ABSTRACT
Purpose:
To explore the efficacy and safety of venetoclax-based combination therapy for older patients with newly diagnosed acute myeloid leukemia (AML).
Methods:
We performed a systematic review and meta-analysis of clinical trials comparing venetoclax plus hypomethylating agents (HMAs) or low-dose cytarabine (LDAC) with mono-HMAs or LDAC. The random or fixed effects model was applied to the studies based on heterogeneity. Dichotomous data were summarized using the risk ratio (RR) and 95% confidence interval (CI). Continuous variable data were reported as weighted mean differences (WMDs).
Results:
Nine studies, including a total of 1232 patients, were included in this meta-analysis. Thec complete remission (CR)/complete remission with incomplete hematological recovery (CRi) rate of the venetoclax (Ven) + azacytidine (Aza) group was significantly greater than that of the Aza monotherapy group (RR: 2.42; 95% CI: 1.85–3.15; P < 0.001). Similarly, the CR/CRi rate of the Ven + LDAC group was also significantly greater than that of the LDAC monotherapy group (RR: 2.57; 95% CI: 1.58–4.17; P = 0.00). The same results were observed for OS among these groups. However, the incidence of febrile neutropenia was greater in the Ven + Aza group than in the Ven + Decitabine (Dec) or monotherapy Aza group (RR: 0.69; 95% CI: 0.53–0.90; P = 0.006 and RR: 2.19; 95% CI: 1.58–3.03; P < 0.001, respectively). In addition, the Ven + LDAC group had significantly greater rates of constipation, diarrhea, nausea, and vomiting than the LDAC monotherapy group, with RRs and CIs of 0.61 (95% CI 0.44–0.83, P = 0.002), 1.81 (95% CI 1.22–2.67, P = 0.003), 1.39 (95% CI 1.06–1.82, P = 0.016), and 1.80 (95% CI 1.19–2.72, P = 0.005), respectively.
Conclusion:
Venetoclax combined with azacitidine, decitabine, or LDAC significantly improved the CR/CRi and OS of patients with previously untreated AML. However, venetoclax plus azacitidine or LDAC was more likely to lead to increased febrile neutropenia and gastrointestinal toxicity.
Introduction
Acute myeloid leukemia (AML) is a clonal proliferative tumor of immature myeloid cells with a specific immunophenotype characterized by the accumulation of immature myeloid cells in the bone marrow, especially in elderly individuals [Citation1]. Traditional chemotherapy and hematopoietic stem cell transplantation (HSCT), which can markedly improve the outcomes of AML patients, have been the gold standard of care for treatment-naive AML patients [Citation2]. However, some AML patients are incurable and intolerant to intensive chemotherapy, especially older AML patients [Citation3]. Furthermore, low-intensity chemotherapy regimens, such as hypomethylating agents (HMAs) or low-dose cytarabine (LDAC), only achieve a complete remission (CR) rate of approximately 11%, and the survival time is continually unsatisfactory [Citation4,Citation5].
In recent years, patients have begun to prefer targeted therapies instead of standard chemotherapy agents. One such targeted therapy agent, venetoclax, is a highly selective orally accessible BCL-2 inhibitor that targets the intrinsic pathway of mitochondrial apoptosis [Citation6]. Previous studies have shown that the use of venetoclax alone can increase the objective response rate (ORR) by 19%∼29% in patients with relapsed/refractory acute myeloid leukemia [Citation7]. However, venetoclax monotherapies can potentially cause drug resistance due to high levels of BCL-XL and MCL1. A previous study has shown that azacitidine can reduce MCL1 levels, increasing the effectiveness of venetoclax-based therapy. Several studies have further shown that the use of venetoclax combined with HMAs results in a 43%∼46% ORR in patients with relapsed/refractory acute myeloid leukemia [Citation8,Citation9]. Therefore, venetoclax combined with low-dose chemotherapy treatments, such as HMAs and LDAC, must be developed for newly diagnosed AML patients, and these treatments must be acceptable in clinical trials.
