Abstract
Olanzapine (OLZ), one of the second-generation antipsychotics, is effective in stress accompanying with chronic psychiatric disease. But the effect of chronic long-standing use of OLZ administration on the liver and its association with metabolic side effects plus weight gain is still unclear. The objective of our study was to evaluate the antioxidant effect of Hypericum perforatum (HP) on OLZ-induced oxidative stress, biochemical disturbance, and histological alteration in the rat’s liver. Forty male wistar albino rats were allocated as follows: normal control rats (group 1), rats treated with OLZ at a dose of 27.0 mg/kg/day (group 2), group 3 (treated with HP at a dose of 81.0 mg/kg/day) and group 4 (received HP plus OLZ at the same doses). The results revealed that the OLZ-administration led to a significant elevation (***P < 0.001) in the levels of MDH, blood glucose, triglycerides, total cholesterol, liver function parameters and weight gain while a significant decrease (***P < 0.001) in activities of liver SOD and GSH was found. In addition to structural damage in the liver’s tissue. These findings were evaluated the toxic effects of chronic administration of OLZ. While HP supplementation caused a significant improvement (++P <0.05, +++P < 0.001) in liver biomarkers, metabolic parameters, and oxidative stress index besides inhibit the histological damage of liver tissue.
1. Introduction
The medication of antipsychotic drugs is considered a basic therapeutic choice of some chronic psychoses and schizophrenia [Citation1]. OLZ is described as atypical antipsychotic drugs, thienobenzodiazepine class (the prototype of the second generation) [Citation2]. The administration of OLZ has been related to severe hepatotoxic events [Citation3,Citation4]. Both high and low doses of OLZ have induced injury to the rat livers at the cellular levels in histopathological and stereological studies [Citation5]. Few studies have examined the mechanism(s) of hepatotoxicity induced by OLZ up to now. Atypical drugs as OLZ are associated with different metabolic disorders, including hypertriglyceridemia, hyperglycemia, and weight gain [Citation6]. These metabolic disturbances should get a great concern with the increased hazard of obesity complications and death [Citation7]. Unfortunately, exercise and diet regulation didn’t play an important role in obesity-induced by antipsychotic drugs [Citation8]. The clinical importance of preventing weight gain is clear in consideration that among people with schizophrenia, the rate of obesity even before antipsychotic therapy is more than in the general population two to threefold. This makes the diminishing of antipsychotic drug-related metabolic disorders and obesity, leading to cardiovascular diseases and type II diabetes mellitus, as essential as the actual schizophrenia treatment [Citation9]. Herbal medications play an important role as antioxidant treatment [Citation10]. Hypericum perforatum (HP) is typically classified as either a member of the family Clusiaceae or the family Hypericaceae with the genus Hypericum consisting of over 370 species and 4 subspecies. In contemporary herbalism HP is known as wort of St. John’s, the taxonomy of HP is debated. It has been used for over 2000 years to contusions, treat wounds, hemorrhoids, menstrual problems, and kidney stones [Citation11]. HP extract is very efficient as an antidepressant medication with a potent antioxidant effect, and therefore it is useful in treating the pathological changes induced by antipsychotic drugs [Citation12]. So, we chose HP because it is already used as an antidepressant treatment and thus in addition to its improving the toxic effects resulting from the long-term use of OLZ, it also could contribute to enhance the medical impact of OLZ as being an antidepressant. Our study was designed not only to investigate hepatotoxicity of OLZ but also to assessment the ameliorative effects of HP as an antioxidant therapy in hepatocytes of male rats treated with OLZ.
2. Methodology
2.1. Animals of the experiment
Forty male albino Wistar rats (Rattus norvegicus) weighting (90-170 g) were purchased from the Holding Company for Vaccines and Biological Products (VACSERA, Cairo, Egypt). They kept in the animal house of the Department of Zoology (Beni-Suef University, Egypt), at room temp. (20-25 C0) and natural daily 12 h light–dark cycles throughout the experiment. They were given a normal diet. The animals were weighed weekly and gain of body weight calculated at the end of each week of the experiment. Animal experiments were done and guided according to the protocols of the Institutional Animal Care and Committee of Beni-Suef University (IACUC Permit Number: BSU-FS-2018-8).
