ABSTRACT
Objectives
Relapsed/refractory acute B-cell lymphoblastic leukemia (R/R B-ALL) often responds poorly to induction chemotherapy. However, recent research has shown a novel and effective drug treatment for R/R B-ALL.
Methods
A total of eight patients with R/R B-ALL were enrolled in the study from November 2021 to August 2022. All patients received chemotherapy based on a combination regimen of venetoclax and azacitidine. The regimen was as follows venetoclax 100 mg d1, 200 mg d2, 400 mg d3–14, azacitidine 75 mg/m2 d1–7.
Results
Five of eight patients achieved very deep and complete remission (CR) with minimal residual disease (MRD) less than 0.1%. One patient achieved partial remission. Two patients did not achieve remission. There were no serious adverse events and all patients were well tolerated. Three patients were eligible for consolidation chemotherapy and were bridged to CAR-T therapy.
Conclusions
The combined regimen of venetoclax and azacitidine may be beneficial for patients with R/R B-ALL.
Introduction
The prognosis of patients with relapsed/refractory acute lymphoblastic leukemia (ALL) remains poor owing to higher frequency of adverse genomic features and increased resistance to treatments. Second-rescue complete response (CR) rates are reportedly as low as 44% in pediatric patients and 18% in adult patients with relapsed/refractory (R/R) ALL [Citation1]. Conventional treatments include induction chemotherapy followed by further consolidative chemotherapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although monoclonal antibodies targeting cell surface antigens (CD19, CD20, and CD22), bispecific antibodies, and chimeric antigen receptor T cell (CAR-T) therapy have shown promise in B-ALL therapy, relapses and drug resistance remain major causes for treatment failure because of diminished expression of target antigens or target antigen loss [Citation2]. Thus, novel effective treatments for B-ALL are needed to improve patient remission rates and prolong patient survival [Citation3].
Bcl-2 family proteins are important regulators of the apoptotic signaling pathway, which plays an important role in regulating the survival and proliferation of leukemia cells [Citation4]. Myeloid leukemia 1 (MCL-1), an antiapoptotic protein of the BCL-2 family, prevents apoptosis by binding with pro-apoptotic BCL-2 proteins [Citation3]. Targeted inhibition of Bcl-2 activity can promote apoptosis of leukemia cells and exert a strong anti-leukemia effect [Citation5]. Similar to acute myeloid leukemia (AML), BCL-2 overexpression was reported in acute lymphocytic leukemia [Citation6], including during the early stages of leukemia initiation [Citation7]. Ph+ ALL tends to be more dependent on other BCL-2 proteins like MCL-1 and shows lower sensitivity to BCL-2 inhibition than Ph – ALL [Citation8]. Venetoclax (VEN), a first-generation selective Bcl-2 inhibitor, can induce apoptosis of leukemia cells and is currently widely used in treating chronic lymphocytic leukemia and AML [Citation7]. At the same time, it also shows good efficacy against R/R AML [Citation9]. Several studies have suggested that the addition of VEN to chemotherapy agents for R/R T-ALL treatment may be safe and efficacious [Citation10]. VEN as a single agent reportedly improves the prognosis of T-ALL patients; however, the rapid emergence of resistance limits the use of VEN monotherapy [Citation11]. Some recent case reports have suggested that adding VEN to chemotherapy drugs of different lines of treatment for R/R ALL may help patients achieve CR and may even turn them negative for minimal residual disease (MRD) [Citation12,Citation13]. Hypomethylating drugs like azacytidine (AZA) can reduce the activity of Mcl-1 [Citation14], which plays a key role in acquired resistance to VEN [Citation15]. Rahmat et al. [Citation16] reported that a patient with ETP-ALL who relapsed after transplantation achieved complete remission after salvage treatment with a combination of VEN and demethylating drugs. The addition of VEN to AZA resulted in a significant survival benefit in vivo, showing the therapeutic potential of this combination to improve the outcomes of children with KMT2A-rearranged ALL [Citation17].