In 2018, the first phase 1b study reported the benefit of venetoclax with an HMA treatment regimen. The CR/complete remission with incomplete hematological recovery (CRi) of this combined treatment could reach 61%, with initial dose of venetoclax varying from 20, 50, or 100 mg on the first day of week 1 with a weekly dose ramp-up schedule, venetoclax dosage could be increased to 400, 800, or 1200 mg, which demonstrated that venetoclax plus HMAs is safe and provides durable responses [Citation10]. Moreover, among patients who progressed after venetoclax treatment, a high proportion of patients achieved an overall response [Citation11]. Venetoclax combined with HMAs for newly diagnosed AML patients results in a CR/CRi of up to 59∼66.4% [Citation11,Citation12]. In addition, venetoclax combined with LDAC for previously untreated patients with AML also results in a CR/CRi of up to 65.9% [Citation13].
At present, no study has investigated which venetoclax-based combination therapy is most effective. In addition, there is no summary analysis of neutropenic toxicity and gastrointestinal toxicity caused by venetoclax-based combination therapy. Therefore, the identification of the optimal treatment to ensure minimal toxicity and side effects is urgently needed. To understand the predictive value of venetoclax combined with other therapeutic agents for AML, this meta-analysis was carried out to determine the efficacy and safety profile of venetoclax combined with HMAs or LDAC for previously untreated AML patients.
Materials and methods
The meta-analysis was performed according to the Cochrane group [Citation14] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation15]. In addition, the review protocol was registered with PROSPERO (CRD42022331475).
Data sources and searches
We performed a comprehensive literature search of the PubMed, Embase, Cochrane Library, Web of Science, and Medline databases from inception to October 1st, 2023, with the following terms: ‘acute myeloid leukemia’ or ‘AML,’ ‘venetoclax’ or ‘ABT-199’ or ‘GDC-0199’. A manual search of the reference lists of the included articles and previously published reviews was also performed to retrieve articles not covered by the database search. HBH imported all the search results to Endnotes and excluded duplicates, including software and manual checks. HBH and XJW independently screened the titles and abstracts of the remaining studies based on the inclusion and exclusion criteria. HBH and XJW independently screened full texts that met these criteria, and HYZ resolved any disagreements.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Studies must have been randomized controlled trials (RCTs) or cohort studies. (2) Patients must be considered ineligible for intensive induction chemotherapy when they were older than 60 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance status of 2–3, had a history of congestive heart failure, or had any other comorbidity that was intolerable with intensive chemotherapy. (3) Studies must have assessed the efficacy and safety of venetoclax combined with azacitidine or decitabine or LDAC versus azacitidine, decitabine, or LDAC in patients with untreated AML. (4) Studies must have reported the CR or CR/CRi rates or OS or AEs, and (5) the full texts for the studies were available. In addition, some studies were excluded because they were (1) case reports, reviews, meta-analyses, comments, meeting abstracts, or nonhuman studies; (2) duplicate publications; or (3) had insufficient data to calculate CR or CR/CRi rates or OS or AEs.
Data extraction
Two reviewers (HBH and XJW) independently extracted the data from each eligible study, including the first author, publication year, classification, number of patients, age, ECOG performance status, number of prior HMA treatments, treatment protocol, adverse events and outcomes. If any discrepancies existed, the two review authors (HBH and XJW) discussed the study with another investigator (HYZ). We selected the CR and CR/CRi rates as the primary outcomes and AEs, including neutropenia, febrile neutropenia, anemia, thrombocytopenia, constipation, diarrhea, nausea, vomiting and hypokalemia, as the secondary outcomes. In addition, we also collected the median OS of patients in the different venetoclax combination treatment groups for a prognostic meta-analysis.