2.2. Materials
Olanzapine was purchased from PHARAONIA PHARMACEUTICALS the commercial name is Lanzapine. The chemical structure of OLZ is 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b] [1,5] benzodiazepine. OLZ at a dose of 27.0 mg/kg was administered orally and daily for six weeks. Hypericum perforatum (HP) was come by ATOS-Pharma-Egypt. For four weeks HP was administered orally 3times/week at a dose of 81.0 mg/kg. The above-mentioned drugs were supplemented orally in therapeutic equivalent doses (per kg body weight of rat) as calculated with the aid of conversion table formulated by Paget and Barnes [Citation13].
2.3. Design of the experiment
Animals of the experiment were divided into four groups. The first group: (10 animals) served as normal control and was orally and daily given the equivalent volume of the distal (H2O) for six weeks. The second group: (10 animals) acts as a control of OLZ. For six weeks it was orally given OLZ at a dose of 27.0 mg/kg 3 times/week and was given the equivalent volume of the distilled (H2O) for four weeks (from the third week for the sixth week) after two hours of taking OLZ. The third group: (10 animals) served as a positive control of HP. For the first two weeks, it was given the equal volume of distilled (H2O) 3 times/week. Then for the last four weeks it was daily orally administered HP at the dose of 81.0 mg/kg. The fourth group: (10 animals) it was orally given dissolved OLZ at a dose of 27,0 mg/kg 3 times /week for six weeks. From the third week until the sixth week (end of the experiment) HP was given daily 2 hrs. after the first administration of OLZ of that day at a dose of 81.0 mg/kg. Design of the experiment is according to Abd El-Hameed et al. [Citation14].
2.4. Tissue samples and blood collection
All group animals were sacrificed by cervical dislocation under light ether anesthesia. The connective tissue was removed, and the liver was taken out and cleaned in deioniser water to eliminate remaining blood. In phosphate buffer (pH 7.4) the specimens of liver tissue were homogenized. Then at certain rates and for 1 h the homogenate was centrifuged at 3000 r.p.m. to remove cellular debris [Citation15]. Into separate Eppendorf tubes, the supernatants were collected for analysis following centrifugation. The samples of blood were left to coagulate and for 15 min were centrifuged at 3000 r.p.m. Serum obtained were used to measure all parameters needed.
2.5. Detection of body weight
During the experimental period, the body weight of all experimental groups was recorded weekly. From the initial point of treatment (start) to the end of treatment (final). For each rat body weight was determined once a week (g) during the complete study.
2.6. Biochemical analysis
Serum glucose was determined as stated in the schemes of Trinder [Citation16] using kits come by Diamond Company (Egypt). Serum triglycerides were estimated as reported by Pinter and Hayashi [Citation17] using reagent kits come by SPINREACT Company (Spain). Serum total cholesterol was estimated by Watson’s [Citation18] method using chemical kits come by Biomerieux Chemical Company (France). The intensity of the colour formed is equivalent to the concentration of glucose, triglycerides, and cholesterol in the sample. The colour measured at the wavelength of 505 nm. Determination of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were done calorimetrically as described by Reitman and Frankel [Citation19]. According to Belfield and Goldberg [Citation20], serum alkaline phosphatase (ALP) activity was estimated. As described by Ohkawa et al. [Citation21], lipid peroxides as malondialdehyde (MDH) concentration was measured calorimetrically. SOD (superoxide dismutase) activity was estimated according to the procedure of Sun et al. [Citation22]. According to the method of Beutler et al. [Citation23], liver glutathione content (GSH) was estimated.
2.7. Histological studies
The liver was rapidly excised and cut into small pieces of 1 mm3 fixed in 10% neutral buffered formalin for 24 h. They were thereafter routinely processed for histopathological examination by embedding in wax; sectioning at 5 m and stained with eosin and hematoxylin [Citation24].
