ABSTRACT
Introduction: Over the years, chewing gum has developed from a candy towards an oral health-promoting nutraceutical. This review summarizes evidence for the oral health benefits of chewing gum, emphasizing identification of active ingredients in gum that facilitate prevention and removal of oral biofilm.
Areas covered: Chewing of sugar-free gum yields oral health benefits that include clearance of food debris, reduction in oral dryness, increase of biofilm pH and remineralization of enamel. These basic effects of chewing gum are attributed to increased mastication and salivation. Active ingredients incorporated in chewing gums aim to expand these effects to inhibition of extrinsic tooth stain and calculus formation, enhanced enamel remineralization, reduction of the numbers of bacteria in saliva and amount of oral biofilm, neutralization of biofilm pH, and reduction of volatile sulfur compounds.
Expert opinion: Evidence for oral-health benefits of chewing gum additives is hard to obtain due to their relatively low concentrations and rapid wash-out. Clinical effects of gum additives are overshadowed by effects of increased mastication and salivation due to the chewing of gum and require daily chewing of gum for prolonged periods of time. Future studies on active ingredients should focus on specifically targeting pathogenic bacteria, whilst leaving the healthy microbiome unaffected.
1. Introduction
Many oral diseases, most notably caries, gingivitis, and periodontitis, are caused by oral biofilms. The development of a pathogenic biofilm depends for a major part on the amount of biofilm and the relative prevalence of good and bad microorganisms within oral biofilms. The formation of oral biofilm constitutes the transition of bacteria from their freely suspended or planktonic state in saliva to an adhering or sessile state on oral hard and soft tissues. In the oral cavity, due to the abundant presence of salivary proteins, bacteria never adhere to bare surfaces but always to an adsorbed salivary conditioning film (SCF), within the dental field sometimes called ‘pellicle.’ Adsorbed SCFs mainly consist of a layer of specific proteins, such as glycoproteins or mucins [Citation1], on the one hand serving functions in lubrication, hydration, and protection of the tooth surface, while on the other hand providing the first anchor for bacteria to adhere to. Formation and composition of SCFs are largely determined by the environment and can be influenced by the presence of specific components that influence adhesion of bacteria [Citation2]. Small differences in the forces by which different strains of bacteria are attracted to a SCF, as determined by the bacterial surface charge and hydrophobicity [Citation3], play an important role in the composition of biofilms on intraoral surfaces [Citation4]. Upon further growth of the biofilm, more strains and species become incorporated in a biofilm through co-adhesion with other colonizers, governed by an interplay between specific ligand–receptor binding and nonspecific bacterial interactions [Citation5]. Oral diseases develop when the balance within the oral microbiome is lost and pathogenic bacteria are commencing to dominate. This occurs for instance, when cariogenic strains in a biofilm produce an excess of acids through the fermentation of environmental sugars causing enamel demineralization or when periodontopathogens residing mostly in gingival pockets, cause gingivitis or in more advanced state, periodontitis [Citation6].
Although much has been achieved with respect to the prevention of oral diseases like caries, gingivitis, and periodontitis [Citation7,Citation8], maintenance of an effective oral hygiene by toothbrushing, using advanced toothpaste formulations and mouthrinses, remains beyond reach for many people. Therefore, a variety of other mechanical aids, in addition to toothbrushing, such as toothpicks, floss wire, and chewing gum have been promoted for the removal of oral biofilm [Citation9]. In this review, we evaluate the potential benefits on oral health of chewing gum for the delivery of oral therapeutics with special emphasis on the identification of active ingredients incorporated in gum that facilitate prevention and removal of oral biofilm.
1.1. History and development of chewing gum
Throughout history, different materials have been used by people to chew on in order to refresh their breath or relieve oral dryness. Early types of chewing gum are based on tree resins. It was not until 1870 that Thomas Adams was able to successfully market chewing gum on a mass scale [Citation10,Citation11]. Since its first introduction, chewing gum has developed into a multibillion dollar industry [Citation10] aided by the invention of rubbers in the 1930s and 1940s [Citation11]. In the 1970s, chewing gum developed more and more from a candy toward a functional food aiming for niche markets. For example, nonstick gum for denture wearers or gums with tooth-whitening properties but also as a delivery system for drugs with systemic effects [Citation12-Citation14]. The current portfolio of chewing gums meets the specific demands of various consumer groups and follows the need to differentiate in a competitive world [Citation15]. Following compliance with different consumer requirements, chewing gum has become recognized for its role in maintenance and improvement of oral health [Citation10]. Nevertheless, it must be emphasized that chewing gum is intended as an addendum to regular daily-oral hygiene procedures, and not a replacement of any other procedure.
Current chewing gums consist of several basic ingredients, as summarized in [Citation14,Citation16]. The gum base provides elasticity to a gum and should not dissolve during chewing. Moreover, it should allow a gum to be chewed for relatively long periods of time without major changes in structure. Generally, gum base consists of a mixture of elastomers, like polyvinylacetate or polyisobutylene, that are complemented with softeners, emulsifiers, and plasticizers. Molecular weight of the polymer ingredients, together with the interaction with other ingredients, determines gum viscosity. Hydrophilicity of the base system is an important determinant for the ability of gums to take up water or saliva, which influences chewing gum texture. The tendency of gums to absorb saliva is mainly determined by emulsifiers, which create a stable mixture of normally immiscible ingredients. Formulation of the latter ingredients is adjusted based on desired functionality.
Table 1. Overview of chewing gum ingredients with their functions and estimates of percentages [Citation14–Citation16].
Approximately 70% of all gums marketed do not contain conventional sweeteners, like sucrose, but have sugar substitutes like xylitol, sorbitol, erythritol, mannitol, and/or maltitol and are consequently considered to be ‘sugar-free’ gums [Citation17]. Aspartame, acesulfame-K, and glycerin add an extra degree of sweetness and also provide longer lasting flavor duration [Citation14,Citation15,Citation18]. Polyols are widely used instead of conventional sugars and their main advantage is that they are not or hardly fermented by bacteria, classifying them as non-acidogenic and cariostatic [Citation19]. The replacement of conventional sugars to create sugar-free gums was the most important development advancing chewing gum from a candy to a nutraceutical with specific oral health benefits.