Leukemia stem cells (LSCs) reportedly have an increase in amino acid uptake and metabolism. Jones et al. [Citation18] reported that a combination of hypomethylating agents with the selective Bcl-2 inhibitor VEN (HMA – VEN) killed LSCs by reducing amino acid uptake and disrupting the tricarboxylic acid cycle via the inhibition of electron transport chain complex II. Therefore, the synergistic mechanism of HMA – VEN may be that the drug inhibits amino acid metabolism, reduces oxidative phosphorylation, and ultimately induces LSC death. Co-administration of hypomethylating agents with VEN has emerged as a highly effective treatment option for patients with AML in both de novo and R/R settings [Citation19]. The largest study reported on this issue is a retrospective study of only 32 patients with AML (19 R/R and 13 de novo) [Citation20]. Sandhu et al. concluded that patients with R/R AML after HMA – VEN treatment could be successfully bridged to allogeneic hematopoietic stem cell transplantation (allo-HSCT), which reportedly has favorable outcomes.
Presently, there is an urgent need to develop targeted therapies that can rapidly induce significant clinical response that is well tolerated and potent in R/R B-ALL. With the above rationale, we explored the efficacy and safety of a combination of VEN and AZA (VEN – AZA) in patients with R/R B-ALL. Herein, we report the findings of this combined treatment and its follow-up in eight patients.
Materials and methods
We enrolled a total of eight patients with R/R B-ALL between November 2021 and August 2022. The study comprised six men and two women (median age, 44.2 years; age range, 16–59 years). All were based on the WHO 2016 diagnostic standard of R/R B-ALL and NCCN Guidelines 2021 Version 1.0 [Citation21] Among the eight patients, seven had relapsed B-ALL and one had primary refractory B-ALL. Among the seven patients with relapsed B-ALL, four patients had previously received allo-HSCT and three patients had received CAR-T therapy before the regimen. One patient had central nervous system and thoracic metastases. A patient developed kidney metastasis. Patients’ baseline characteristics and prior treatment regimens are shown in . The patients did not receive blinatumomab, inotuzumab, and other tyrosine kinase inhibitors because of their financial limitations. All patients were treated with chemotherapy based on VEN – AZA of 1–2 courses after obtaining their informed consent. The treatment regimen was as follows: VEN 100 mg d1, 200 mg d2, 400 mg d3-14; AZA 75 mg/m2 qd d1–7. The study was approved by the hospital’s institutional review board (approval no. HEYLL202112). Mutations in the ABL kinase region of the BCR/ABL fusion gene were detected by nested PCR coupled with sequencing. TaqMan probe real-time fluorescence quantitative PCR was used to detect the fusion gene. Antibiotics were used for prophylaxis before the onset of neutropenia. Written informed consent was obtained from all patients. Adverse events (AEs) were assessed per the National Cancer Institute Common Terminology Criteria for Adverse Events 4.0 (NCI-CTCAE 4.0).
Table 1. Baseline of patients.
Results
The median follow-up period was 7 months for all patients and 6.4 months for survivors. They all received one treatment course of VEN + AZA (VEN – AZA) in the hospital. The eight patients’ dynamic responses to therapies since the initiation of VEN – AZA are shown in . The patients showed varying degrees of response to the treatment, with five achieving CR, one attaining partial remission (PR), and two showing on-remission (NR). The overall remission rate (ORR) following one course of treatment was 62.5% (See ). Patients 1, 2, 3, 5, and 8 achieved complete remission with MRD below 0.1% (See and A). The percent of leukemia-associated fusion gene decreased dramatically from patients 1–4 (See B). Patients 3 and 8 achieved CR and were then prepared for consolidation chemotherapy. Patient 4 achieved partial remission and was bridged to CAR-T therapy. Patients 6 and 7 showed no response and stayed refractory state. Finally, patients 1 and 7 died of infection. Patient 5 immediately was bridged to treatment with CD22+CD38+ CAR-T therapy after achieving CR; however, he died of severe infection after 6 months. Patient 2 died of original cardiac failure (See ).
Figure 1. Summary of treatment response. (A) Minimal Residual Disease measured by flow cytometry. (B) Leukemia-associated fusion genes measured by real-time quantitative PCR.