2.8. Statistical analysis
Using statistical software IBM SPSS Statistics 20 (IBM Corporation, NY, USA) data was interpreted and shown as mean ± standard error (SE). To compare the data between experimental groups One-way analysis of variance (ANOVA) was run and supported by the least significant difference multiple comparison test. At P < 0.05 differences were considered significant.
3. Results
3.1. Biochemical analysis
Table shows a marked increase of liver enzymes (AST, ALT, and ALP) in the group given OLZ alone compared to the control group with marked improvement of liver enzymes in the group treated with combined OLZ and HP.
Table 1. Effect of HP on serum ALT, AST, and ALP levels in OLZ-administered rats.
Table shows that the recorded values of OLZ-administered rats (group2) displayed a significant increase at P < 0.001 in values of lipid peroxidation (LPO). A significant difference in values of liver glutathione content (GSH) and superoxide dismutase (SOD) of group 2 as compared to the normal control group (group1). After 4 weeks, the treatment of OLZ-administered rats with HP (groups 4) make the values of GSH and SOD closer to that of group 1. With concern to the treatment of OLZ-administered rats with HP (groups 4) we found a significant decrease in the values of MDA.
Table 2. Effect of HP on liver oxidative stress and antioxidant enzymes activity of OLZ-administered rats.
Table shows significant increase in serum (glucose, triglyceride, and cholesterol) in the group treated with olanzapine with significant improvement of the results in the group treated by both HP and OLZ.
Table 3. Effect of HP on serum triglyceride, cholesterol, and glucose of OLZ administered rats.
3.2. Weight gain
The values in Table demonstrate the effect of HP on body weight gain of OLZ administered rats. From the results, there is no statistic significant difference between groups at the beginning of the experiment. After 4 and 6 weeks of OLZ-administration a significant increase in the weight of the OLZ-administered rats compared to the control ones was observed. It was found that body weight gain was reduced significantly in the group treated by both HP and OLZ during the fourth and the sixth week. While there are no significant differences on body weight gain between rats treated with HP compared to control rats during different measurement periods which indicate to the safety effect of HP.
Table 4. Effect of HP on body weight gain of OLZ administered rats.
3.3. Histological studies: microscopic examination of the liver
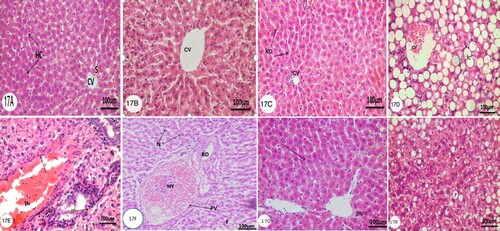
Microscopically, examinations of the liver sections stained with hematoxylin and eosin of different groups (1,2,3 and 4) illustrated that liver tissue of the Control group (group 1) and HP groups (group 3) showed a normal hepatic architecture (Figs. 17A, B and C). The normal liver consists of a few poorly defined hepatic lobules. From the central vein (CV), cords of hepatocytes are radiated for each lobule. By narrow blood sinusoids (S) lined by Kupffer Cells (KC) and endothelial cells, the cell cords are separated. The Kupffer Cells are small with dense nuclei showing several processes and irregular stellate outlines. The hepatocytes (HC) are large polyhedral with acidophilic cytoplasm and rounded. After 6 weeks of OLZ-administration histological assessment of the liver showed many hepatocytes undergo fatty changes (fc) and have the characteristics signet ring appearance and congested central vein (CV) (Fig.17D). The lesions appeared more severe near the portal areas, moreover, the inflammatory cell infiltration (IF) in the portal areas in addition to dilated portal vein (PV) with swelling endothelium (Fig.17E), The other noticed changes, degenerative, necrotic changes (N) of hepatocytes and hyperemic dilated portal vein (HY) (Fig.17F). Microscopical investigation of the liver of OLZ-administered rats supplemented with HP for 4 weeks showed that the liver hepatocytes became more organized in hepatic cells, mild degenerative changes of hepatocytes and the number of hepatocytes that undergoes fatty change decreased (Fig.17G, H) (Figure ).