2. Potential benefits of chewing gum on oral health for delivery of nutraceuticals
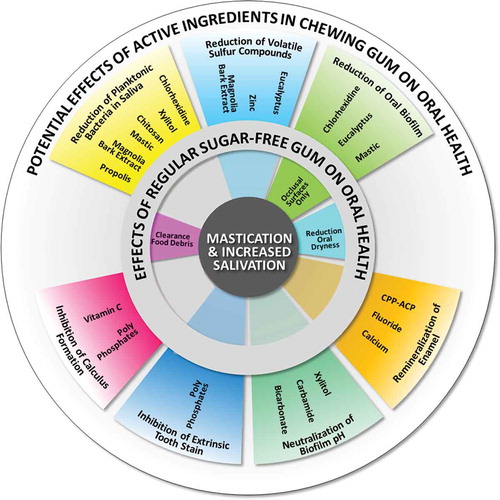
Chewing of gum stimulates the salivary glands, causing an approximately 10-fold increase in salivary flow over unstimulated salivation during the first 5 min of chewing [Citation20]. Increased salivation together with the mechanical action of mastication provides the basis for many effects of chewing gum on oral health (see and for an overview). Furthermore, chewing gum can be a vehicle for administering active ingredients to the oral cavity. Potential oral health benefits of the chewing of gum are summarized in the different circle segments in , together with the active ingredients assumed responsible for these benefits.
Table 2. Overview of active ingredients used in different chewing gums and oral health benefits as reported in the literature, including EFSA support of specific claims where available.
Figure 1. Basic effects of the chewing of regular sugar-free gum on oral health are displayed in the inner ring, and are predominantly due to increased mastication and salivation. Potential effects of active ingredients used in chewing gum on oral health are displayed in the outer ring, of which some are an extension of the effects of regular sugar-free gum.
A release profile of active ingredients from chewing gum is dependent on various factors such as, chew rate, gum base composition, and characteristics of the active ingredients, such as solubility in water, potentially making their prolonged presence and substantive action in the oral cavity possible [Citation14]. In general, active ingredients soluble in water will be released in the first minutes of chewing, opposed to less soluble ingredients, that will bind more strongly to the gum base [Citation16]. At the same time, rapid dilution of active ingredients in the oral cavity occurs, being adsorbed to oral tissues and swallowed at a high salivary flow rate of approximately 2 ml/min [Citation20]. This makes high concentrations of active ingredients in the oral cavity difficult to achieve during chewing and their clinical efficacy hard to demonstrate over the basic effects of increased salivation and mastication.
2.1. Reduction of oral dryness
Individuals, who suffer from xerostomia or the subjective feeling of dry mouth and have secretory capacity, can relieve their symptoms by the use of regular sugar-free chewing gum (). Chewing gum is generally preferred by dry mouth patients over the use of artificial saliva, although there is no evidence that either one is more effective than the other [Citation21,Citation22]. Symptom relief is related to mastication and increased salivation, and not related to any specific flavor or additive incorporated in chewing gums [Citation22,Citation23]. Importantly, the claim that the chewing of gum reduces dry mouth perception [Citation22,Citation24] is supported by the European Food Safety Authority (EFSA).
2.2. Clearance of food debris
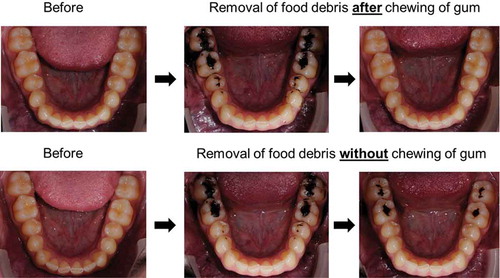
The chewing of gum can stimulate removal of food debris left after consumption, as illustrated in . Removal is partly due to direct attachment of debris to the gum but also due to increased mastication and salivation which aids to wash away debris [Citation25,Citation26]. Since debris left after food consumption often contains fermentable sugars, used by bacteria to produce acids that desorb calcium (Ca2+) and phosphates from the enamel within minutes after consumption, quick removal of debris is necessary to prevent or reduce demineralization [Citation20,Citation27–Citation29]. Removal of food debris constitutes one of the clear oral health benefits of chewing gum ().
Figure 2. Removal of food debris (Oreo cookie, Nabisco, East Hanover, NJ, USA) from lower jaw occlusal surfaces by the chewing of gum (TOP row) and by natural oral cleansing mechanisms only without chewing of gum (BOTTOM row). A photograph was taken before consumption (LEFT column) and directly after consumption (MIDDLE column). Subsequently, the volunteer was requested to chew regular sugar-free gum for 2 min (TOP row) or wait for 2 min without the chewing of gum (BOTTOM row) and a third photograph was taken afterwards, illustrating the difference in removal of food debris (RIGHT column). Photographs taken with thanks to the Department of Orthodontics, University Medical Center Groningen, The Netherlands.
2.3. Inhibition of calculus formation
Calculus formation involves the formation of calcium phosphate mineral salts that calcify and harden the oral biofilm. Among other factors, biofilm pH and salivary calcium phosphate saturation play an important role in the rate of calculus formation [Citation30,Citation31].
Chewing of regular sugar-free gum did not have a pronounced effect on inhibiting calculus formation and it has even been suggested that calculus formation is promoted by chewing sugar-free gum, due to higher biofilm pH and salivary calcium phosphate saturation [Citation32,Citation33]. Therefore, active ingredients have been incorporated in chewing gums aiming to maintain calcium phosphate deposits in an amorphous state, preventing hardening and facilitating removal. When chewing vitamin C supplemented chewing gum at least 5 times per day for 3 months, a reduction in supragingival calculus formation was found compared to a control gum and no chewing gum. The acidic properties of vitamin C were proposed to account for the reduction of calcium phosphate deposits [Citation33]. It should be noted that it was not studied whether the acidic properties of vitamin C adversely affected enamel demineralization. Similar results were obtained for pyro/triphosphates supplemented chewing gum after 6-week use [Citation34], related to the calcium sequestering properties of polyphosphates at the enamel surface [Citation35]. Nevertheless, since reductions in calculus formation were only demonstrated for supragingival surfaces, and not for gingival margins and interproximal spaces that matter most in oral health, a negative verdict was released by the EFSA on oral health claims of sugar-free gums regarding a reduction in calculus formation [Citation36] ().
2.4. Inhibition of extrinsic tooth stain
Esthetics, including the appearance of white teeth, is more and more considered as an important component of oral health. Extrinsic tooth stain is caused by chromogens from food, drinks, or smoking that adsorb in superficial enamel layers (or calculus). Extrinsic tooth stain is more susceptible to whitening regimens than intrinsic tooth stain, but usually still requires professional removal, depending on the causative chromogen. Chewing of regular sugar-free gum multiple times per day for 4 weeks or longer has been shown to prevent and remove extrinsic tooth stain caused by chromogens [Citation12,Citation37,Citation38], likely again as a result of increased salivation ().