Quality assessment
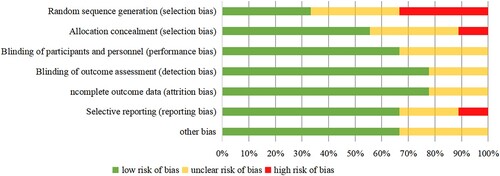
Two reviewers independently evaluated the methodological quality of each included study. The systematic bias of the included randomized controlled trial articles and control studies, including random sequence generation selection bias, allocation selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias, was evaluated by using Cochrane's risk of bias tool [Citation16]. Each item was subjected to a detailed review based on the following criteria: low risk of bias, high risk of bias, and unclear risk. The lower the risk of bias was, the greater the quality of the literature. Disagreements were resolved by discussion.
Statistical analysis
The statistical analysis in this study was performed using STATA MP version 17. Dichotomous data were summarized using the risk ratio (RR) and 95% confidence interval (CI). Continuous variable data are reported as weighted mean differences (WMDs), and the heterogeneity of CIs in all the included studies was assessed using the Cochrane Q and I2 statistics. If P < 0.1 or I2 > 50%, a random-effects model was used; otherwise, a fixed-effects model was applied [Citation17]. Moreover, the publication bias of the studies was evaluated by funnel plots and Egger's tests.
Results
Study selection and characteristics
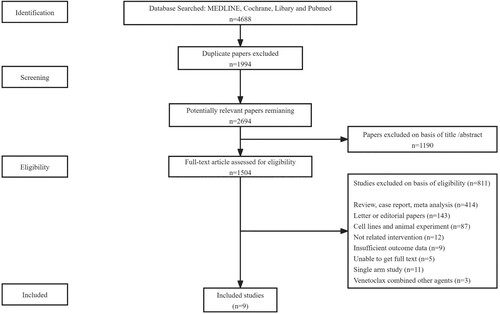
The literature screening process is shown in . The Embase, MEDLINE, Cochrane Library and PubMed databases were screened, and 4688 articles were identified to be included in the endnotes. A total of 1994 articles were excluded due to duplication, and 1190 articles were excluded due to the abstracts and topics identified. Finally, nine studies with 1232 patients were included in the meta-analysis [Citation11,Citation18–25]. These studies were published between 2019 and 2021, and the sample sizes ranged from 15 to 427, with a median of 115. Six studies were randomized controlled trials, and three were control studies. Among these trials, the treatment protocols of five trials included venetoclax combined with HMAs, and the other treatments of four trials included venetoclax and LDAC. The details of all the trials are shown in . The Cochrane risk of bias tool was used to assess the RCTs and control trials. The detailed biases are presented in . Forest plots of meta-analyses with high, moderate, low and very low quality of evidence are shown in .
Table 1. Baseline characteristics of AML patients treated with venetoclax based combination therapy among the included studies.
Table 2. Summary of High, Moderate, Low and Very Low-Quality Evidence Meta-analysis on efficiency and AE of AML patients.
Efficacy meta-analysis
CR/CRi
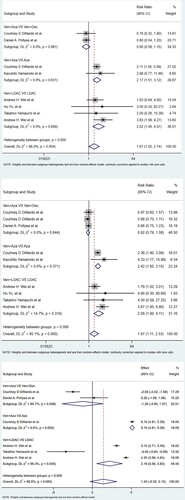
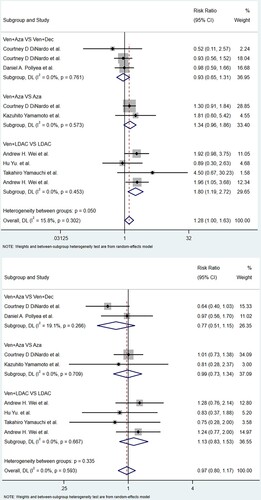
For the Ven + Aza vs. Aza group, which included two studies (22.2%) with 464 patients (37.7%), the CR/CRi rate of the Ven + Aza group was considerably greater than that of the Aza group (RR: 2.42; 95% CI: 1.85-3.15; P = 0.000), with no significant heterogeneity (I2 = 0.0%, P = 0.371). For the Ven + LDAC vs. LDAC group, which included four studies (44.4%) with 463 patients (37.9%), the CR/CRi rate of the Ven + LDAC group was still greater than that of the LDAC single agent group (RR: 2.56; 95% CI: 1.60-4.11; P < 0.001), with no heterogeneity (I2 = 14.7%, P = 0.319). For the Ven + Aza vs. Ven + Dec group, three studies (33.3%) with 305 patients (24.8%) assessed CR/CRi and were included. There was no significant difference in the CR/CRi in this group (RR: 0.92; 95% CI: 0.79-1.08; P = 0.317), with no significant heterogeneity (I2 = 0.0%, P = 0.844). .
