?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Insomnia is a significant difficulty and is reported by large proportion of people with tinnitus. Although cognitive behavioural therapy for insomnia (CBTi) might be an effective treatment, no controlled studies had been conducted to date. This randomised controlled trial evaluated the benefits of CBTi on a sample of 102 people with tinnitus-related insomnia. Participants were randomised to 1) CBTi, 2) Audiology-Based Care (ABC) or 3) Sleep Support Group (SSG). Primary outcomes included insomnia, sleep efficiency and total sleep time. Secondary outcomes measured sleep onset latency, sleep quality, tinnitus distress, psychological distress, functioning and quality of life. CBTi was superior at reducing insomnia and increasing sleep efficiency compared to ABC post-intervention and at 6-month follow-up. ABC was superior at reducing insomnia and increasing sleep efficiency compared to SSG. Both CBTi and ABC reported increased total sleep time compared to SSG at 6-month follow. More than 80% of participants in the CBTi group reported clinically meaningful improvements compared to 47% in ABC and 20% for those receiving social support. CBTi was more effective in reducing tinnitus distress and improving sleep quality, functioning and some aspects of mental health. CBTi and ABC offer effective treatments for tinnitus-related sleep disorder but CBTi offers a sizeable benefit.
Tinnitus, the perception of a sound in the absence of external acoustical stimuli, is a common symptom with prevalence estimates of 10–13% in the UK (range 11.9–30.3% globally: McCormack et al., Citation2016). Intrusive tinnitus represents a significant public health burden (Baguley et al., Citation2013), frequently accompanied by difficulties with anxiety, depression, concentration, hearing, functioning and sleep. Sleep disturbance is reported by up to 80% of tinnitus sufferers (Asnis et al., Citation2018), with 27% meeting formal diagnostic criteria for insomnia (Miguel et al., Citation2014). By comparison, estimates of insomnia in the general population range from 6% to 10%. Tinnitus severity is correlated with sleep disturbance (Schecklmann et al., Citation2015), although few studies adequately assess insomnia (Asnis et al., Citation2018).
Insomnia is characterised by regular, sustained difficulties in initiating, sustaining or obtaining qualitatively satisfying sleep, despite adequate opportunity (Edinger et al., Citation2004). Day-time effects include difficulties with fatigue, attention, activity, memory and mood (Buysse et al., Citation2007). Insomnia is commonly assessed by self-reported impact and severity of sleep disturbance. Sleep patterns can be tracked for example, by measuring total sleep time (TST), Sleep Efficiency (SE) and Sleep Onset Latency (SOL). TST offers good face validity but can be an unreliable indicator of insomnia due to significant individual variability and variable correlation with sleep satisfaction. SE refers to time in bed spent asleep (often reported as a percentage) and is often associated with more satisfactory sleep. SE is affected by SOL (time taken to fall asleep) and waking after sleep onset. A SE of <85% can indicate insomnia (Edinger et al., Citation2004) and as lower SE indicates more time in bed spent awake and this may interact with time spent aware of tinnitus and related distress.
Polysomnography indicates that sleep architecture is similar in people with insomnia both with and without tinnitus (Burgos et al., Citation2005), with equivalent outcomes on physiological and subjective measures of sleep and daytime impact, although tinnitus sufferers report significantly longer subjective SOL (Crönlein et al., Citation2007). Insomnia and tinnitus have similar precipitants (depression, stress, autonomic arousal) (Attanasio et al., Citation2013; Wallhäusser-Franke et al., Citation2013), and both have similar cognitive behavioural maintaining processes, namely negative cognitions and unhelpful changes in behaviour, attention and emotion (Harvey, Citation2002; McKenna et al., Citation2014). Specific worries common in insomnia are also common in tinnitus patients with sleep disturbance (Crönlein et al., Citation2016). Many tinnitus patients with insomnia tend to attribute sleep disturbance to the tinnitus noise, but evidence for a causal link is unclear.
The most effective treatment for insomnia is Cognitive Behavioural Therapy for Insomnia (CBTi), which targets cognitive behavioural maintenance factors. Clinical trials demonstrate medium-to-large effect sizes of CBTi on various outcome measures assessing insomnia (Morin et al., Citation2006; Okajima et al., Citation2011). Similar sizes of effect are reported in trials of CBTi treating insomnia comorbid with chronic conditions such as pain (Jungquist et al., Citation2010) and cancer (Espie et al., Citation2008). Since sleep deprivation can make comorbid symptoms more distressing, it is likely that improving sleep reduces symptom interference in pain (Tang et al., Citation2012), and the same could be true of tinnitus.
The prevalent and disabling nature of tinnitus-related insomnia indicates a need for effective treatments, but evidence is lacking. To date, CBTi has not been rigorously tested in tinnitus-related insomnia. Meta-analysis of CBT for tinnitus (Curtis et al., Citation2021) shows that small but significant improvements in insomnia can occur as secondary outcomes in CBT, but this has not been tested on participants with clinical insomnia nor have mean improvements met clinically meaningful reductions, so the applicability of CBT to clinical levels of insomnia is unclear (Hoare et al., Citation2015). One uncontrolled evaluation of CBTi for tinnitus-related insomnia offers initial support (Marks et al., Citation2019a), with 22 patients reporting clinically significant improvements following treatment in insomnia, sleep patterns, tinnitus distress and psychological distress.
Standard care for tinnitus-related insomnia lacks evidence and treatment guidelines. Most patients attend audiology centres, where treatment varies widely, but usually includes education, supportive counselling, relaxation and sleep hygiene advice from an audiologist. This is counter to evidence that sleep hygiene alone is ineffective for general insomnia (Edinger et al., Citation2021). Another intervention for tinnitus involves sound enrichment, again with limited evidence for improved sleep and tinnitus handicap (National Institute for Health and Care Excellence, Citation2020; Wakabayashi et al., Citation2018).