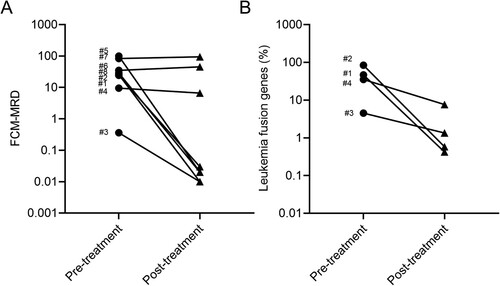
Table 2. Response rates for patients who received ven/aza.
Table 3. Treatment-emergent adverse events.
These patients experienced no serious complications except neutropenia and thrombocytopenia. Six patients developed grade 2/3 anemia. All patients developed neutropenia, five patients experienced agranulocytosis and the median time to neutrophil recovery (absolute neutrophil count ≥ 0.5 × 109/L) was 8 days (range, 0–24 days). Patients 1, 5, 6 and 7 had febrile neutropenia, and three patients developed respiratory infections. Patient 6 developed mild renal disfunction. Five patients experienced grade 4 thrombocytopenia. There were no events of tumor lysis syndrome (See ).
Discussion
With new chemotherapy regimens, better supportive care, and the widespread use of HSCT, survival rates for adults with ALL have slightly improved. However, the prognosis remains poor. Achieving CR is the key to successful HSCT, which is the only curative option for patients with R/R B-ALL after salvage therapy. Induction of HMA – VEN is now considered the standard of care for patients with AML due to fewer comorbidities, and it may also play a role in the aged patients with R/R AML. HMA – VEN contributes to the potential ability to combine allo-HSCT and CAR-T therapy. Therefore, the efficacy, safety, and tolerability of this combination therapy in R/R B-ALL is worth investigating. Currently, clinical trials evaluating the clinical efficacy of HMA – VEN are limited. In our study, we identified VEN – AZA in patients with R/R B-ALL as a potential approach to improve treatment outcomes while reducing the toxicity of chemotherapy regimens.
With the overexpression of BCL-2 in ALL, the use of BCL-2 inhibitors for the treatment of ALL, including R/R ALL, is a feasible approach. The Bcl-2 protein family comprises pro – and antiapoptotic molecules. According to the function of the protein, it can be divided into three categories, namely pro-apoptotic proteins (e.g. Bak and Bax), antiapoptotic proteins (e.g. Bcl-2, Bcl-x, and Bcl-w), and BH-3-only proteins (e.g. Bim, Bad, and Bid) [Citation22]. VEN induces apoptosis of leukemia cells probably by downregulating the activation of the Bax/Bak pathway. In addition, it can also increase cell stress response signals by inhibiting the oxidative phosphorylation respiratory chain or mitochondrial deoxyribonucleic acid release, which ultimately lead to apoptosis [Citation23]. Hypomethylating agents like AZA can decrease Mcl-1 levels and reduce antimicrobial resistance to VEN, and thus, a combination of VEN and hypomethylating agents may enhance the anti-leukemia effect. Notably, VEN – AZA can prevent leukemia progression and synergistically induce apoptosis of leukemia cells, which has been demonstrated in both cell lines and animal models [Citation24]. Klocke et al. reported a case wherein relapsed of a T/myeloid mixed-phenotype acute leukemia after allo-HSCT was successfully treated with VEN + decitabine [Citation25].
A meta-analysis assessed the efficacy and safety of VEN plus hypomethylating agents or low-dose cytarabine for 440 untreated AML patients who are ineligible for intensive chemotherapy. It showed that CR and Complete Remission with incomplete hematologic recovery (CR/CRi) rates were 0.40 and 0.64, respectively. The median overall survival was 11.7 months [Citation26]. In patients with R/R AML, the overall response rate for VEN – AZA was 21% – 75%, and the median survival was 3–16.6 months [Citation27,Citation28].
Taken together, we innovatively applied VEN – AZA to patients with R/R B-ALL. In this study, five out of eight patients achieved deep CR, demonstrating certain therapeutic effects of the combination in patients with R/R B-ALL. MRD with measurable recurrence is associated with high relapse rates and poor survival [Citation29]. Five patients attained deep CR and one patient achieved disease-free survival of 7 months before undergoing HSCT. We observed a significant decline in the expression of BCR/ABL and MLL/AF4 genes in four patients. This indicates that the VEN – AZA treatment markedly impacted these patients and offered a potential treatment avenue before opting for HSCT or CAR-T therapy for patients with R/R B-ALL. Therefore, patients probably achieved longer-term survival benefit.