Figure 1. Photomicrographs of transverse section (T. S) (5 µm) of liver of rats of control group, HP-administered group, OLZ-administered group, and animal treated with OLZ and HP. Characteristic figures were stained with H&E. The original magnification was x100. (A and B) Control group, Arrow, (CV) and (S) indicate normal hepatic cell, central vein, and sinusoids, respectively. (C) Animals treated with 81.0 mg/kg /day HP three times a week for 4 weeks. Illustrating normal Kupffer Cells (KC) lining the sinusoids. (D, E, and F) animals treated with 27.0 mg/kg/day OLZ daily for 6 weeks showing severe fatty changes (FC) and dilated congested central vein (CV) hyperemic dilated portal vein (HY), bile duct (BD) and necrotic cells (N). In addition to inflammatory cells infiltration (IF) in the portal areas and dilated portal vein (pv) with arrow refers to swelling endothelium. (G and H) rats were given OLZ at a dose of 27.0 mg/kg/day for 6 weeks plus with HP at a dose of 81.0 mg/kg/day three times a week for last 4 weeks revealing normal hepatic parenchyma indicated by an arrow without fatty changes and dilated portal vein (PV).
3. Discussion
The atypical antipsychotics are considered the first line in the treatment of psychotic disorders [Citation25]. Although the atypical antipsychotic represented a principal advancement in the treatment of psychotic disorders, OLZ and others induce dyslipidemia, hyperglycemia, obesity, and body weight gain [Citation26]. Our investigation aims to view the beneficial antioxidant effect of HP in minimizing OLZ toxicity side effects. Treatment with HP without exposure to OLZ revealed no significant changes in almost investigated parameters showing its safe use. This study showed that the administration of OLZ induces metabolic disorder including an elevation in fasting glucose, serum triglycerides and total cholesterol levels besides an increase in body weight. Regarding the treatments with HP, our results showed a marked decrease in these metabolic parameters and body weight as compared with OLZ-administered rats after 4 weeks of treatments. Our results agree with the results of Deng and Al-Sayed et al. [Citation27,Citation28] which revealed that obesity was observed frequently in OLZ-treated patients (64%) than in patients treated by conventional antipsychotics (30%) or by other atypical antipsychotics (56%) among the schizophrenic patient besides a significant increase in concentrations of triglycerides in 56% of OLZ-treated patients. These results were recently established in different investigations comparing OLZ with other atypical antipsychotic drugs [Citation29]. Generally, weight gain is significantly evident in treatment with atypical antipsychotic drugs especially in the first month [Citation8]. The most accepted mechanism of action is related to 5-HT2C antagonism and/or other receptors by antipsychotic drugs, which is leading to an increasing food intake [Citation8]. Many studies proposed that the beginning of diabetes that associate antipsychotic drugs are secondary to the weight gain effect associated with antipsychotic therapy [Citation30], most likely both are because of histamine antagonism [Citation31]. Excess free fatty acids (FFA) release from hypertrophic adipocytes results from the increase in adiposity leading to higher FFA concentrations [Citation32]. Also, in accordance with our results Shirzadi et al. [Citation33] results found that there is an elevation in serum lipids relates to body weight increase in the animal group treated with OLZ. Our results about HP are supported by García et al. [Citation34] as his results revealed that some HP solenoids’ extracts help in decreasing body weight gain and serum metabolic parameters (glucose, triglycerides) and results of Arokiyaraj et al. [Citation32] that found a decrease in serum glucose and increase serum insulin besides a decrease in serum triglycerides and total cholesterol in STZ-diabetic rats treated with HP extract. The oxidative stress may be the possible mechanism by which these differential metabolic adverse effects occur. Miljevic et al. [Citation35] reported that oxidative stress may be a mechanism by which OLZ induces its adverse effects that increase the risk of metabolic syndrome and diabetes. Increasing levels of protein and lipids oxidation result from oxidative cell injury, which is induced by atypical antipsychotics as shown recently in many studies [Citation36]. In this study, we found that there is a significant increase in the level of malondialdehyde which is an indicator of lipid peroxides and