Aims
This randomised controlled trial aimed to examine whether CBTi for tinnitus-related insomnia is effective. CBTi was compared with (i) Audiology-based care (ABC), which was based on best possible treatment as usual and (ii) Sleep support group (SSG), a contact control condition. The primary hypothesis was that CBTi would lead to a significantly greater reduction in insomnia than ABC pre-to-post-treatment and at six-month follow-up. Secondary hypotheses were that the same pattern would be observed when comparing CBTi to SSG, and that in comparison to either control, a greater proportion of CBTi participants would show reliable clinical change in insomnia and significantly greater mean changes on SOL, sleep quality, dysfunctional sleep beliefs, tinnitus distress, tinnitus catastrophising, measures of mental health, functioning and quality of life after treatment and at follow-up. It was hypothesised that ABC would lead to significantly greater improvements in outcomes than SSG. Psychosocial interventions tend not to improve tinnitus volume (NICE, Citation2020) so it was hypothesised that tinnitus volume would be unchanged.
Methods
This three-group, randomised controlled trial was conducted at the Royal National ENT Hospital, London, from June 2017 to December 2019. Ethical approval was provided by the UK NHS research committee, and the trial was registered with clinicaltrials.gov (NCT03386123). The full trial protocol was published (Marks et al., Citation2019b).
Interventions
Interventions were delivered on the same day, at different times, with timing counterbalanced across cohorts. Two clinical psychologists experienced in tinnitus, insomnia and CBT delivered all treatments. Sessions were 120 min (follow-ups 90 min). Treatment completion was pre-set as attendance at 50% of sessions. Participants kept psychotropic and hypnotic medication stable throughout the study. The group interventions were (1) Cognitive Behavioural Therapy for Insomnia (CBTi), a six-session multicomponent treatment for insomnia, adapted for tinnitus, delivered over eight weeks. A bedside sound generator was provided. (2) Audiology-Based Care (ABC) gave advice on tinnitus, sleep hygiene and relaxation in two sessions, eight weeks apart. A bedside sound generator was provided. (3) Sleep Support Group (SSG) involved six sessions of supportive counselling, delivered over eight weeks. This active control offered non-specific benefits of social and clinical support without specific clinical advice (Pryce et al., Citation2019). For more information see Supplementary Material.
Inclusion criteria
Inclusion criteria were (1) aged 18–70years; (2) Clinically significant insomnia (minimum of 15 on Insomnia Severity Index) (Bastien et al., Citation2001) caused by tinnitus, present for at least 3 months; (3) at least moderately distressing tinnitus for at least 6 months (minimum of 8 on Mini Tinnitus Questionnaire) (Hiller & Goebel, Citation2004); (4) Sufficient English language and hearing ability; (5) Completed medical investigations. Exclusion criteria were (1) Organic sleep disorders indicated; (2) Severe physical or psychiatric comorbidities; (3) Risk to self or others; (4) Substance dependence; (5) Pregnancy/breast-feeding.
Ethical considerations
Participants provided full, informed consent. Alternative treatment or referral was available to those opting out and after the final follow-up.
Randomisation and masking
Randomisation was in cohort groups of 15–20. Treatment was delivered in cohorts, split into three groups, each containing an average of six participants. Anonymised details were sent to an independent researcher, who completed randomisation with stratification for gender using a customised computer algorithm. Participants were informed of their group number (1,2,3) three weeks prior to commencement and informed of treatment type on first attendance. Neither participants nor therapists could be blinded to allocation, but participants were blinded to the content of alternative treatments. An independent statistician was masked to group for all analyses.
Procedures
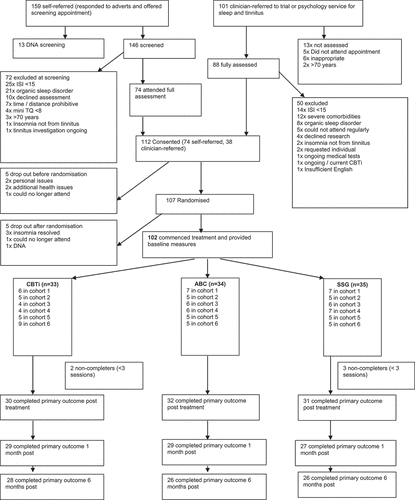
Participants responding to advertisements completed telephone pre-screening with a research assistant, who checked age, ability to attend, ISI, mini-TQ and organic sleep disorder scores (see measures section). Participants from the routine referrals route were pre-screened by their clinician. Full assessment by a clinical psychologist was completed, followed by informed consent and sleep diary training. shows the CONSORT flow diagram.
After randomisation, participants were contacted to confirm attendance and prompted to keep a sleep diary for the 14 days prior to session one. Outcome measures were collected four times: Time 1 (T1) for baseline measures completed on the day before treatment session one (3-weeks post randomisation); Time 2 (T2) at 10-weeks (end of treatment); Time 3 (T3) at 14-weeks (1 month follow-up); Time 4 (T4) at 34 weeks (6-month follow-up).
Treatment fidelity
A randomly selected sample of sessions (selected using a computer-generated random number sequence) were audio recorded. A clinical psychologist from the research team experienced in CBTi and SAC, rated each audio recording for adherence to the treatment, by indicating on coding sheets the extent to which each facilitator covered the relevant topic and if they included information not appropriate for that treatment. Coding sheets indicated appropriate intervention components for each type of treatment (e.g. psycho-education, relaxation). No breaches of fidelity were reported.