To enhance extrinsic stain prevention and removal [Citation12], sugar-free chewing gum has been supplemented with polyphosphates [Citation39]. Sodium hexametaphosphate () in a sugar-free gum reduced stain formation better than a control gum in short-term (2 days) studies during which volunteers chewed 8 times two tablets of gum throughout the day [Citation39–Citation41]. When chewing 3 times two tablets per day for 6 weeks or longer, tooth stain prevention has been shown for hexametaphosphate, pyrophosphate, and tripolyphosphate supplemented sugar-free gums [Citation42,Citation43]. Stain prevention by polyphosphates has been attributed to adsorption of these highly negatively charged and hydrophilic molecules to the SCF, which makes incorporation of chromogens in superficial enamel layers more difficult. Administration of sodium hexametaphosphate through the chewing of gum produced a more hydrophilic tooth surface in vivo than a control gum [Citation44], while sodium hexametaphosphate caused desorption of proteins from SCFs and created a more open film structure in vitro [Citation45,Citation46].
2.5. Reduction of volatile sulfur compounds
Oral malodor, or halitosis, results from the production of volatile sulfur compounds (VSCs), such as hydrogen sulfide and methyl-mercaptan by anaerobic gram-negative bacteria adhering to the tongue or associated with periodontitis [Citation47]. Regular sugar-free chewing gum has been shown to successfully reduce VSCs and thereby freshen breath [Citation13] (). Besides the reduction of VSCs by the regular chewing of sugar-free gum, active ingredients incorporated in a gum have aimed to further reduce halitosis either by directly interacting with VSCs or by targeting bacteria responsible for oral malodor (). Zinc has high affinity for sulfur compounds [Citation48] and, especially in combination with allyl isothiocyanate [Citation49], results in reduced VSC levels directly after chewing compared to a control gum [Citation50], although this could not be confirmed in another study [Citation13]. Furthermore, magnolia bark extract and eucalyptus both target the viability of bacteria producing VSC and were shown to be effective against oral malodor in a chewing gum [Citation51,Citation52], especially when magnolia bark extract was combined with zinc [Citation53] ().
2.6. Neutralization of biofilm pH
The pH buffering ability of saliva counteracts acids produced in oral biofilm and is therefore of importance to maintain the intraoral balance between enamel re- and demineralization. Bicarbonate
provides the main buffering system of saliva and neutralizes oral biofilm pH [Citation54]. Neutralization of biofilm pH is also achieved via a different mechanism involving carbamide ([NH2]2CO) or urea. Oral bacteria that produce urease hydrolyze and convert carbamide into ammonia and create a more alkaline environment. Chewing of regular sugar-free gum has been shown to increase biofilm pH, as was recognized by the EFSA [Citation55]. Furthermore, it increases the resting pH of oral biofilm and resistance of the enamel surface to acid challenges [Citation56] as a result of increased salivation. This effect was enhanced by the addition of xylitol to chewing gum [Citation57] ().
Addition of other actives such as bicarbonate to chewing gum also caused an increase in the buffering capacity of saliva [Citation58]. Accordingly, interproximal biofilm pH, after a sucrose challenge, was elevated more rapidly and maintained at a higher level compared to a gum without bicarbonate [Citation59]. Furthermore, chewing of carbamide supplemented gum yielded a concentration-dependent rise in biofilm pH [Citation60,Citation61]. The EFSA has concluded that the claim that chewing gum containing carbamide stimulates biofilm pH neutralization directly after chewing is justified when the gum contained at least 20 mg of carbamide and was chewed for 20 min after food intake [Citation62]. However, when effects of the chewing of carbamide supplemented gum were evaluated for 4 weeks or longer, no change in acid production by oral biofilm was observed [Citation61], neither were caries preventive effects observed after 3 years of use in terms of a reduced number of decayed, missing or filled surfaces [Citation63]. This shows that direct, short-term effects cannot be readily extrapolated to long-term effects, most likely because short-term studies do not include enamel demineralization due to food and drink consumption, effects that are influential in long-term studies.
2.7. Enamel remineralization
Saliva is rich in calcium and phosphates, facilitating enamel remineralization and preventing demineralization [Citation10]. Chewing of regular sugar-free gum can enhance calcium and phosphate levels in the oral cavity through increased salivation. Long-term clinical studies showed that, in addition to normal oral hygiene, chewing of regular sugar-free gum multiple times per day can result in lower caries incidence, particularly after a meal. In view of this, the daily frequency is more important than the single chewing time [Citation63–Citation65]. This effect on caries incidence was acknowledged by the EFSA [Citation24,Citation66] ().
In order to increase the effects of the chewing of gum on remineralization, calcium has been added to chewing gums either in the form of ionic calcium or casein–calcium conjugates (casein phosphopeptide - amorphous calcium phosphate (CPP-ACP)) (). In situ studies, with demineralized enamel slabs placed in the oral cavity using specific intraoral appliances which were removed after the chewing of gum that was supplemented with calcium phosphates, demonstrated increased remineralization compared to chewing of regular sugar-free gum [Citation67,Citation68]. Unfortunately, in these studies, the intraoral appliances were worn only for approximately 40 min after the chewing of gum or were removed during food intake. Therewith, demineralization is largely left out of consideration [Citation69]. A review on calcium phosphate supplemented chewing gum concluded that these additives to chewing gum did not yield increased caries prevention [Citation70]. In accordance with the latter study, the EFSA does not support an oral health claim on increased remineralization of chewing gum containing calcium phosphates as compared to regular, sugar-free gums [Citation71].
CPP-ACP has been suggested to deposit a calcium and phosphate reservoir on the tooth surface and the surface of oral biofilm, inhibiting enamel demineralization and promoting remineralization [Citation72]. Similar to calcium phosphates, significant, dose-dependent, enamel remineralization and increased resistance against demineralization were reported in situ after use of chewing gum containing CPP-ACP compared to a control gum [Citation73–Citation75]. However, contrary to calcium phosphates, the caries preventive effect of CPP-ACP could be demonstrated in a long-term, 2-year study involving 2720 volunteers, showing that when CPP-ACP gum was chewed 3 times per day, there was 18% less chance of a tooth surface progressing to caries compared to a control gum [Citation76]. Nonetheless, there is no unanimous positive judgment on the remineralization potential of CPP-ACP in chewing gum and while most studies on CPP-ACP were done by the same research group, studies by others did not confirm beneficial effects of chewing CPP-ACP supplemented gums on remineralization [Citation77,Citation78], indicating that the ingredient is promising but large independent randomized controlled trials in vivo are still necessary [Citation79].
