hb Boghé (HBA2:c.177C > A, α58His > Gln) [Citation13,Citation14] and Hb Kirklareli (HBA1:c.176A > T, α58His > Leu) [Citation11,Citation15]. Hb M-Boston is reported to be relatively frequent and consistently causes cyanosis with low oxygen saturation and markedly elevated levels of methaemoglobin (MetHb). The other three variants have only been reported in a small number of heterozygotes, with diverse clinical presentations. However, available data suggest that Hb Kirklareli exhibits a lower oxygen affinity and denatures very rapidly under physiological conditions due to autoxidation and increased haem loss from the mutant α subunits [Citation11]. Hb Flurlingen is likely to cause a reduction in mRNA levels, potentially due to altered mRNA stability [Citation13].
The novel Hb DG-Nancheng represents the fifth mutation identified at α58 position. mRNA analysis suggests that the decrease in α-globin chain production is not attributable to changes in mRNA levels. The Hb DG-Nancheng variant likely exerts a destabilising effect due to alterations in the haem pocket (). MALDI-TOF detected abnormal α-chains only in the proband, not the carrier father. This observation may be explained by the likely high instability of the mutant α-chains, leading to rapid catabolisation. Additionally, trans-inheritance of the --SEA deletion might contribute by reducing the normal pool of free α-chains, potentially forcing the abnormal α-chains to form more stable dimers or tetramers. These dimers or tetramers may be partially or transiently stabilised when incorporated into haemoglobin molecules [Citation5].
Owing to the proband’s young age, further observation and follow-up are required to fully characterise the clinical features of HbH disease with the --SEA/αDG-Nanchengα genotype. In light of the rapid catabolism of the mutant globin, the clinical course is likely to be similar to that of deletional HbH disease (such as --SEA/-α3.7 and --SEA/-α4.2). Individuals with deletional HbH disease typically lead normal lives without any overt clinical symptoms [Citation16]. Therefore, prenatal diagnosis of --SEA/αDG-Nanchengα may not be unwarranted.
In clinical practice, it is not rare that individuals with positive thalassaemia screening results have negative results from routine PCR-based genetic testing. Clinicians usually recommend further testing for rare mutations. However, further testing is usually not recommended for those with borderline MCV, MCH, and a normal hemoglobin pattern, which suggests a possible rare α+-thal mutation. It is important to note that different variants in one of the four α-globin genes often cause similar haematological changes, certain types (such as Hb QS) can cause severe clinical presentations when occurring in compound heterozygosity with an α0-thal mutation, or even in homozygous state. Even for hypomorphic allele (such as Hb WS), confirmation in parents in a prenatal stage can provide medical preparation of potential conditions after birth. Therefore, further testing for rare mutations is advisable in individuals with borderline blood indices and negative primary genotyping, especially if their spouse carries an α0-thal mutation.
In summary, this study reported a novel α-chain variant (HBA2:c.175C > A) in a Chinese family. The prenatal and postnatal clinical pathway of this case offers a valuable reference for thalassaemia prevention and diagnosis. Analysis of the clinical and genetic data offers insights into the variant’s pathogenicity and the role of the distal α-globin chain histidine residue.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Giardine BM, Joly P, Pissard S, et al. Clinically relevant updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2021;49(D1):D1192–D1196. doi:10.1093/nar/gkaa959
- Harteveld CL, Achour A, Arkesteijn SJG, et al. The hemoglobinopathies, molecular disease mechanisms and diagnostics. Int J Lab Hematol. 2022;44(S1):28–36. doi:10.1111/ijlh.13885
- Thom CS, Dickson CF, Gell DA, et al. Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harb Perspect Med. 2013;3(3):a011858. doi:10.1101/cshperspect.a011858
- Williamson D. The unstable haemoglobins. Blood Rev. 1993;7(3):146–163. doi:10.1016/0268-960X(93)90002-L
- Steinberg MH. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. 2nd Cambridge: Cambridge Univ Pr; 2009. p. 826.
- Wajcman H, Traeger-Synodinos J, Papassotiriou I, et al. Unstable and thalassemic α chain hemoglobin variants: a cause of Hb H disease and thalassemia intermedia. Hemoglobin. 2008;32(4):327–349. doi:10.1080/03630260802173833
- Zhang Q, Wang G, Sun D, et al. Maldi–TOF–MS for rapid screening and typing of β-globin variant and β-thalassemia through direct measurements of intact globin chains. Clin Chem. 2022;68(12):1541–1551. doi:10.1093/clinchem/hvac151
- Sun M, Lou J, Zhag Y, et al. Polymorphisms of alpha-globin genes compromise polymerase chain reaction-based alpha-thalassemia genotyping in three chinese families. Hemoglobin. 2019;43(2):101–106. doi:10.1080/03630269.2019.1607372
- Birukou I, Schweers RL, Olson JS. Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin. J Biol Chem. 2010;285(12):8840–8854. doi:10.1074/jbc.M109.053934
- Mathews AJ, Rohlfs RJ, Olson JS, et al. The effects of E7 and E11 mutations on the kinetics of ligand binding to R state human hemoglobin. J Biol Chem. 1989;264(28):16573–16583. doi:10.1016/S0021-9258(19)84745-2
- Bissé E, Schaeffer-Reiss C, Van Dorsselaer A, et al. Hemoglobin Kirklareli (αH58L), a new variant associated with iron deficiency and increased CO binding. J Biol Chem. 2017;292(6):2542–2555. doi:10.1074/jbc.M116.764274
- Chen J, Zhao H, Deng X, et al. Hereditary methaemoglobinemia caused by haemoglobin M Boston. Lancet Haematol. 2020;7(12):e912. doi:10.1016/S2352-3026(20)30289-1
- Qadah T, Finlayson J, Dennis M, et al. Experimental characterization of Hb flurlingen (HBA2:c.177C > G, p.His > Gln) and Hb Boghe (HBA2:c.177C > A, p.His > Gln) reveals contradictory HBA2 expression and translation patterns despite identical amino acid substitutions. Hemoglobin. 2015;39(5):340–345.
- Lacan P, Francina A, Souillet G, et al. Two new alpha chain variants: Hb Boghe [α58(E7)His–>Gln, α2], a variant on the distal histidine, and Hb CHarolles [α103(G10)His-Tyr, α1]. Hemoglobin. 1999;23(4):345–352. doi:10.3109/03630269909090750
- Pedroso GA, Fernandes P, Jorge SEDC, et al. Hemoglobin Kirklareli [α2 59(E7) His > Leu; HBA2:c.176A > T] in a Brazilian child with severe dyspnea and low O2 saturation. Ann Hematol. 2019;98(12):2853–2855. doi:10.1007/s00277-019-03839-z
- Chen FE, Ooi C, Ha SY, et al. Genetic and clinical features of hemoglobin H disease in Chinese patients. N Engl J Med. 2000;343(8):544–550. doi:10.1056/NEJM200008243430804