Primary outcomes
Changes on the Insomnia Severity Index (ISI score) (Morin et al., Citation2011), a self-report questionnaire assessing characteristics, impact and severity of sleep disturbance over the last two weeks. Seven items on a 5-point Likert Scale are totalled, indicating insomnia as non-clinical (0–7), sub-threshold (8–14) moderate (15–21) or severe (22–28). Internal consistency is excellent (Cronbach 0.91). Clinically significant change is a reduction in at least 6 points (Yang et al., Citation2009).
Sleep diaries tracked sleep pattern. Two weeks before each measurement point, participants recorded daily sleep characteristics (time to bed, SOL, waking, time out of bed). Fortnightly mean averages were calculated for TST, SE and SOL. The CBTi group kept sleep diaries continuously as part of treatment.
Secondary outcomes
The Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., Citation1989) assessed global sleep quality. Seven component scores produce a global index score (0–21). Higher scores indicate lower quality; scores >5 indicate “poor sleep”.
The Dysfunctional Beliefs and Attitudes about Sleep questionnaire (abbreviated) (DBAS-16) (Morin et al., Citation2007) identifies unhelpful sleep-beliefs. Sixteen statements about sleep are rated on a 10-point Likert scale and the mean score calculated. Psychometric properties are robust (α = .8).
Tinnitus severity was assessed using the Tinnitus Questionnaire (TQ) (Hallam, Citation1996), with 41 items rated from 0 to 2, contributing to a total score. Reliable change is a reduction of at least 11 points (McKenna et al., Citation2018). Test–retest reliability (r = .94) and internal consistency (α=.93) are high. Perceived Tinnitus Loudness was measured using a standard 10 cm visual analogue scale (VAS). The Tinnitus Catastrophizing Scale (TCS) (Cima et al., Citation2011) assessed negative tinnitus cognitions.
Psychological distress was measured using the pan-diagnostic, 34 item Clinical Outcome in Routine Evaluation—Outcome Measure (CORE-OM). Items are rated on a 5-point scale, with the mean then multiplied by 10. Internal reliability is good (ranging from α > .75 to <.95). Scores >10 are clinically significant with reliable change indicated by a change >5 points (Evans et al., Citation2000). Depression was assessed on the Patient Health Questionnaire-9 (PHQ-9), where a total of >10 indicates “caseness” (Kroenke et al., Citation2001). Anxiety was assessed on the Generalized Anxiety Disorder Assessment-7 (GAD-7), where a total of >8 indicates ‘caseness’ (Kroenke et al., Citation2007).
The EQ-5D-3 L (Herdman et al., Citation2011) measured Health-related quality of life (HRQL). The EQ5D descriptive system contains five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with three problem levels (none, some, extreme), contributing to an index score. The EQ visual analogue scale (VAS) records perceived health on a scale from 0 to 100 (worst to best imaginable health state). The Work and Social Adjustment Scale (WSAS) (Mundt et al., Citation2002) assessed functioning, with a total score >10 indicating clinically significant impairment.
Information on age, gender, ethnicity, education, tinnitus duration, hearing loss, sleep, other audiological problems, other health problems and previous treatments was collected. Following treatment, participants indicated usefulness, relevance and acceptability of treatment (0 to 10).
Sample size
CBTi for primary insomnia shows medium (d = 0.44) to large (d = 1.09) effect sizes on subjective measures (Morin et al., Citation2006; Okajima et al., Citation2011). Power analysis was conducted prior to commencement of the trial. Assuming an effect size of 0.8, a sample of 102 participants (34 per group) provided 80% power, allowing for 10% loss to follow up, and accounting for a cohort design with 6 participants per group, a within-group correlation of 7% and baseline to outcome correlation of 25% (estimated from Marks et al., Citation2019a).
Missing data
Statistical analysis was planned prior to completion of recruitment. It was completed by a statistician blinded to treatment group. We used modified intention to treat (all allocated to treatment included). Missing data were treated as missing at random (MAR) as appropriate for clinical trial data. The impact of missing data was assessed for all measures, with multiple imputation performed as a sensitivity analysis, using the R package mice (3.1.2), with 100 imputations for each missing observation. Estimated parameters and confidence intervals were not materially different from those obtained using all available data, so results reported are on available observations. The proportion of individuals with missing observations for the primary outcome ISI was broadly similar in each group: 21%, 24% and 19% for treatments 1, 2 and 3, respectively.
Statistical analysis
Statistical analysis was performed in R (3.6.1) using lme4 (1.1) to fit linear mixed models, with a random effect for participant to account for repeated measurements on each participant. Covariate terms were time, treatment and time × treatment interaction. Ninety-five per cent Confidence Intervals (95% CI) for the fixed effect coefficients were obtained from 1000 bootstrap samples. For multi-arm trials in which distinct treatments are compared, there is no consensus on whether adjustment for multiple testing is appropriate (Juszczak et al., Citation2019), so we report all comparisons made, without adjusting confidence intervals for multiple comparisons. Owing to the cohort structure of the trial, a sensitivity analysis was performed with an additional random effect term included for treatment cohort. As there was no evidence for cohort-to-cohort variability, analysis proceeded without a random effect for cohort. In line with Cochrane recommendations, we report effect sizes as standardised mean differences. Effect sizes for relevant variables are available in the supplementary material.
Deviation from protocol
Plans had been made to gather objective sleep measures from a subsample of participants using actiwatches. Unfortunately, hardware failure prevented such analysis. To compensate, the analysis was expanded from protocol to include assessment of SOL, as delayed SOL is a frequent complaint in tinnitus.