Fluoride () hardens the enamel as it is incorporated in the hydroxyapatite lattice network of the crystallites, creating less soluble fluorohydroxyapatite [Citation80]. Its incorporation in a chewing gum was shown in 4-week in situ studies to enhance remineralization of enamel compared to a control gum [Citation81], likely to be more effective on the side of the dentition which is used mostly for chewing [Citation82]. The EFSA considers that the general health claims with respect to the use of fluoride also apply to fluoridated chewing gum [Citation83]. However, the chewing of fluoridated gum did not yield additional benefits when used in combinations with a regular oral hygiene with fluoridated products [Citation84].
2.8. Reduction of oral biofilm formation and impact on biofilm composition
Oral bacteria adhere to SCFs in order to avoid being washed away by salivary flow, and adhesion is governed by the forces by which specific planktonic bacteria are attracted to the SCF [Citation4]. Planktonic initial colonizers adhere directly to the SCF and later colonizers co-adhere with initial colonizers to yield a multispecies biofilm [Citation5]. Disease usually develops when the composition of oral biofilm shifts toward a predominance of specific pathogens. Active ingredients in chewing gums () can affect oral biofilm formation at various stages either by reducing the number of specific planktonic bacteria, preventing their adhesion, or reducing growth of adhering bacteria to yield less biofilm or biofilm with a different microbial composition, ultimately trying to reduce the pathogenic potential of the biofilm. Here, we discuss the effects of the active ingredients at the various stages of biofilm formation.
2.8.1. Effects on planktonic bacteria in saliva
Planktonic bacteria suspended in saliva constitute the source of bacteria for initial adhesion and biofilm formation on oral surfaces. Therefore, the effects of active ingredients in chewing gum on the number of salivary pathogens, such as cariogenic Streptococcus mutans or Streptococcus sobrinus commonly referred to as mutans streptococci, are often used as an indicator of potential oral health benefits. Chewing of regular sugar-free gum had no specific effect on salivary mutans streptococcal concentrations [Citation85,Citation86]. However, some artificial polyol sweeteners, particularly xylitol when used exclusively, were reported to reduce salivary mutans streptococcal numbers [Citation87]. A minimum of 6 g of xylitol per day during 5 weeks was necessary to reach a significant reduction in these mutans streptococcal concentrations [Citation88–Citation90]. Xylitol should be preferred over other sweeteners such as sorbitol which have been reported to be low cariogenic, while sorbitol should be preferred over conventional sugars [Citation91]. The mechanism of reduction has not been unraveled entirely, but xylitol, together with erythritol, have been suggested to interfere with the adhesion of oral streptococci [Citation92].
Other ingredients incorporated in chewing gum such as chlorhexidine, chitosan, magnolia bark extract, mastic, and propolis were also shown to lower the concentration of planktonic bacteria in saliva compared to a control gum [Citation86,Citation93–Citation97].
2.8.2. Effects on bacterial adhesion to oral surfaces
Adhesion of planktonic bacteria to oral surfaces is the first step in the formation of oral biofilm and is mediated by attractive forces between oral surfaces and adhering bacteria. Accordingly, the properties of the oral surfaces play a major role in the development of these adhesion forces and changing the forces may impact the amount and composition of oral biofilm formed [Citation4,Citation98]. Chewing a gum containing polyphosphates made SCFs more hydrophilic and more negatively charged as compared with other gums. Since most oral bacterial strains are negatively charged [Citation44,Citation46], this implies weaker adhesion of oral bacteria and polyphosphates have been demonstrated to even promote detachment of bacteria from SCFs on enamel surfaces [Citation99].
2.8.3. Effects on biofilm formation, composition, and removal
Chewing of regular sugar-free gum dislodges loosely bound bacteria from the oral mucosa [Citation100] and inhibits regrowth and maturation of oral biofilm on occlusal surfaces [Citation101] (). Nonetheless, there is no unanimous judgment on a chewing gum-induced reduction of biofilm regrowth on smooth lingual and buccal surfaces and a relation between biofilm removal directly after a single gum chew has not been firmly established [Citation102–Citation104], not even when abrasive agents were included in the gum [Citation105]. Therefore, the EFSA has concluded that the claim that the chewing of regular sugar-free gum ‘reduces oral biofilm formation’ is unsubstantiated [Citation102], although this conclusion does not rule out the possibility that the chewing of gum can modify oral biofilm composition toward a less cariogenic one.
Chlorhexidine is the most effective antimicrobial for the chemical control of oral biofilm [Citation106]. Its antimicrobial properties are based on disturbing the bacterial cell membrane and its binding to intraoral surfaces ensures substantive action [Citation106]. Chlorhexidine tastes bitter, alters long-term taste perception, and causes (reversible) tooth stain [Citation106]. Antimicrobially effective chewing gums containing chlorhexidine with acceptable taste can be made [Citation107], but consumer hesitance remains and in certain countries, chewing gums containing chlorhexidine are only available on prescription [Citation56,Citation108]. Application of chlorhexidine in chewing gum not only reduces planktonic levels of mutans streptococci directly after chewing [Citation93], but also reduces oral biofilm formation. Incorporation of chlorhexidine in chewing gum inhibited oral biofilm growth in a 4-day study when only two pieces of gum were chewed per day in absence of other oral hygiene measures [Citation108]. When used for 1 year, chewing gum containing chlorhexidine showed a stronger reduction in gingival index and amount of oral biofilm formed than a chewing gum containing xylitol [Citation109,Citation110], but concerns remain about long-term consumption of potent antimicrobial agents such as chlorhexidine.
Similar to chlorhexidine, xylitol also resulted in reduction of salivary mutans streptococcal concentrations when used for 5 weeks, but this was too short to result in a change in composition of oral biofilm [Citation87,Citation111]. Also, in combination with regular brushing, no effects of chewing gum containing xylitol on biofilm and gingivitis scores were observed compared to chewing gum base only [Citation112]. However, 6 months chewing of a gum containing xylitol caused a decrease in the acidogenicity of oral biofilm [Citation57], indicative of a change in biofilm composition. These clinical studies, particularly among children, showed that chewing gum containing xylitol reduced caries rates more effectively than sorbitol sweetened gum, even after the chewing regime was discontinued [Citation113–Citation115]. In general, oral health-care benefits of xylitol are broadly documented and the EFSA supports the claim that consumption of 2–3 g of chewing gum containing xylitol, 3 times per day after meals, reduces the risk of caries in children [Citation89,Citation116]. Yet, the debate still continues in literature on whether the effects of chewing gum containing xylitol are solely due to increased salivation or to the addition of xylitol [Citation85,Citation117–Citation119]. Also erythritol, though not clinically evaluated in chewing gum, has shown promising results in caries prevention when used in candies, in which it performed better than xylitol [Citation120].