CR
For the Ven + Aza vs. Aza group, two studies (22.2%) with 464 patients (37.7%) showed that the CR rate of the Ven + Aza group was even greater than that of the Aza monotherapy group (RR: 2.17; 95% CI: 1.15-3.12; P < 0.001), with no heterogeneity (I2 = 0.0%, P = 0.631). For the Ven + LDAC vs. LDAC group, four papers (44.4%) with 463 patients (37.6%) showed that the CR rate of the Ven + LDAC group was significantly greater than that of the LDAC monotherapy group (RR: 2.52; 95% CI: 1.45-4.37; P = 0.001), with no heterogeneity (I2 = 16.8%, P = 0.659). Three studies (88.9%) with 160 patients (13%) reported the CR rate and were eligible for the Ven + Aza group vs. the Ven + Dec group. There was no significant difference in CR in this group (RR: 0.80; 95% CI: 0.56-1.15; P = 0.230), with no heterogeneity (I2 = 0.0%, P = 0.961).
OS
For the Ven + Aza vs. Aza group, which included only one study with 427 patients (34.7%), the OS (RR: 5.10; 95% CI: 4.81–5.39; P = 0.00). For the Ven + LDAC vs. LDAC group, which included three studies (33.3%) with 448 patients (36.4%), the OS of the Ven + LDAC group was much greater than that of the monotherapy LDAC group (RR: 2.19; 95% CI: 0.56–3.83; P = 0.009), with no heterogeneity (I2 = 96.3%, P = 0.00). Three studies (66.7%) with 162 patients (13.1%) assessed OS and were eligible for the Ven + Aza group vs. the Ven + Dec group. There was no significant difference in OS in this group (RR: – 1.26; 95% CI: – 3.90–1.37; P = 0.348), with significant heterogeneity (I2 = 85.7%, P = 0.008).
Safety meta-analysis
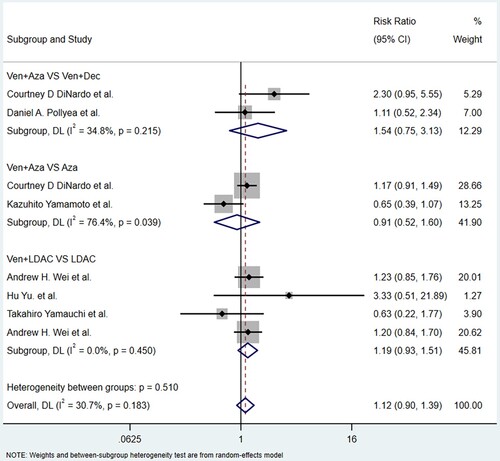
Nine studies reported AEs, including four common hematological AEs of grade ≥ 3 and five non-hematological AEs of any grade. The hematological AEs included anemia, thrombocytopenia, neutropenia and febrile neutropenia, and the nonhematological AEs included nausea, constipation, diarrhea, hypokalemia and vomiting. The details of the analysis of the AEs caused by venetoclax with or without other agents are shown in and .