Results
Between June 2017 and April 2019, there were 260 respondents to the trial. Of these, 162 were fully assessed and 112 consented. Five withdrew consent pre-randomisation, and five dropped out post-randomisation without providing baseline measures. Treatment commenced with 102 (34 CBTi, 33 ABC, 35 SSG). Attrition from therapy was low (5% overall), and non-completers similar between groups (6% CBTi, 0% ABC, 9% SSG) ().
Demographics and baseline characteristics were equivalent between groups (). Tinnitus was problematic overall (mean TQ score ranged 42.8–46.4) as was insomnia (mean ISI ranged 18.1–19.1) and psychological distress (mean CORE ranged 10.3–11.7). Medical complexity of the group was reflected by self-reporting of 44% with hearing loss, 12% with other audiological conditions and 53% with other health conditions. Pre-trial, 30% had tried other tinnitus treatments and 40% had tried other sleep treatments. A logistic regression analysis found age to be the only predictor of failure to complete (all non-completers were under 50, whereas 52.5 was the median age of the full sample) ().
Table 1. Demographics and baseline characteristics for each group.
Table 2. Outcomes reported as comparison between groups.
Effects on primary outcomes
Insomnia severity index (ISI)
As hypothesised, CBTi led to significantly greater mean reduction in ISI at T2 (adjusted for baseline) than ABC (−6.9 points, 95% CI [−9.1, −4.4]) and SSG (−10.3 points, 95% CI [−12.6, −8.0]). This was maintained at T4, with CBTi showing a greater mean reduction (−5.5 points) than ABC (95% CI [−7.9, −3]) and SSG (−9.7 points, 95% CI [−11.9, −7.1]). Mean reduction in ISI was significantly greater in ABC than SSG at all time points. There were large effect sizes when comparing CBTi to ABC on the ISI at T2 (SMD −2.22 95% CI [−2.95, −1.44]) and at T4 (SMD −1.79 95% CI [−2.57, −0.97]).
CBTi led to a higher proportion of individuals showing clinically meaningful improvement on the ISI (>6-point reduction) and is summarised in . By T4, clinically meaningful improvement was reported by 82% of CBTi participants, compared with 47% in ABC and this was statistically significant (odds-ratio 5.1, 95% CI [1.7,15.5]) and 20% in SSG, which was also a statistically significant difference (odds-ratio 18, 95% CI [5.4,60.5]).
Table 3. Proportion showing reliable change (RC) on each measure and adverse change (AC) proportions on each measure ISI – Insomnia Severity Index (RC > 6 points); TQ – Tinnitus Questionnaire (RC > 11 points); CORE – Clinical Outcomes in Routine Evaluation (RC > 5 points).
Sleep diary – total sleep time and sleep efficiency
All groups showed some increase in Total Sleep Time (TST). At T2, there were no significant differences between groups. By T3 and T4, the CBTi group had greater increase in TST than ABC and SSG. At T4, the CBTi group obtained an average increase in TST of 66 min per night, whilst ABC reported a 30-min increase and SSG a 5-min increase on average. The greater increase in TST in CBTi compared to ABC was significant at T3 (29.3 min, 95% CI [4.1,54.6]) but not at T4 (25.9 min, 95% CI [−1,53.4]). CBTi showed significantly greater TST compared to SSG at T3 (50.3 min, 95% CI [23.8,76.2]) and T4 (67 min, 95% CI [38.9,92.9]). ABC led to significantly greater increases in TST than SSG at T2 (29.3 min, 95% CI [4.4,56.1]) and T4 (41.1 min, 95% CI [12.4,67.8]).
Sleep efficiency has scale with a finite range of values, evident in its distribution, which is distinctly non-normal. As this would violate the assumptions of the linear mixed model, sleep efficacy was transformed using the arcsine transformation, the variance-stabilizing transformation for a proportion. Mean differences for sleep efficiency are reported on the untransformed scale. As hypothesised, sleep efficiency increased more in CBTi when compared to ABC at T2: 9.5%, 95% CI [5.2,13.9] and T4: 8.8%, 95% CI [4.3,14] and when compared to SSG at T2: 18.7%, 95% CI [13.9,23.5] and T4: 18.3%, 95% CI [13.5,23.1]. Sleep efficiency improved significantly more in ABC than SSG.
Effects on secondary outcomes
Effects on sleep onset latency, sleep quality and sleep beliefs
In CBTi, mean Sleep Onset Latency (SOL) was reduced from 42 min at baseline to 16 min at T4. SOL was significantly more reduced in CBTi compared to ABC at T2 (−10.9 min, 95% CI [−17.5,-4.7]), and T4 (−14.5 min, 95% CI [−22.6,-7.7]), and compared to SSG at T2 (−14.4 min, 95% CI [−22,-7.4]) and T4 (−19.4 min, 95% CI [−28.8,-12.6]). SOL was not significantly different between ABC and SSG.
As predicted, the mean reduction in global sleep distress (PSQI) was significantly greater in CBTi when compared to ABC at T2 (−3.5, 95% CI[−4.7,-2.3]) and T4 (−2.7, 95% CI[−4.1,-1.3]) and to SSG at T2 (−5.6, 95% CI [−6.9,-4.2]) and T4 (−3.8, 95% CI [−5,-2.4]). The mean reduction in dysfunctional beliefs about sleep (DBAS) was significantly greater in CBTi at all time points when compared to ABC at T2 (−2.1, 95% CI [−2.7,-1.4]), T4 (−2.3, 95% CI [−4.1,-1.3]) and SSG at T2 (−2.9, 95% CI [−3.6,-2.2]), T4 (−3, 95% CI [−3.6,-2.2]).