Of the other ingredients mentioned above to lower planktonic levels of bacteria, only chewing gum containing mastic and eucalyptus were hinted to successfully reduce oral biofilm formation better than a control gum under the artificial condition of refraining from other oral hygiene measures [Citation121–Citation123]. In general, the research on the use of natural products in oral health-care products, such as in chewing gum, is relatively new and gaining more interest. Besides the natural ingredients mentioned above, other natural products such as coffee, cacao, and extracts from fruit or tea are currently being investigated for their potential to prevent caries [Citation124].
3. Disadvantages of chewing gum
The exclusion of conventional sugars as an additive to chewing gum has removed a major disadvantage of the chewing of gum [Citation91]. However, there is also debate surrounding possible general health effects of artificial (high intensity) sweeteners [Citation125], despite evidence that they are safe for human consumption [Citation126]. Also, not related to any gum ingredient, excessive chewing of gum for more than 3 h/day has been suggested to contribute to symptoms of temporomandibular disorder [Citation127]; however, a clear causal relationship has not been demonstrated [Citation128].
4. Conclusions
Over the last decades, chewing gum developed from a candy toward an oral health promoting nutraceutical to be used as an adjunct to regular oral hygiene. The basic beneficial effects of the chewing of gum on oral health have been well documented and are officially approved by the EFSA (see and ). The same cannot be said about the delivery of oral therapeutics that are incorporated in chewing gums to enhance the oral health benefits perceived, mainly because most effects aimed for by chewing gum additives are overshadowed by effects of increased salivation and mastication, as readily achieved by the chewing of regular, sugar-free gum without oral therapeutics added. Due to the relatively small effects of active ingredients and the difficulty of translating short-term outcomes to long-term effects, it is necessary to chew gum for prolonged periods of time in order to perceive possible benefits. Therefore, official approval of the EFSA for active ingredients on relevant outcome parameters, such as caries prevention, requires large and long-term clinical studies with proper control gums, as was done for xylitol.
5. Expert opinion
The main hurdle in demonstrating oral health-care benefits of active ingredients added to chewing gums is their necessarily low potency and rapid washout from the oral cavity, combined with the health benefits achieved by the chewing of regular, sugar-free gum that may easily overshadow the additional benefits of added active ingredients unless used for prolonged periods of time. Potent antimicrobial additives are undesirable to add to chewing gums as unlike to mouthrinse ingredients that are spit out; chewing gum additives are mostly swallowed and enter the gastrointestinal tract.
This implies that when evaluated in vitro, chewing gum with active ingredients added will, like many nutraceuticals, always do significantly less well than more potent ‘positive controls,’ such as mouthrinses containing chlorhexidine. The in vitro comparison of nutraceuticals, including chewing gum additives, with more potent therapeutic drugs as a positive control, however, is not a valid one, as nutraceuticals are seldom or never used therapeutically but only as a regular component of peoples lifestyle to act prophylactically against disease. Therefore, proper benefit assessment of active ingredients in chewing gum can only be done in clinical studies, using proper control gums, preferentially lasting more than 1 year, as exemplified by large-scale studies done on xylitol [Citation63,Citation76,Citation115] which adds a major cost factor to the translation of new additives to the market. Nevertheless, with respect to the clinical demonstration of oral health-care benefits, oral health is influenced by many other factors that are hard to control over prolonged periods of time. Therefore, observed effects, also in long-term studies, will always be marginal.
Currently, antimicrobial ingredients in oral health-care products target the entire oral microbiome. The generally low efficacy of antimicrobials added to chewing gum, although disappointing at a first glance, may be advantageous when more selective antimicrobials targeted to specific oral pathogens are applied to gradually change the composition of oral biofilm into a less pathogenic one [Citation129]. Gradual is the preferred way of changing a microbiome in order to make changes lasting [Citation130] and to maintain a symbiotic relation of the microbiome with the host [Citation129]. One suggested approach is based on repeated administration of low-level dosage of antibacterial substances, such as triclosan, which showed inhibition of bacteria associated with disease without affecting bacteria implicated in health [Citation129]. Also, inclusion of additives that increase the surface hydrophobicity of specific pathogens may yield more selective entrapment of bacteria within a piece of gum, than hitherto demonstrated [Citation131], similar to the effects of a toothpaste containing triclosan in combination with a mouthrinse based on essential oils [Citation132]. As a final option to advance chewing gum further to a nutraceutical that drives the oral microflora into a healthy direction, probiotics such as lactobacilli can be added [Citation133] to compete for a position on oral surfaces with oral pathogens, similar to the events occurring in the gastrointestinal tract between probiotics and other members of the gastrointestinal microbiome [Citation134,Citation135].
In summary, whereas evidence for oral health-care benefits of chewing gum additives is hard to obtain vis-à-vis additives in advanced toothpaste formulations or mouthrinses, the basic benefits of the long-term chewing of sugar-free gum due to increased mastication and salivation are beyond dispute. Given the fact that the chewing of gum nonspecifically removes bacteria from the oral cavity by entrapment in gum with only temporal effects, it seems feasible to construct gum formulations that entrap more specific oral pathogens. Therewith, long-term use of such gums may aid to restore and maintain a healthier oral microbiome, further contributing to the recognition of chewing gum as a nutraceutical.
Article highlights
Sugar-free chewing gum can be a valuable addendum to oral hygiene. However, it is not a replacement for traditional oral hygiene procedures, such as toothbrushing, toothpicks, floss wire and mouthrinses.
Oral health effects of chewing sugar-free gum center around the stimulation of salivary flow.
Effects of chewing sugar-free gum, such as reduction of oral dryness, neutralization of biofilm pH, remineralization of enamel and reduced caries incidence are recognized by the European Food Safety Authority.
Several active ingredients have been incorporated in chewing gum to enhance the effects of sugar-free gum, but the clinical efficacy of these effects are difficult to prove and require long term studies as potential benefits are easily overshadowed by effects of salivary flow stimulation.
A potential future strategy for chewing gum additives is the targeted removal of pathogenic bacteria to gradually change the composition of the oral biofilm, whilst leaving bacteria associated with health unaffected.
This box summarizes key points contained in the article
Declaration of interest
This work was funded by Wm. Wrigley Jr. Co, Chicago, USA and SASA BV, Thesinge, NL. Authors were employed by their own organizations. HJ Busscher is also director-owner of a consulting company SASA BV. A Maitra and MWJ Dodds are employees of the Wm. Wrigley Jr. Company. Opinions and assertions contained herein are those of the authors and are not meant to be construed as necessarily representing views of the organizations to which the authors are affiliated. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Kreth J, Merritt J, Qi F. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009;28:397–403.