Figure 4. Meta-analysis for neutropenia, febrile neutropenia, anemia, and thrombocytopenia of the overall study. (a) neutropenia; (b) febrile neutropenia; (c) anemia; (d) thrombocytopenia. Note: Ven: Venetoclax; Aza: Azacitidine; Dec: Decitabine; LDAC: Low-dose cytarabine.
Figure 5. Meta-analysis for constipation, diarrhea, nausea, vomiting, and hypokalemia of the overall study. (a) constipation; (b) diarrhea; (c) nausea; (d) vomiting; (e) hypokalemia. Note: Ven: Venetoclax; Aza: Azacitidine; Dec: Decitabine; LDAC: Low-dose cytarabine.
Hematological adverse events
For neutropenia, two studies (22.2%) with 464 patients (37.7%) reported neutropenia, which showed that the incidence of neutropenia in the Ven + Aza group was much greater than that in the monotherapy Aza group (RR: 1.49; 95% CI: 1.12–1.97; P = 0.006). Similarly, four studies (44.4%) with 462 patients (37.5%) showed that the incidence of neutropenia in the Ven + LDAC group was greater than that in the monotherapy LDAC group (RR: 2.74; 95% CI: 1.84–4.09; P < 0.001). For febrile neutropenia, three studies (33.3%) with 305 patients (24.8%) reported febrile neutropenia, which indicated that the incidence of febrile neutropenia in the Ven + Aza group was much greater than that in the Ven + Dec group (RR: 0.69; 95% CI: 0.53–0.90; P = 0.006). Two studies (22.2%) with 464 patients showed that the incidence of febrile neutropenia in the Ven + Aza group was significantly greater than that in the Aza monotherapy group (RR: 2.19; 95% CI: 1.58–3.03; P < 0.001). However, there were no significant differences in anemia or thrombocytopenia among these groups.
Nonhematological adverse events
Four studies (44.4%) with 462 patients (37.5%) showed that the incidence of constipation in the Ven + LDAC group was greater than that in the monotherapy LDAC group (RR: 0.61; 95% CI: 0.44–0.83; P = 0.002). Four papers (44.4%) with 462 patients (37.5%) reported that the incidence of diarrhea in the Ven + LDAC group was much greater than that in the monotherapy LDAC group (RR: 1.81; 95% CI: 1.22–2.67; P = 0.003). Moreover, four studies (44.4%) with 462 patients (37.5%) showed that the incidence of nausea in the Ven + LDAC group was much greater than that in the monotherapy LDAC group (RR: 1.39; 95% CI: 1.06–1.82; P = 0.016). In addition, four studies (44.4%) with 462 patients (37.5%) indicated that the incidence of vomiting in the Ven + LDAC group was significantly greater than that in the monotherapy LDAC group (RR: 1.80; 95% CI 1.19–2.72; P = 0.005). However, there was no significant difference in the incidence of adverse hypokalemia among these groups.
Publication bias
Since only nine studies were included, we did not construct a funnel plot.
Discussion
The meta-analysis showed that venetoclax combined with azacitidine or LDAC could achieve greater CR and CR/CRi than azacitidine or LDAC alone. Similarly, OS was much longer in the venetoclax combination group than in the monotherapy group. However, the incidence of neutropenia and febrile neutropenia was greater in the Ven + Aza group than in the Aza monotherapy group. This means that the side effects in the Ven + Aza group could be on the alert. Furthermore, the Ven + LDAC group had higher incidence rates of constipation, diarrhea, nausea, and vomiting than did the monotherapy LDAC group.