Tinnitus outcomes
The reduction in TQ was significantly greater in CBTi than ABC post-treatment at T2 (−10.2 points, 95% CI [−15.3,-4.9]) and T4 (−6.1 points, 95% CI [−11.6,-0.8]). The same pattern was seen when comparing CBTi to SSG. By T4, clinically meaningful improvement was reported by 75% of CBTi participants, compared with 47% in ABC and the difference between the groups was statistically significant (odds-ratio 3.5, 95% CI [1.2,9.9]) and 26% in SSG, which was also a statistically significant difference (odds ratio 9, 95% CI [3, 27]). There were large to moderate effect sizes when comparing CBTi to ABC on the TQ at time 2 (SMD −0.76, 95% CI [−1.14,-0.37]) and at time 4 (SMD −0.45, 95% CI [−0.87,-0.06]).
Counter to hypotheses, there was a significant difference in reduced tinnitus loudness between groups; CBTi participants reported significantly greater reductions in tinnitus loudness on the VAS at T2 compared to ABC (−1.4, 95% CI [−2.5,-0.3]) and compared to SSG (−2.8, 95% CI [−3.8,-1.8]). This remained significant up to T4 for CBTi vs SSG (−1.6, 95% CI [−2.8,-0.5]).
The mean reduction in tinnitus catastrophising was significantly greater in CBTi than in ABC at T2 (−5.9, 95% CI [−9.8,-1.7]) and T3 (−6, 95% CI [−10.1,-1.8]), but not at T4. The mean reduction in tinnitus catastrophising was greater in CBTi than SSG at T2 (−8.7, 95% CI [−12.9,-4.6]) and T4 (−7.1, 95% CI [−11.3,-2.8]).
Effects on psychological distress
Partly in line with hypotheses, psychological distress (CORE) showed a significantly greater reduction in CBTi compared to ABC at T2 only (−2.9, 95% CI [−4.7,-1]). There was a moderate effect size when comparing CBTi to ABC on the CORE at T2 (SMD 0.48, 95% CI [0.17,0.78]). Psychological distress was significantly reduced in CBTi compared to SSG up to T4 (−2.9, 95% CI [−4.7,-0.8]).
At T2, clinically meaningful improvement (a reduction >5 points) was reported by 30% of CBTi participants, compared with 18% in ABC and this was not statistically significant (odds-ratio 2, 95% CI [0.6, 6.3]) and 11% in SSG, where the difference between groups was not statistically significant (odds ratio 3.4, 95% CI[0.9,12.2]). By T4, clinically meaningful improvement was reported 36% of CBTi participants, compared with 15% in ABC and this was statistically significant (odds-ratio 3.3, 95% CI [1,10.8]) and 9% in SSG, and the difference between the groups was statistically significant (odds ratio 6.1, 95% CI [1.5, 24.2]).
In line with hypotheses, the mean reduction in depression (PHQ9) was significantly greater in CBTi than ABC at T2 (−2.2, 95% CI [−4.4,-0.1]) and T4 (2.5, 95% CI [−4.8,-0.5]), and significantly greater in CBTi than SSG at T2 (−5.4, 95% CI [−7.4,-3.2]) and T4 (−4.5, 95% CI [−6.7,-2.3]). Counter to hypotheses, the mean reduction anxiety (GAD7) was not significantly different between CBTi or ABC at any time point. There was a significantly greater reduction in anxiety in CBTi compared to SSG at T2 (−3.1, 95% CI [−5,-1.2]) and T4 (−3.2, 95% CI [−5.1,-1.3]).
Effects on quality of life and functioning
Index values were calculated based on available population norms (Szende et al., Citation2007) using the cran.r-project.org for the EQ5D; no significant differences between groups were found here. However, the EQ VAS measure showed change, and CBTi had significantly greater increases in QoL compared to ABC at T2 (11.7, 95% CI [4.8,18.1]) and T4 (7.2, 95% CI [0.1,14.5]). CBTi significantly greater improvements in EQ VAS compared to SSG, but only at T2 (11, 95% CI [3.9,17.6]). No significant differences between ABC and SSG groups were found on the EQ VAS. Following treatment, the mean impairment in functioning (WSAS) was significantly smaller in CBTi compared to ABC at both T2 (−5.9, 95% CI [−8.3,-2]) and T4 (−4.2, 95% CI [−7.4,-0.9]) and significantly smaller in CBTi than SSG at both T2 (−11.2, 95% CI [−14.4,-8.1]) and T4 (−9.9, 95% CI [−12.9,-6.9]).
Adverse events & acceptability
No major adverse events or clinically significant deteriorations on primary outcomes were reported (). CBTi was rated highly for acceptability, relevance and usefulness (>9.5/10 on all measures). Ratings were lower for ABC (7.7–8.6/10) and SSG (6.4–7.4/10) (see ).
Discussion
CBTi for tinnitus-related insomnia led to significant, clinically meaningful improvements in insomnia and tinnitus distress. CBTi is more effective than commonly available treatments (ABC) including a contact control (SSG). The effect size was striking: 82% of CBTi participants reported clinically significant reductions in insomnia by the end of treatment, maintained for six months (compared to 47% of ABC and 20% of SSG participants). CBTi led to significant improvements on SE and SOL (on average SOL reduced by 26 min by T4). Hypotheses that ABC would lead to significantly greater improvements in insomnia and sleep diary measures when compared to the SSG were also broadly supported, indicating that a lower intensity treatment of this sort may offer important benefits.