- Cheaib Z, Rakmathulina E, Lussi A, et al. Impact of acquired pellicle modification on adhesion of early colonizers. Caries Res. 2015;49:626–632.
- Wade WG, Slayne MA. Controlling plaque by disrupting the process of plaque formation. Periodontol 2000. 1997;15:25–31.
- Wessel SW, Chen Y, Maitra A, et al. Adhesion forces and composition of planktonic and adhering oral microbiomes. J Dent Res. 2013;93:84–88.
- Kolenbrander PE, Palmer RJ, Periasamy S, et al. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–480.
- Jakubovics NS, Kolenbrander PE. The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 2010;16:729–739.
- Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38:182–191.
- Serrano J, Escribano M, Roldán S, et al. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis. A systematic review and meta-analysis. J Clin Periodontol. 2014;42:S106–S138.
- Crocombe L, Brennan D, Slade G, et al. Is self interdental cleaning associated with dental plaque levels, dental calculus, gingivitis and periodontal disease? J Periodontal Res. 2012;47:188–197.
- Imfeld T. Chewing gum - facts and fiction: a review of gum-chewing and oral health. Crit Rev Oral Biol Med. 1999;10:405–419.
- Redclift M. Chewing gum - The fortunes of taste. London: Routledge; 2004.
- Yankell S, Emling R. Efficacy of chewing gum in preventing extrinsic tooth staining. J Clin Dent. 1997;8:169–172.
- Rösing C, Gomes S, Bassani D, et al. Effect of chewing gums on the production of volatile sulfur compounds (VSC) in vivo. Acta Odontol Latinoam. 2009;22:11–14.
- Chaudhary SA, Shahiwala AF. Medicated chewing gum - a potential drug delivery system. Expert Opin Drug Deliv. 2010;7:871–885.
- Fritz D. Formulation and production of chewing and bubble gum. Cambridge: Woodhead Publishing Ltd.; 2006.
- Aslani A, Rostami F, Ghannadi A. Medicated chewing gum, a novel drug delivery system. J Res Med Sci. 2015;20:403–411.
- Variety of gum sold. International chewing gum association, 2011 [Internet]; 2011. Available from: http://www.gumassociation.org/default/index.cfm/about-icga/our-mission/
- Hyrup B, Andersen C, Andreasen LV, et al. The MediChew technology platform. Expert Opin Drug Deliv. 2005;2:927–933.
- Maguire A, Rugg-Gunn A. Xylitol and caries prevention - is it a magic bullet? Br Dent J. 2003;194:429–436.
- Edgar M, Dawes C, O’Mullane D. Saliva and oral health. 4th ed. Chapter 1, 3 and 5 ed. London: BDJ Books; 2004.
- Bots CP, Brand HS, Veerman ECI, et al. The management of xerostomia in patients on haemodialysis: comparison of artificial saliva and chewing gum. Palliat Med. 2005;19:202–207.
- Furness S, Worthington H. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;12:1–106.
- Dawes C, Macpherson LM. Effects of nine different chewing-gums and lozenges on salivary flow rate and pH. Caries Res. 1992;26:176–182.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to sugar-free chewing gum and dental and oral health, including gum and tooth protection and strength (ID 1149), plaque acid neutralisation (ID 1150), maintenance of tooth mineralisation (ID 1151), reduction of oral dryness (ID 1240), and maintenance of normal body weight (ID 1152) pursuant to Article 13 (1)of Regulation (EC) No 1924/2006. Efsa J. 2009;7:1271.
- Kakodkar P, Mulay S. Effect of sugar-free gum in addition to tooth brushing on dental plaque and interdental debris. Dent Res J (Isfahan). 2011;7:64–69.
- Fu Y, Li X, Ma H, et al. Assessment of chewing sugar-free gums for oral debris reduction: a randomized controlled crossover clinical trial. Am J Dent. 2012;25:118–122.
- Jensen M. Responses of interproximal plaque pH to snack foods and effect of chewing sorbitol-containing gum. J Am Dent Assoc. 1986;113:262–266.
- Sbordone L, Bortolaia C. Oral microbial biofilms and plaque-related diseases: microbial communities and their role in the shift from oral health to disease. Clin Oral Investig. 2003;7:181–188.
- Leme A, Koo H, Bellato C. The role of sucrose in cariogenic dental biofilm formation - new insight. J Dent Res. 2006;85:878–887.
- Nancollas G, Johnsson M. Calculus formation and inhibition. Adv Dent Res. 1994;8:307–311.
- White DJ. Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur J Oral Sci. 1997;105:508–522.
- Fure S, Lingström P, Birkhed D. Effect of three months’ frequent use of sugar-free chewing gum with and without urea on calculus formation. J Dent Res. 1998;77:1630–1637.
- Lingström P, Fure S, Dinitzen B, et al. The release of vitamin C from chewing gum and its effects on supragingival calculus formation. Eur J Oral Sci. 2005;113:20–27.
- Porciani P, Grandini S. A six-week study to evaluate the anti-calculus efficacy of a chewing gum containing pyrophosphate and tripolyphosphate. J Clin Dent. 2003;14:11–13.
- White D, Cox E, Suszcynskymeister E, et al. In vitro studies of the anticalculus efficacy of a sodium hexametaphosphate whitening dentifrice. J Clin Dent. 2002;13:33–37.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to sugar-free chewing gum with pyro- and triphosphates and reduction of calculus formation (ID 1309) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. Efsa J. 2011;9:2268.
- Ozcan M, Kulak Y, Kazazoglu E. The efficacy of two prototype chewing gums for the removal of extrinsic tooth stain. Int Dent J. 2003;53:62–66.
- Milleman JL, Milleman KR, Kleber CJ, et al. Crossover clinical investigation of a whitening chewing gum for inhibiting dental stain formation in conjuction with tooth brushing. J Clin Dent. 2014;25:37–42.
- Walters P. Benefits of sodium hexametaphosphate-containing chewing gum for extrinsic stain inhibition. J Dent Hyg. 2004;78:1–9.
- Bartizek R, Walters P, Biesbrock A. The prevention of induced stain using two levels of sodium hexametaphosphate in chewing gum. J Clin Dent. 2002;14:77–81.
- Biesbrock A, Walters P, Bartizek R. A chewing gum containing 7.5% sodium hexametaphosphate inhibits stain deposition compared with a placebo chewing gum. Compend Contin Educ Dent. 2004;25:253–254.