Efficacy
The CR/CRi rates of the Ven + Aza and Ven + LDAC groups were much greater than those of the Aza and LDAC monotherapy groups. Recently, a meta-analysis revealed that only 42% of AML patients achieved CR/CRi when HMA monotherapy was applied; this percentage was less than that in the venetoclax plus HMAs group in our study (up to 66%) [Citation26]. Another study demonstrated that the median OS was 13.5 months for patients treated with Ven + Aza therapy for untreated AML patients [Citation13], whereas for patients treated with HMAs alone, the median OS was only 7.7-10.4 months [Citation4,Citation5]. Similarly, LDAC monotherapy only achieved CR/CRi rates of 11% to 19%, which were much lower than those of the Ven + LDAC group (up to 35%) in our study [Citation27,Citation28]. The median OS of patients receiving LDAC monotherapy was only approximately 5.5 months, which was also lower than that of patients in the Ven + LDAC group (14.2 months) in our study [Citation5,Citation28,Citation29]. Venetoclax, a potent, selective, orally bioavailable, small-molecule inhibitor of BCL-2 [Citation30], has been shown to have single-agent clinical activity, with a response rate of 19% for relapsed and refractory AML in a phase 2 trial [Citation7]. The BCL-2 protein is well known to be overexpressed in leukemia stem cells and promotes cell survival. Due to the high level of BCL-2 protein that is persistently overexpressed in AML, venetoclax can replace proapoptotic proteins and rapidly trigger apoptosis to achieve a profound response [Citation31]. In vitro and in vivo studies have shown that leukemia stem cells are sensitive to BCL-2 inhibition [Citation32,Citation33]. The overexpression of BCL-2 in AML was associated with the inhibition of cell apoptosis, and venetoclax promoted the apoptosis of leukemia cells by inhibiting the activity of BCL-2, thereby achieving therapeutic effects [Citation34]. In previous studies, the combination of venetoclax and chemotherapeutic agents (such as azacitidine) was shown to increase treatment efficacy, improve clinical remission rates and prolong survival in AML patients [Citation35]. Additionally, some studies have indicated that azacitidine can reduce the level of MCL-1, which enhances the sensitivity of cells to venetoclax and synergistically eliminates leukemia cells [Citation36,Citation37]. Later, venetoclax combined with azacytidine or decitabine exhibited synergistic effects on preclinical models of AML cells [Citation38]. Since then, venetoclax-based combination therapy has been applied in the clinical setting and has proven to be more effective than mono-chemotherapy. The venetoclax combination treatment regimens, including the Ven + Aza, Ven + Dec and Ven + LDAC regimens, achieved a high remission rate and improved OS in AML patients. Combination therapy with venetoclax has been strongly recommended for AML patients, especially for elderly patients who cannot tolerate intensive chemotherapy.
Safety
The incidence of febrile neutropenia was greater in the Ven + Aza group than in the Ven + Dec or Aza single agent group. A meta-analysis evaluating adverse events associated with Aza monotherapy in AML patients revealed that 25% of patients had febrile neutropenia [Citation39], which was considerably lower than the percentage in the Ven + Aza group (44.6%) in our study. This result indicated that Ven + Aza combination therapy increased the incidence of febrile neutropenia. A study by DiNardo et al. [Citation19] also showed that the incidence of febrile neutropenia was significantly greater in the Ven + Aza regimen group than in the azacitidine plus placebo group (42% vs. 19%). Similarly, in this meta-analysis, compared with mono-Aza treatment, Ven + Aza also caused severe neutropenia or febrile neutropenia. Therefore, good supportive care should be provided, and prophylactic antimicrobial agents should be recommended for patients receiving azacitidine plus venetoclax [Citation40,Citation41]. In addition, the combination of venetoclax and azacitidine may enhance the toxicity to hematopoietic stem cells and normal blood cells, resulting in hematotoxic side effects. It should also be noted that there is variability in venetoclax metabolism and tolerance among patients. In the future, there might be clinical trials of the combination of oral azacitidine and venetoclax to further evaluate the incidence of toxicity and side effects of the combined treatment protocols [Citation42]. This may account for the greater rate of febrile neutropenia in the Ven + Aza group than in the Ven + Dec group. Physicians should carefully manage neutropenia and febrile neutropenia in AML patients in the Ven + Aza group.