Changes in TST were mixed, probably due to treatment design and the unreliability of TST as a measure of insomnia. CBTi and ABC were superior at increasing TST compared to SSG. However, CBTi was only superior to ABC at T3. It is worth noting, however, that the raw scores indicate noteworthy improvements in CBTi, increasing on average by 66 min per night in CBTi (compared to 30 min in ABC and 4 min in SSG). One possible explanation is that whilst ABC focuses on sleep hygiene, CBTi focuses on SE, which involves restricting time in bed until SE has increased to 90%. This means CBTi shortens sleep time initially, reversing over the longer term (Edinger et al., Citation2021). TST can also be an unreliable measure of sleep satisfaction or insomnia, and the results at T2 where CBTi group show significantly reduced ISI scores and improvements in sleep quality despite having little increase in TST, support this conclusion.
Secondary outcomes
CBTi was far more effective in reducing tinnitus distress than the control conditions, with 76% reporting reliable reduction by six months. This compares well with other tinnitus-specific psychological treatments (McKenna et al., Citation2017). It is noteworthy that tinnitus improved following treatment that focused on insomnia rather than tinnitus. The finding aligns with observations in other chronic conditions such as pain (Tang et al., Citation2015), where CBTi improves pain, depression and sleep, and indicates that early intervention for insomnia can have broad physical and mental health benefits.
Unexpectedly, CBTi led to greater reductions in tinnitus loudness than ABC or SSG. As this pattern was mirrored for tinnitus catastrophizing, it is possible more efficient sleep and less SOL, meant that participants had less time to spend awake in bed, bothered by tinnitus. This could have reduced the stress arousal and attentional processes thought to contribute to perceived tinnitus loudness (McKenna et al., Citation2014).
As predicted, CBTi led to significantly greater improvements than SSG in psychological distress, anxiety and depression. Differences between CBTi and ABC were clear on measures of mood, but less apparent on measures of distress and anxiety. This could relate to evidence that insomnia may contribute more to depression than anxiety (Staner, Citation2010), although causal relationships between anxiety, depression and insomnia are unclear (Alvaro et al., Citation2013) and further research is warranted.
Improvements in quality of life and functioning were significantly greater in CBTi than the controls, although a significant difference in the EQ VAS did not continue beyond T2. In contrast, there was little change and no significant group differences on the EQ-5D. This could be the result of a ceiling effect, as baseline scores were high. Researchers recommend the 5-level scale for improved responsiveness (Herdman et al., Citation2011).
Strengths and limitations
Strengths of the study include the novel and robust design, which for the first time compared CBTi to a contact control and an active treatment. Findings thus indicate that outcomes from CBTi are superior to existing treatments and not due to clinical contact alone. Attrition rates were low across all conditions, and ratings of acceptability and usefulness were high, indicating clinical relevance. Recruitment led to a diverse and complex sample, many with hearing loss, and previous experience of treatment, suggesting CBTi may be appropriate for use within standard care. The proportion of reported hearing loss (44%) was low for a tinnitus population, where hearing loss tends to be more prevalent. It is important to note, however, that this was assessed by self-report rather than audiological tests at the time of treatment, meaning mild hearing loss may not have been accurately captured. The proportions with hearing loss were balanced across groups suggesting that it is unlikely that this variable accounts for differences between groups. As “treatment as usual” is highly variable, a specific form of ABC had to be developed as a comparator for this trial. It was based on best possible practice and thus offered an intensified version of standard care. This should be regarded as a strength, as it shows that CBTi is superior to a high quality, active control. While ABC was used as an accurate representation of audiological care likely to be available, it is worth noting that from a methodological point of view, it would have been fairer if all treatments had contained an equal number of sessions; however, this would have decreased representativeness of clinical practice.
There is a risk of bias despite randomization, as the study was not double blind, a common and unavoidable issue in psychological trials (Berger, Citation2015). Unfortunate hardware failure made it impossible to corroborate self-report sleep data with objective measures, but consistency between diary and questionnaire measures suggested participants could indicate sleep improvement without electronic corroboration. This does not limit conclusions relating to insomnia, as satisfaction is more important in assessing insomnia than are objective measures. Finally, although the trial was adequately powered for between-group analysis, the sample size is relatively small for a three-arm RCT.
Conclusions
CBTi for tinnitus-related insomnia leads to greater improvements in insomnia and tinnitus distress than treatment as usual, which in turn leads to greater improvements than supportive group counselling. CBTi for tinnitus-related insomnia should be a standard treatment for patients with this complaint.
Supplemental Material
Download MS Word (23.5 KB)Acknowledgements
We thank Roland Schaette for conducting randomisation and to all of the participants who took part in the study.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/16506073.2022.2084155
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Alvaro, P. K., Roberts, R. M., & Harris, J. K. (2013). A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep, 36(7), 1059–1068. https://doi.org/10.5665/sleep.2810
- Asnis, G. M., Majeed, K., Henderson, M. A., Sylvester, C., Thomas, M., & La Garza, R. D. (2018). An examination of the relationship between insomnia and tinnitus: A review and recommendations. Clinical Medicine Insights: Psychiatry, 9, 1179557318781078. https://doi.org/10.1177/1179557318781078
- Attanasio, G., Russo, F. Y., Roukos, R., Covelli, E., Cartocci, G., & Saponara, M. (2013). Sleep architecture variation in chronic tinnitus patients. Ear and Hearing, 34(4), 503–507. https://doi.org/10.1097/AUD.0b013e31827bc436
- Baguley, D., Andersson, G., McFerran, D., & McKenna, L. (2013). Tinnitus: A multidisciplinary approach. John Wiley & Sons.