- Porciani P, Grandini S. Whitening effect by stain inhibition from a chewing gum with sodium hexametaphosphate in a controlled twelve-week single-blind trial. J Clin Dent. 2006;17:14–16.
- Porciani P, Perra C, Grandini S. Effect on dental stain occurrence by chewing gum containing sodium tripolyphosphate-a double-blind six-week trial. J Clin Dent. 2010;21:4–7.
- van der Mei HC, Kamminga-Rasker HJ, De Vries J, et al. The influence of a hexametaphosphate-containing chewing gum on the wetting ability of salivary conditioning films in vitro and in vivo. J Clin Dent. 2003;14:14–18.
- Veeregowda DH, Busscher HJ, Vissink A, et al. Role of structure and glycosylation of adsorbed protein films in biolubrication. PLoS One. 2012;7:1–8.
- Busscher HJ, White DJ, Kamminga-Rasker HJ, et al. Influence of oral detergents and chlorhexidine on soft-layer electrokinetic parameters of the acquired enamel pellicle. Caries Res. 2003;37:431–436.
- Loesche WJ, Kazor C. Microbiology and treatment of halitosis. Periodontol 2000. 2002;28:256–279.
- Blom T, Slot D, Quirynen M, et al. The effect of mouthrinses on oral malodor: a systematic review. Int J Dent Hyg. 2012;10:209–222.
- Tian M, Hanley A, Dodds M, et al. Chewing gum containing allyl isothiocyanate from mustard seed extract is effective in reducing volatile sulfur compounds responsible for oral malodor. Am J Dent. 2013;26:180–184.
- Wåler SM. The effect of zinc-containing chewing gum on volatile sulfur-containing compounds in the oral cavity. Acta Odontol Scand. 1997;55:198–200.
- Greenberg M, Urnezis P, Tian M. Compressed mints and chewing gum containing magnolia bark extract are effective against bacteria responsible for oral malodor. J Agric Food Chem. 2007;55:9465–9469.
- Tanaka M, Toe M, Nagata H, et al. Effect of eucalyptus-extract chewing gum on oral malodor: a double-masked, randomized trial. J Periodontol. 2010;81:1564–1571.
- Porciani P, Grandini S. The effect of zinc acetate and magnolia bark extract added to chewing gum on volatile sulfur-containing compounds in the oral cavity. J Clin Dent. 2012;23:76–79.
- Polland KE, Higgins F, Orchardson R. Salivary flow rate and pH during prolonged gum chewing in humans. J Oral Rehabil. 2003;30:861–865.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to sugar free chewing gum and neutralisation of plaque acids which reduces the risk of dental caries pursuant to Article 14 of Regulation (EC) No 1924/2006. Efsa J. 2010;8:1776.
- Dodds M. The oral health benefits of chewing gum. J Ir Dent Assoc. 2012;58:253–261.
- Campus G, Cagetti MG, Sacco G, et al. Six months of daily high-dose xylitol in high-risk schoolchildren: a randomized clinical trial on plaque pH and salivary mutans streptococci. Caries Res. 2009;43:455–461.
- Anderson L, Orchardson R. The effect of chewing bicarbonate-containing gum on salivary flow rate and pH in humans. Arch Oral Biol. 2003;48:201–204.
- Igarashi K, Lee IK, Schachtele CF. Effect of chewing gum containing sodium bicarbonate on human interproximal plaque pH. J Dent Res. 1988;67:531–535.
- Imfeld T, Birkhed D, Lingström P. Effect of urea in sugar-free chewing gums on pH recovery in human dental plaque evaluated with three different methods. Caries Res. 1995;29:172–180.
- Smith CA, Higham SM, Smith PW, et al. The effect of chewing urea-containing gum on plaque acidogenic and alkaligenic parameters. Caries Res. 2004;38:124–129.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to sugar-free chewing gum with carbamide and plaque acid neutralisation (ID 1153) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. Efsa J. 2011;9:2071.
- Machiulskiene V, Nyvad B, Baelum V. Caries preventive effect of sugar-substituted chewing gum. Community Dent Oral Epidemiol. 2001;29:278–288.
- Szoke J, Banoczy J, Proskin HM. Effect of after-meal sucrose-free gum-chewing on clinical caries. J Dent Res. 2001;80:1725–1729.
- Dong Y, Yin W, Hu D, et al. Remineralization of early caries by chewing sugar-free gum: a clinical study using quantitative light-induced fluorescence. Am J Dent. 2014;27:291–295.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to sugar free chewing gum and reduction of tooth demineralisation which reduces the risk of dental caries pursuant to Article 14 of Regulation (EC) No 1924/2006. Efsa J. 2010;8:1775.
- Suda R, Suzuki T, Takiguchi R, et al. The effect of adding calcium lactate to xylitol chewing gum on remineralization of enamel lesions. Caries Res. 2006;40:43–46.
- Kitasako Y, Tanaka M, Sadr A, et al. Effects of a chewing gum containing phosphoryl oligosaccharides of calcium (POs-Ca) and fluoride on remineralization and crystallization of enamel subsurface lesions in situ. J Dent. 2011;39:771–779.
- Dodds MWJ, Chidichimo D, Haas MS. Delivery of active agents from chewing gum for improved remineralization. Adv Dent Res. 2012;24:58–62.
- Lingström P, Holm A, Mejàre I, et al. Dietary factors in the prevention of dental caries: a systematic review. Acta Odontol Scand. 2003;61:331–340.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to sugar-free chewing gum with calcium phosphoryl oligosaccharides and maintenance of tooth mineralisation (ID 337) pursuant to Article 13 (1)of Regulation (EC) No 1924/2006. Efsa J. 2011;9:2267.
- Gurunathan D, Somasundaram S, Kumar S. Casein phosphopeptide-amorphous calcium phosphate: a remineralizing agent of enamel. Aust Dent J. 2012;57:404–408.
- Shen P, Cai F, Nowicki A, et al. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–2070.
- Cai F, Manton DJ, Shen P, et al. Effect of addition of citric acid and casein phosphopeptide-amorphous calcium phosphate to a sugar-free chewing gum on enamel remineralization in situ. Caries Res. 2007;41:377–383.
- Prestes L, Souza BM, Comar LP, et al. In situ effect of chewing gum containing CPP-ACP on the mineral precipitation of eroded bovine enamel-a surface hardness analysis. J Dent. 2013;41:747–751.
- Morgan MV, Adams GG, Bailey DL, et al. The anticariogenic effect of sugar-free gum containing CPP-ACP nanocomplexes on approximal caries determined using digital bitewing radiography. Caries Res. 2008;42:171–184.