In our study, the Ven + LDAC group had significantly greater incidences of constipation, diarrhea, nausea, and vomiting than the LDAC single agent group (P < 0.05). Previous studies also indicated a greater incidence of gastrointestinal adverse effects in the Ven + LDAC group, but these papers did not report significant differences [Citation13,Citation23,Citation43]. Another meta-analysis showed that common AEs of any grade were related to gastrointestinal symptoms in the Ven + LDAC group [Citation44]. Gastrointestinal toxicity might be associated with a high pill burden, which may limit venetoclax dose escalation and continuous therapy [Citation10]. This may also be related to the clinical use of antifungal drugs. When antifungal drugs are combined with venetoclax, the blood concentration of venetoclax increases. Therefore, it is necessary to monitor blood levels of venetoclax [Citation45]. However, there was no significant difference regarding hypokalemia in our study. In summary, all these results showed that increased hematological and gastrointestinal toxicity were adverse events observed in patients treated with venetoclax combined with HMAs or LDAC.
There were several limitations of our study that should be acknowledged. First, some of the eligible studies had a limited number of participants, which increases the risk of overfitting. Second, not all included trials were randomized controlled trials, which increased the risk of bias. Finally, the number of included studies was small enough that heterogeneity and sensitivities among these studies could not be addressed.
In conclusion, venetoclax combined with HMAs or LDAC could be beneficial in previously untreated AML patients who are ineligible for intensive chemotherapy and who have a high proportion of patients who achieved CR/CRi. However, venetoclax-based combination therapy also has hematological and gastrointestinal effects. Physicians should be more careful to monitor febrile neutropenia and gastrointestinal toxicity when administering venetoclax combined with azacitidine or the LDAC regimen.
Availability of data and materials
The data supporting this study's findings are available on request from the corresponding author.
Authors’ contributions
HH, XW Writing – original draft; HH, XW, HZ Writing – review & editing; HH, XW, HZ Data curation; HH, XW Formal analysis; HH, XW, HZ Project administration.
Consent for publication
All authors have read and approved the final manuscript.
Acknowledgments
HBH and XJW contributed equally to this work. HBH and XJW take responsibility for the content of the manuscript. XJW was involved in the conception, hypothesis delineation, and design of the study and in the acquisition and analysis of the data. HBH wrote and edited the manuscript. HBH was involved in designing the study, analyzing the data, and writing and editing the manuscript. All authors read and approved the final manuscript. HYZ is the guarantor of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016. doi:10.1016/j.lrr.2016.06.001
- Levin-Epstein R, Oliai C, Schiller G. Allogeneic hematopoietic stem cell transplantation for older patients with acute myeloid leukemia. Curr Treat Options Oncol. 2018. doi:10.1007/s11864-018-0577-2
- Krug U, Büchner T, Berdel WE, et al. The treatment of elderly patients with acute myeloid leukemia. Dtsch Arztebl Int. 2011. doi:10.3238/arztebl.2011.0863
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015. doi:10.1182/blood-2015-01-621664
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012. doi:10.1200/JCO.2011.38.9429
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discovery. 2008. doi:10.1038/nrd2658
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016. doi:10.1158/2159-8290.CD-16-0313
- Ram R, Amit O, Zuckerman T, et al. Venetoclax in patients with acute myeloid leukemia refractory to hypomethylating agents-a multicenter historical prospective study. Ann Hematol. 2019. doi:10.1007/s00277-019-03719-6
- Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019. doi:10.1002/ajh.25567
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018. doi:10.1016/S1470-2045(18)30010-X
- DiNardo CD, Maiti A, Rausch CR, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukemia: a single-center, phase 2 trial. Lancet Haematol. 2020. doi:10.1016/S2352-3026(20)30210-6
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020: 1), doi:10.1056/NEJMoa2012971