- Bastien, C. H., Vallières, A., & Morin, C. M. (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. https://doi.org/10.1016/S1389-9457(00)00065-4
- Berger, D. (2015). Double-Blinding and bias in medication and cognitive-behavioral therapy trials for major depressive disorder. F1000research, 4, 638. https://doi.org/10.12688/f1000research.6953.1
- Burgos, I., Feige, B., Hornyak, M., Härter, M., Weske-Heck, G., Voderholzer, U., & Riemann, D. (2005). Chronic tinnitus and associated sleep disturbances. Somnologie-Schlafforschung Und Schlafmedizin, 9(3), 133–138. https://doi.org/10.1111/j.1439-054X.2005.00056.x
- Buysse, D. J., Reynolds, C. F., III, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4
- Buysse, D. J., Thompson, W., Scott, J., Franzen, P. L., Germain, A., Hall, M., Moul, D. E., Nofzinger, E. A., & Kupfer, D. J. (2007). Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Medicine, 8(3), 198–208. https://doi.org/10.1016/j.sleep.2006.10.006
- Cima, R. F., Crombez, G., & Vlaeyen, J. W. (2011). Catastrophizing and fear of tinnitus predict quality of life in patients with chronic tinnitus. Ear and Hearing, 32(5), 634–641. https://doi.org/10.1097/AUD.0b013e31821106dd
- Crönlein, T., Langguth, B., Geisler, P., & Hajak, G. (2007). Tinnitus and insomnia. Progress in Brain Research, 166, 227–233. https://doi.org/10.1016/S0079-6123(07)66021-X
- Crönlein, T., Langguth, B., Pregler, M., Kreuzer, P. M., Wetter, T. C., & Schecklmann, M. (2016). Insomnia in patients with chronic tinnitus: Cognitive and emotional distress as moderator variables. Journal of Psychosomatic Research, 83, 65–68. https://doi.org/10.1016/j.jpsychores.2016.03.001
- Curtis, F., Laparidou, D., Bridle, C., Law, G. R., Durrant, S., Rodriguez, A., Pierzycki, R. H., & Siriwardena, A. N. (2021). Effects of cognitive behavioural therapy on insomnia in adults with tinnitus: Systematic review and meta-analysis of randomised controlled trials. Sleep Medicine Reviews, 56, 101405. https://doi.org/10.1016/j.smrv.2020.101405
- Edinger, J. D., Bonnet, M. H., Bootzin, R. R., Doghramji, K., Dorsey, C. M., Espie, C. A., Jamieson, A. O., McCall, W. V., Morin, C. M., & Stepanski, E. J. (2004). Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep, 27(8), 1567–1596. https://doi.org/10.1093/sleep/27.8.1567
- Edinger, J. D., Arnedt, J. T., Bertisch, S. M., Carney, C. E., Harrington, J. J., Lichstein, K. L., Sateia, M. J., Troxel, W. M., Zhou, E. S., Kazmi, U., Heald, J. L., & Martin, J. L. (2021). Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. Journal of Clinical Sleep Medicine, 17(2), 263–298. https://doi.org/10.5664/jcsm.8988
- Espie, C. A., Fleming, L., Cassidy, J., Samuel, L., Taylor, L. M., White, C. A., Douglas, J., Engleman, H. M., Kelly, H.-L., & Paul, J. (2008). Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of Clinical Oncology, 26(28), 4651–4658. https://doi.org/10.1200/JCO.2007.13.9006
- Evans, C., Mellor-Clark, J., Margison, F., Barkham, M., Audin, K., Connell, J., & McGrath, G. (2000). CORE: Clinical outcomes in routine evaluation. Journal of Mental Health, 9(3), 247–255. https://doi.org/10.1080/713680250
- Hallam, R. S. (1996). Manual of the tinnitus questionnaire, The Psychological Corporation. Brace & Co.
- Harvey, A. G. (2002). A cognitive model of insomnia. Behaviour Research and Therapy, 40(8), 869–893. https://doi.org/10.1016/S0005-7967(01)00061-4
- Herdman, M., Gudex, C., Lloyd, A., Janssen, M. F., Kind, P., Parkin, D., Bonsel, G., & Badia, X. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research, 20(10), 1727–1736. https://doi.org/10.1007/s11136-011-9903-x
- Hiller, W., & Goebel, G. (2004). Rapid assessment of tinnitus-related psychological distress using the Mini-TQ. International Journal of Audiology, 43(10), 600–604. https://doi.org/10.1080/14992020400050077
- Hoare, D. J., Broomhead, E., Stockdale, D., & Kennedy, V. (2015). Equity and person-centeredness in provision of tinnitus services in UK National Health Service audiology departments. European Journal for Person Centered Healthcare, 3(3), 318–326. https://doi.org/10.5750/ejpch.v3i3.984
- Jungquist, C. R., O’Brien, C., Matteson-Rusby, S., Smith, M. T., Pigeon, W. R., Xia, Y., Lu, N., & Perlis, M. L. (2010). The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Medicine, 11(3), 302–309. https://doi.org/10.1016/j.sleep.2009.05.018
- Juszczak, E., Altman, D. G., Hopewell, S., & Schulz, K. (2019). Reporting of multi-arm parallel-group randomized trials: Extension of the CONSORT 2010 statement. Jama, 321(16), 1610–1620. https://doi.org/10.1001/jama.2019.3087
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ‐9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
- Kroenke, K., Spitzer, R. L., Williams, J. B., Monahan, P. O., & Löwe, B. (2007). Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Annals of Internal Medicine, 146(5), 317–325. https://doi.org/10.7326/0003-4819-146-5-200703060-00004