Current treatments for adult ALL are associated with significant toxicity, particularly in older patients, which limits further intensive therapy. However, VEN – AZA was well tolerated in our study. DiNardo et al. reported that gastrointestinal and hematological AEs were the most common toxicities, and no tumor lysis syndrome was observed in patients with AML [Citation7]. Similar to their results, the most common AE in our study was hematological toxicity. Almost all patients experienced some degree of myelosuppression, which is mainly characterized by neutropenia and anemia. Other common AEs were infections (3/8 patients), general fatigue (3/8 patients), and gastrointestinal disorders like nausea and vomiting (2/8 patients). All patients received prophylactic antibiotics and antifungals. There were no serious AEs during treatment. This means that VEN – AZA did not cause any serious AEs in patients. Patients who have undergone HSCT and immunotherapy can tolerate the dose of combination therapy.
Unfortunately, three of five patients with CR died; one patient died of pneumorrhagia and one patient died of infections, and both of them died because of not achieving remission. One patient died of cardiac failure. We speculated the cause of death from infection to be bone marrow suppression caused by leukemic infiltrate. Receiving the combination treatment at an earlier stage of disease and achieving early complete remission may improve the survival chances of patients. Our 14-day regimen with VEN depends greatly on the patient’s myelosuppression to avoid more severe hematological toxicity. Therefore, the initial cycle duration of VEN dosage may need to be modified for cytopenia to shorten the period of neutropenia.
In summary, VEN – AZA is a novel and promising combination for patients with R/R B-ALL and provides a bridge to treatments like HSCT and CAR-T. Using VEN – AZA revealed its tolerable safety profile and favorable survival outcomes in patients with R/R B-ALL. Although our report is limited by the small number of patients and heterogeneous patient characteristics, these preliminary results suggest that this combination regimen is beneficial for patients with R/R B-ALL. Given the significant variability in response rates, larger prospective and randomized studies of AZA + VEN as salvage therapy are warranted to verify our findings.
Ethical approval
The study was approved by the hospital’s institutional review board (approval no. HEYLL202112).
Informed consent
Written informed consent was obtained from all patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- O'Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113(11):3186–3191.
- Mejstrikova E, Hrusak O, Borowitz MJ, et al. Cd19-negative relapse of pediatric b-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659. doi:10.1038/s41408-017-0023-x
- DeFilipp Z, Advani AS, Bachanova V, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: Updated 2019 evidence-based review from the American society for transplantation and cellular therapy. Biol Blood Marrow Transplant. 2019;25(11):2113–2123. doi:10.1016/j.bbmt.2019.08.014
- Wu H, Medeiros LJ, Young KH. Apoptosis signaling and bcl-2 pathways provide opportunities for novel targeted therapeutic strategies in hematologic malignances. Blood Rev. 2018;32(1):8–28. doi:10.1016/j.blre.2017.08.004
- Reed JC. Bcl-2-family proteins and hematologic malignancies: History and future prospects. Blood. 2008;111(7):3322–3330. doi:10.1182/blood-2007-09-078162
- Robinson BW, Behling KC, Gupta M, et al. Abundant anti-apoptotic bcl-2 is a molecular target in leukaemias with t(4;11) translocation. Br J Haematol. 2008;141(6):827–839. doi:10.1111/j.1365-2141.2008.07100.x
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. doi:10.1182/blood-2018-08-868752
- Hohtari H, Kankainen M, Adnan-Awad S, et al. Targeting apoptosis pathways with bcl2 and mdm2 inhibitors in adult b-cell acute lymphoblastic leukemia. Hemasphere. 2022;6(3):e701. doi:10.1097/HS9.0000000000000701
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase ii study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117. doi:10.1158/2159-8290.CD-16-0313
- Pullarkat VA, Lacayo NJ, Jabbour E, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11(6):1440–1453. doi:10.1158/2159-8290.CD-20-1465
- La Starza R, Cambo B, Pierini A, et al. Venetoclax and bortezomib in relapsed/refractory early t-cell precursor acute lymphoblastic leukemia. JCO Precis Oncol. 2019;3:PO.19.00172.