a significant decrease in the antioxidant enzymes’ activity like Glutathione (GSH) and Superoxide dismutase in liver tissues of OLZ treated rats which suggest oxidative stress status. Increasing lipid peroxides’ level may be due to the activation of superoxide production [Citation37]. Our findings are supported by the results of Todorović et al., [Citation38] that found an increase in ROS production with OLZ administration and supported by Beasley C. who found that antipsychotic drugs cause oxidative stress and cell damage in some organs as liver in animals treated with OLZ. The supplementation of HP plus OLZ for 4 weeks has made the content of liver GSH and the values of SOD near to the values of the normal control group and minimizing the elevation of lipid peroxidation as compared to OLZ-administered group. The important detoxification mechanism is the conjugation of reactive drug metabolites to glutathione that can be mediated spontaneous and/or spontaneous by GSTs (glutathione S-transferases) [Citation39]. The antioxidant activity of HP extracts is well recognized, and it is discussed because of its high content of phenolic compounds with the excellent radical scavenging ability [Citation40]. So, the extracts of HP inhibit lipid peroxidation in vivo [Citation41]. Our study revealed that OLZ-treated rats showed a significant rise in serum transaminases AST, ALT and ALP activities after 4 weeks of the treatment. These results reflect liver cell damage and degeneration or destruction of cellular architecture and the increase in plasma ALP activities might be due to cellular necrosis or the increased permeability of plasma membrane and this indicates to stress condition in the liver of rats treated with OLZ [Citation42]. The present study recorded a marked decrease in serum transaminases and ALP after administration of HP. Our results are parallel to the findings of Bitiren et al. [Citation43] who found a significant increase of liver enzymes (AST, ALT, and ALP) levels in the plasma has occurred after OLZ treatment and it was markedly lowered by HP extract administration. HP supplementation overcome the biochemical changes by protecting the antioxidant mechanism and suppressing the formation of reactive oxygen species [Citation43]. By histopathological examination of the OLZ treated animals compared to untreated normal controls we found an increased vacuolization of hepatocytes, severe fatty changes, inflammatory infiltration, and an increase in congestion of the portal area. These findings referred to marked changes in liver’s tissue because of OLZ administrations. Primarily may be by the injury to the different membrane components of the cell by the generation of reactive oxygen species. These changes are correlated to the biochemical changes as the increased level of lipid peroxidation and alter serum activities of liver marker enzymes. Also, we showed that these histological changes were diminished after HP administration and become more like that of the normal control group. In the same line with our results Bitiren et al. [Citation43] revealed that the histopathological examination of the liver showed a severe fibrosis in CCL4 treated animal group and less fibrosis in groups treated with HP.
3. Conclusion
When administered to Wistar albino rats at the indicated dose and time-period, OLZ disrupted the cellular antioxidant defense system and caused lipid peroxidation in the liver (Table ) which, leads to metabolic disorder including weight gain (Table ). Therefore, it is likely that previous studies have been underestimating OLZ’s side effects and it will be important to consider these results going forward. On the other hand, HP, as a powerful antioxidant that revealed many beneficial effects in the hepatotoxicity of rat liver, exhibited a positive effect that alleviated/eliminated the oxidative stress induced by OLZ and attenuated the histopathological changes of the liver. Based on the findings obtained in the present study, it is suggested that (i) to monitor body weight, blood glucose, lipid profile and antioxidant parameters in patients undergoing therapy with olanzapine. (ii) HP could be used as a food additive for protection from hepatotoxicity risk of OLZ. (iii) the present study may constitute a reference for future mechanistic animal research to be investigated on the molecular mechanism of HP active component.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Patel KP, Cherian J, Gohil K, et al. Schizophrenia: Overview and treatment Options. pharmacology and Therapeutics. 2014;39(9):638–645.