- Marks, E., McKenna, L., & Vogt, F. (2019a). Cognitive behavioural therapy for tinnitus-related insomnia: Evaluating a new treatment approach. International Journal of Audiology, 58(5), 311–316. https://doi.org/10.1080/14992027.2018.1547927
- Marks, E., Hallsworth, C., & McKenna, L. (2019b). Cognitive behavioural therapy for insomnia (CBTi) as a treatment for tinnitus-related insomnia: Protocol for a randomised controlled trial. Trials, 20(1), 1–11. https://doi.org/10.1186/s13063-019-3778-5
- McCormack, A., Edmondson-Jones, M., Somerset, S., & Hall, D. (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research, 337, 70–79. https://doi.org/10.1016/j.heares.2016.05.009
- McKenna, L., Handscomb, L., Hoare, D. J., & Hall, D. A. (2014). A scientific cognitive-behavioral model of tinnitus: Novel conceptualizations of tinnitus distress. Frontiers in Neurology, 5, 196. https://doi.org/10.3389/fneur.2014.00196
- McKenna, L., Marks, E. M., Hallsworth, C. A., & Schaette, R. (2017). Mindfulness-Based cognitive therapy as a treatment for chronic tinnitus: A randomized controlled trial. Psychotherapy and Psychosomatics, 86(6), 351–361. https://doi.org/10.1159/000478267
- McKenna, L., Marks, E. M., & Vogt, F. (2018). Mindfulness-Based cognitive therapy for chronic tinnitus: Evaluation of benefits in a large sample of patients attending a tinnitus clinic. Ear and Hearing, 39(2), 359–366. https://doi.org/10.1097/AUD.0000000000000491
- Miguel, G. S., Yaremchuk, K., Roth, T., & Peterson, E. (2014). The effect of insomnia on tinnitus. Annals of Otology, Rhinology & Laryngology, 123(10), 696–700. https://doi.org/10.1177/0003489414532779
- Morin, C. M., Bootzin, R. R., Buysse, D. J., Edinger, J. D., Espie, C. A., & Lichstein, K. L. (2006). Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep, 29(11), 1398–1414. https://doi.org/10.1093/sleep/29.11.1398
- Morin, C. M., Vallières, A., & Ivers, H. (2007). Dysfunctional beliefs and attitudes about sleep (DBAS): Validation of a brief version (DBAS-16). Sleep, 30(11), 1547–1554. https://doi.org/10.1093/sleep/30.11.1547
- Morin, C. M., Belleville, G., Bélanger, L., & Ivers, H. (2011). The Insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34(5), 601–608. https://doi.org/10.1093/sleep/34.5.601
- Mundt, J. C., Marks, I. M., Shear, M. K., & Greist, J. M. (2002). The work and social adjustment scale: A simple measure of impairment in functioning. The British Journal of Psychiatry, 180(5), 461–464. https://doi.org/10.1192/bjp.180.5.461
- National Institute for Health and Care Excellence. (2020). Tinnitus: Assessment and management. https://www.nice.org.uk/guidance/NG155
- Okajima, I., Komada, Y., & Inoue, Y. (2011). A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms, 9(1), 24–34. https://doi.org/10.1111/j.1479-8425.2010.00481.x
- Pryce, H., Moutela, T., Bunker, C., & Shaw, R. (2019). Tinnitus groups: A model of social support and social connectedness from peer interaction. British Journal of Health Psychology, 24(4), 913–930. https://doi.org/10.1111/bjhp.12386
- Schecklmann, M., Pregler, M., Kreuzer, P. M., Poeppl, T. B., Lehner, A., & Crönlein, T., Langguth, B., Frank, E., Landgrebe, M., & Langguth, B. (2015). Psychophysiological associations between chronic tinnitus and sleep: A cross validation of tinnitus and insomnia questionnaires. BioMed Research International, 2015, 1–6. https://doi.org/10.1155/2015/461090
- Staner, L. (2010). Comorbidity of insomnia and depression. Sleep Medicine Reviews, 14(1), 35–46. https://doi.org/10.1016/j.smrv.2009.09.003
- Szende, A., Oppe, M., & Devlin, N. J. (2007). EQ-5D value sets: Inventory, comparative review and user guide. Springer.
- Tang, N. K., Goodchild, C. E., Hester, J., & Salkovskis, P. M. (2012). Pain-Related insomnia versus primary insomnia: A comparison study of sleep pattern, psychological characteristics, and cognitive-behavioral processes. The Clinical Journal of Pain, 28(5), 428–436. https://doi.org/10.1097/AJP.0b013e31823711bc
- Tang, N. K., Lereya, S. T., Boulton, H., Miller, M. A., Wolke, D., & Cappuccio, F. P. (2015). Nonpharmacological treatments of insomnia for long-term painful conditions: A systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep, 38(11), 1751–1764. https://doi.org/10.5665/sleep.5158
- Wakabayashi, S., Saito, H., Oishi, N., Shinden, S., & Ogawa, K. (2018). Effects of tinnitus treatments on sleep disorders in patients with tinnitus. International Journal of Audiology, 57(2), 110–114. https://doi.org/10.1080/14992027.2017.1374565
- Wallhäusser-Franke, E., Schredl, M., & Delb, W. (2013). Tinnitus and insomnia: Is hyperarousal the common denominator? Sleep Medicine Reviews, 17(1), 65–74. https://doi.org/10.1016/j.smrv.2012.04.003
- Yang, M., Morin, C. M., Schaefer, K., & Wallenstein, G. V. (2009). Interpreting score differences in the Insomnia severity index: Using health-related outcomes to define the minimally important difference. Current Medical Research and Opinion, 25(10), 2487–2494. https://doi.org/10.1185/03007990903167415