- Zhang X, Li J, Jin J, et al. Relapsed/refractory early t-cell precursor acute lymphoblastic leukemia was salvaged by venetoclax plus hag regimen. Ann Hematol. 2020;99(2):395–397. doi:10.1007/s00277-019-03902-9
- Wan CL, Zou JY, Qiao M, et al. Venetoclax combined with azacitidine as an effective and safe salvage regimen for relapsed or refractory t-cell acute lymphoblastic leukemia: A case series. Leuk Lymphoma. 2021;62(13):3300–3303. doi:10.1080/10428194.2021.1957876
- Kapoor I, Bodo J, Hill BT, et al. Targeting bcl-2 in b-cell malignancies and overcoming therapeutic resistance. Cell Death Dis. 2020;11(11):941. doi:10.1038/s41419-020-03144-y
- Tsao T, Shi Y, Kornblau S, et al. Concomitant inhibition of DNA methyltransferase and bcl-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91(12):1861–1870. doi:10.1007/s00277-012-1537-8
- Rahmat LT, Nguyen A, Abdulhaq H, et al. Venetoclax in combination with decitabine for relapsed t-cell acute lymphoblastic leukemia after allogeneic hematopoietic cell transplant. Case Rep Hematol. 2018;2018:6092646.
- Cheung LC, Aya-Bonilla C, Cruickshank MN, et al. Preclinical efficacy of azacitidine and venetoclax for infant kmt2a-rearranged acute lymphoblastic leukemia reveals a new therapeutic strategy. Leukemia. 2023;37(1):61–71. doi:10.1038/s41375-022-01746-3
- Jones CL, Stevens BM, D'Alessandro A, et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell. 2018;34(5):724–740.e4. doi:10.1016/j.ccell.2018.10.005
- Othman TA, Tenold ME, Moskoff BN, et al. An evaluation of venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine as therapy for acute myeloid leukemia. Expert Rev Hematol. 2021;14(5):407–417. doi:10.1080/17474086.2021.1938533
- Sandhu KS, Dadwal S, Yang D, et al. Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2020;26(12):e322–e327. doi:10.1016/j.bbmt.2020.08.027
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
- Delbridge AR, Grabow S, Strasser A, et al. Thirty years of bcl-2: Translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16(2):99–109. doi:10.1038/nrc.2015.17
- Um HD. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget. 2016;7(5):5193–5203. doi:10.18632/oncotarget.6405
- Jin S, Cojocari D, Purkal JJ, et al. 5-azacitidine induces noxa to prime aml cells for venetoclax-mediated apoptosis. Clin Cancer Res. 2020;26(13):3371–3383. doi:10.1158/1078-0432.CCR-19-1900
- Klocke H, Dong ZM, O'Brien C, et al. Venetoclax and decitabine for t/myeloid mixed-phenotype acute leukemia not otherwise specified (mpal nos). Case Rep Hematol. 2020;2020:8811673.
- Qin Y, Kuang P, Liu T. Venetoclax combined with hypomethylating agents or low-dose cytarabine as induction chemotherapy for patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy: A systematic review and meta-analysis. Clin Exp Med. 2023;23(2):219–227. doi:10.1007/s10238-021-00784-y
- Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019;94(10):E253–E255. doi:10.1002/ajh.25567
- Winters AC, Maloney KW, Treece AL, et al. Single-center pediatric experience with venetoclax and azacitidine as treatment for myelodysplastic syndrome and acute myeloid leukemia. Pediatr Blood Cancer. 2020;67(10):e28398. doi:10.1002/pbc.28398
- Giebel S, Czyz A, Ottmann O, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with philadelphia chromosome-positive acute lymphoblastic leukemia: A position statement of the acute leukemia working party of the european society for blood and marrow transplantation. Cancer. 2016;122(19):2941–2951. doi:10.1002/cncr.30130