- Wei AH, Strickland SA, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019. doi:10.1200/JCO.18.01600
- Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009. doi:10.1371/journal.pmed.1000097
- Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Br Med J. 2011. doi:10.1136/bmj.d5928
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003. doi:10.1136/bmj.327.7414.557
- Wei AH, Panayiotidis P, Montesinos P, et al. 6-month follow-up of VIALE-C demonstrates improved and durable efficacy in patients with untreated AML ineligible for intensive chemotherapy (141/150). Blood Cancer J. 2021. doi:10.1038/s41408-021-00555-8
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020. doi:10.1056/NEJMoa2012971
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019. doi:10.1182/blood-2018-08-868752
- Yamamoto K, Shinagawa A, DiNardo CD, et al. Venetoclax plus azacitidine in Japanese patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy. Jpn J Clin Oncol. 2022. doi:10.1093/jjco/hyab170
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020. doi:10.1182/blood.2020004856
- Yamauchi T, Yoshida C, Usuki K, et al. Venetoclax plus low-dose cytarabine in Japanese patients with untreated acute myeloid leukaemia ineligible for intensive chemotherapy. Jpn J Clin Oncol. 2021. doi:10.1093/jjco/hyab112
- Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long-term follow-up from a phase 1b study. Am J Hematol. 2021. doi:10.1002/ajh.26039
- Hu Y, Jin J, Zhang Y, et al. Zhonghua xueyexue zazhi. 2021. doi:10.3760/cma.j.issn.0253-2727.2021.04.004
- Ji J, Chen M, Han B. Comparison of hypomethylator monotherapy with hypomethylator plus chemotherapy for intermediate/high-risk MDS or AML: A meta-analysis. J Cancer. 2020. doi:10.7150/jca.40614
- Kadia TM, Ravandi F, Borthakur G, et al. Long-term results of low-intensity chemotherapy with clofarabine or cladribine combined with low-dose cytarabine alternating with decitabine in older patients with newly diagnosed acute myeloid leukemia. Am J Hematol. 2021. doi:10.1002/ajh.26206
- Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006. doi:10.1002/cncr.21723
- Colunga-Lozano LE, Kenji Nampo F, Agarwal A, et al. Less intensive antileukemic therapies (monotherapy and/or combination) for older adults with acute myeloid leukemia who are not candidates for intensive antileukemic therapy: A systematic review and meta-analysis. PLoS One. 2022. doi:10.1371/journal.pone.0263240
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016. doi:10.1056/NEJMoa1513257
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007. doi:10.1038/sj.onc.1210220
- Pan R, Ruvolo VR, Wei J, et al. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood. 2015. doi:10.1182/blood-2014-10-604975
- Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014. doi:10.1158/2159-8290.CD-13-0609
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi:10.1038/nm.3048
- Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–778. doi:10.1016/S1470-2045(16)30019-5
- Tsao T, Shi Y, Kornblau S, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012. doi:10.1007/s00277-012-1537-8
- Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017. doi:10.1080/10428194.2017.1283032
- Bogenberger JM, Delman D, Hansen N, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015. doi:10.3109/10428194.2014.910657
- Gao C, Wang J, Li Y, et al. Incidence and risk of hematologic toxicities with hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukopenia: A systematic review and meta-analysis. Medicine (Baltimore). 2018. doi:10.1097/MD.0000000000011860
- Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia. 2019. doi:10.1038/s41375-019-0612-8
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020. doi:10.1182/blood.2019001239
- Döhner H, Wei AH, Roboz GJ, et al. Prognostic impact of NPM1 and FLT3 mutations in patients with AML in first remission treated with oral azacitidine. Blood. 2022;140(15):1674–1685. doi:10.1182/blood.2022016293
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020. doi:10.1182/blood.2020004856
- Qin Y, Kuang P, Liu T. Venetoclax combined with hypomethylating agents or low-dose cytarabine as induction chemotherapy for patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy: a systematic review and meta-analysis. Clin Exp Med. 2022. doi:10.1007/s10238-021-00784-y
- Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359–367. doi:10.1016/j.clinthera.2017.01.003