- Jennings AA, Guerin N, Foley T. Development of a tool for monitoring the prescribing of antipsychotic medications to people with dementia in general practice: a modified eDelphi consensus study. Clin Interv Aging. 2018;13:2107–2117. doi: 10.2147/CIA.S178216
- Telles-Correia D, Barbosa A, Cortez-Pinto H, et al. Psychotropic drugs and liver disease: A critical review of pharmacokinetics and liver toxicity. World J Gastrointest Pharmacol Ther. 2017;8(1):26–38. doi: 10.4292/wjgpt.v8.i1.26
- Eftekhari A, Azarmi Y, Parvizpur A, et al. Involvement of oxidative stress and mitochondrial/lysosomal crosstalk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica. 2015;46(6):369–378.
- Odaci E, Bilen H, Hacimuftuoglu A, et al. Long-term treatments with low and high dose olanzapine change hepatocyte numbers in rats. A stereological and histopathological study. Arch Med Res. 2009;40:139–145. doi: 10.1016/j.arcmed.2009.02.006
- Freyberg Z, Aslanoglou D, Shah R, et al. Intrinsic and antipsychotic drug-induced metabolic Dysfunction in schizophrenia. Front Neurosci. 2017;11:432–445. doi: 10.3389/fnins.2017.00432
- Grajales D, Vitor Ferreira V, Valverde AM. Second-Generation antipsychotics and Dysregulation of glucose Metabolism: Beyond weight gain. Cells. 2019;8:1336–1363. doi: 10.3390/cells8111336
- Dayabandara M, Hanwella R, Ratnatunga S, et al. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatric Disease Treatment Journal. 2017;13:2231–2241. doi: 10.2147/NDT.S113099
- Bradshaw T, Mairs H. Obesity and Serious Mental Ill Health: A Critical Review of the Literature. Healthcare (Basel). Jun; 2014;2(2):166–182. doi: 10.3390/healthcare2020166
- Alok S, Jain S, Verma A, et al. . herbal antioxidant in clinical practice: A review. Asian Pac J Trop Biomed. 2014;4(1):78–84. doi: 10.1016/S2221-1691(14)60213-6
- Raclariu AC, Paltinean R, Vlase L, et al. Comparative authentication of Hypericum perforatum herbal products using DNA metabarcoding, TLC and HPLC-MS. Sci Rep. 2017;7(1291):1–12.
- Wang P. and T. Use of antipsychotics in the treatment of depressive disorders. Shanghai Arch Psychiatry J. 2013;25(3):134–140.
- Paget GE, Barnes JM. Toxicity tests. In: Laurence DR, Bacharach AL, editor. Evaluation of drug activities: pharmacometrics. London: Academic Press; 1964. p. 134–166.
- Abd El-Hameed AM, Soliman HA, Abd El-Reheem ES. Protective role of Wheat Germ Oil in Clozapine-induced oxidative stress and biochemical alterations in liver of male albino rats. Journal of American Science. 2013;9(1):268–174.
- Adel A, Eman SA, Sanaa MA, et al. Assessment of the ameliorative effect of p-coumaric acid and gallic acid on oxidative stress and haematological abnormalities in experimental type 2 diabetes. Gen Med Open. 2018;2(6):1–6.
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. J. Ann. Clin. Biochem. 1969;6:24–27. doi: 10.1177/000456326900600108
- Pinter J, Hayashi J. Determination of triglycerides. J. Arch. Biochem. Biophy. 1967;121(2):404–414. doi: 10.1016/0003-9861(67)90094-X
- Watson D. A simple method for determination of serum cholesterol. J. Clin. Chem. Acta. 1960;5:637–643. doi: 10.1016/0009-8981(60)90004-8
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56
- Belfielda H, Goldberg DM. (1971). Revised assay for serum phenyl phosphate activity using 4-amino-anti-pyrine enzyme; 12: 561–531.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry Journal. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clinical Chemistry Journal. 1988;34(3):497–500. doi: 10.1093/clinchem/34.3.497
- Beutler E, Duron O, Kelly BM. Improved method for determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888.
- Bancroft JD, Gamble M. Theory and practice of histology Techniques. 5th ed. London: Churchill Livingston; 2002.
- Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–179. doi: 10.1093/bmb/ldv017
- Jennings LJ, Arcila ME, Corless C, et al. Guidelines for Validation of Next-generation Sequencing-Based Oncology Panels: A Joint Consensus Recommendation of the association for molecular Pathology and College of American Pathologists. Journal Molecular Diagnosis. 2017;19(3):341–365. doi: 10.1016/j.jmoldx.2017.01.011
- Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am. 2013;42(3):545–563. doi: 10.1016/j.ecl.2013.05.006
- Al-Sayed E, Martiskainen O, Seif el-Din S, et al. Hepatoprotective and antioxidant Tetrachloride-induced hepatotoxicity in Mice and Characterization of Its Bioactive compounds by HPLC-PDA-ESI-MS/MS. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/245171
- Raben AT, Marshe VS, Chintoh A, et al. The Complex Relationship between antipsychotic-induced weight gain and therapeutic Benefits: A Systematic Review and Implications for treatment. Front Neurosci. 2018;11:1–19. doi: 10.3389/fnins.2017.00741
- Holt RG. Association between antipsychotic medication Use and diabetes. Curr Diab Rep. 2019;19(10):96–106. doi: 10.1007/s11892-019-1220-8
- Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry. 2000;61:742–749. doi: 10.4088/JCP.v61n1006
- Arokiyaraj S, Balamurugan R, Augustian P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2011;1(5):386–390. doi: 10.1016/S2221-1691(11)60085-3
- Shirzadi AA, Ghaemi SN. Side effects of atypical antipsychotics: extra pyramidal symptoms and the metabolic syndrome. Harv Rev Psychiatry. 2006;14:152–164. doi: 10.1080/10673220600748486
- García-de CL, Navarrete-Castro A. Hypolipidemic and weight Reducing activity of two Hypericum species extracts in Cafeteria fed overweight rats. Planta Med. 2012;78:6–12. PH6. doi: 10.1055/s-0031-1280228
- Miljevic C, Nikolic M, Nikolic-Kokic CA, et al. Lipid status, antioxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:303–307. doi: 10.1016/j.pnpbp.2009.11.024
- Xu H, Zhuang X. Atypical antipsychotics-induced metabolic syndrome and non-alcoholic fatty liver disease: a critical review. Neuropsychiatr Dis Treat. 2019;15:2087–2099. doi: 10.2147/NDT.S208061
- Beasley C. (2010). Recipient Research Update. Mind foundation of British Colombia. Foundation of the BC Schizophrenia Society.
- Todorović N, Tomanović N, Gass P, et al. Olanzapine modulation of hepatic oxidative stress and inflammation in socially isolated rats. Eur J Pharm Sci. 2016;1(81):94–102. doi: 10.1016/j.ejps.2015.10.010
- Dragovic S, Boerma JS, Bergen LV, et al. Role of human glutathione S-transferases in the inactivation of reactive metabolites of clozapine. Chem Res Toxicol. 2010;23(9):1467–1476. doi: 10.1021/tx100131f
- Abd El-Hameed AM, Mahmoud HS. Cypermethrin induced apoptosis and testicular toxicity by upregulation of p53 in the brain and testis of male rats is alleviated by Sesame oil. Journal of Taibah University for Science. 2020;14(1):1342–1349. doi: 10.1080/16583655.2020.1822057
- Spiteller M, Ozen T, Šmelcerović A, et al. Phenolic constituents and the in vitro antioxidant activity of the flowers of Hypericum venustum. Fitoterapia. 2008;79:191–193. doi: 10.1016/j.fitote.2007.11.012
- Martha Lucinda Contreras-Zentella and Rolando Hernández-Muñoz. Is liver Enzyme release Really associated with cell necrosis induced by Oxidant stress? Oxid Med Cell Longev. 2016;1:1–12.
- Bitiren M, Musa D, Ozgonul A, et al. Protective effects of green tea, Hypercium perforatum on hepatic injury and lymphocyte DNA induced by carbon tetrachloride. International Journal of Pharmacology. 2010;6(3):241–248. doi: 10.3923/ijp.2010.241.248