- Schirrmeister JF, Seger RK, Altenburger MJ, et al. Effects of various forms of calcium added to chewing gum on initial enamel carious lesions in situ. Caries Res. 2007;41:108–114.
- Zero DT. Recaldent - evidence for clinical activity. Adv Dent Res. 2009;21:30–34.
- Yengopal V, Mickenautsch S. Caries preventive effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP): a meta-analysis. Acta Odontol Scand. 2009;67:321–332.
- Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol. 1999;27:31–40.
- Suyama E, Tamura T, Ozawa T, et al. Remineralization and acid resistance of enamel lesions after chewing gum containing fluoride extracted from green tea. Aust Dent J. 2011;56:394–400.
- Sjögren K, Ruben J, Lingström P, et al. Fluoride and urea chewing gums in an intra-oral experimental caries model. Caries Res. 2002;36:64–69.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to sugar-free chewing gum with fluoride and maintenance of tooth mineralisation (ID 1154) pursuant to Article 13 (1)of Regulation (EC) No 1924/2006. Efsa J. 2011;9:2072.
- Tubert-Jeannin S, Auclair C, Amsallem E, et al. Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing dental caries in children. Cochrane Database Syst Rev. 2011;12:1–80.
- Van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 2004;38:286–293.
- Campus G, Cagetti MG, Cocco F, et al. Effect of a sugar-free chewing gum containing magnolia bark extract on different variables related to caries and gingivitis: a randomized controlled intervention trial. Caries Res. 2011;45:393–399.
- Söderling E, Elsalhy M, Honkala E, et al. Effects of short-term xylitol gum chewing on the oral microbiome. Clin Oral Investig. 2014;19:237–244.
- Milgrom P, Ly KA, Roberts MC, et al. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006;85:177–181.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific substantiation of a health claim related to xylitol chewing gum/pastilles and reduction of the risk of tooth decay pursuant to Article 14 of Regulation (EC) No 1924/2006. Efsa J. 2008;852:1–16.
- Milgrom P, Ly K, Rothen M. Xylitol and its vehicles for public health needs. Adv Dent Res. 2009;21:44–47.
- Burt B. The use of sorbitol-and xylitol-sweetened chewing gum in caries control. J Am Dent Assoc. 2006;127:190–196.
- Söderling EM, Hietala-Lenkkeri AM. Xylitol and erythritol decrease adherence of polysaccharide-producing oral streptococci. Curr Microbiol. 2010;60:25–29.
- Marwaha M, Bhat M. Antimicrobial effectiveness of chlorhexidine chewing gums on Streptococcus mutans counts – an in vivo microbiological study. J Clin Pediatr Dent. 2010;35:31–35.
- Hayashi Y, Ohara N, Ganno T, et al. Chitosan-containing gum chewing accelerates antibacterial effect with an increase in salivary secretion. J Dent. 2007;35:871–874.
- Aksoy A, Duran N, Koksal F. In vitro and in vivo antimicrobial effects of mastic chewing gum against Streptococcus mutans and mutans streptococci. Arch Oral Biol. 2006;51:476–481.
- Hayashi Y, Ohara N, Ganno T, et al. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch Oral Biol. 2007;52:290–294.
- Tulsani SG, Chikkanarasaiah N, Siddaiah SB, et al. The effect of propolis and xylitol chewing gums on salivary Streptococcus mutans count: a clinical trial. Indian J Dent Res. 2014;25:737–741.
- Tang G, Yip HK, Samaranayake LP, et al. Direct detection of cell surface interactive forces of sessile, fimbriated and non-fimbriated Actinomyces spp. using atomic force microscopy. Arch Oral Biol. 2004;49:727–738.
- van der Mei HC, White DJ, Cox E, et al. Bacterial detachment from salivary conditioning films by dentifrice supernates. J Clin Dent. 2002;13:44–49.
- Dawes C, Tsang RW, Suelzle T. The effects of gum chewing, four oral hygiene procedures, and two saliva collection techniques, on the output of bacteria into human whole saliva. Arch Oral Biol. 2001;46:625–632.
- Hanham A, Addy M. The effect of chewing sugar-free gum on plaque regrowth at smooth and occlusal surfaces. J Clin Periodontol. 2001;28:255–257.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to sugar free chewing gum and reduction of dental plaque (ID 3084) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. Efsa J. 2010;8:1480.
- Mouton C, Scheinin A, Mäkinen K. Effect on plaque of a xylitol-containing chewing-gum: a clinical and biochemical study. Acta Odontol Scand. 1975;33:33–40.
- Barnes VM, Santarpia P, Richter R, et al. Clinical evaluation of the anti-plaque effect of a commercial chewing gum. J Clin Dent. 2005;16:1–5.
- Pizzo G, Licata ME, La Cara M, et al. The effects of sugar-free chewing gums on dental plaque regrowth: a comparative study. J Dent. 2007;35:503–508.
- Imfeld T. Chlorhexidine-containing chewing gum. Schweiz Monatsschr Zahnmed. 2006;116:476–483.
- Kolahi J, Soolari A. Formulated chlorhexidine gluconate chewing gum that gives both anti-plaque effectiveness and an acceptable taste: a double blind, randomized, placebo-controlled. J Int Acad Periodontol. 2008;10:38–44.
- Ainamo J, Nieminen A, Westerlund U. Optimal dosage of chlorhexidine acetate in chewing gum. J Clin Periodontol. 1990;17:729–733.
- Simons D, Beighton D, Kidd EA, et al. The effect of xylitol and chlorhexidine acetate/xylitol chewing gums on plaque accumulation and gingival inflammation. J Clin Periodontol. 1999;26:388–391.
- Simons D, Brailsford S, Kidd EA, et al. The effect of chlorhexidine acetate/xylitol chewing gum on the plaque and gingival indices of elderly occupants in residential homes. J Clin Periodontol. 2001;28:1010–1015.
- Söderling E, Hirvonen A, Karjalainen S, et al. The effect of xylitol on the composition of the oral flora: a pilot study. Eur J Dent. 2011;5:24–31.
- Keukenmeester R, Slot D, Rosema N, et al. Effects of sugar-free chewing gum sweetened with xylitol or maltitol on the development of gingivitis and plaque: a randomized clinical trial. Int J Dent Hyg. 2014;12:238–244.
- Isokangas P, Tiekso J, Alanen P, et al. Long-term effect of xylitol chewing gum on dental caries. Comm Dent Oral Epidemiol. 1989;17:200–203.
- Hujoel PP, Makinen KK, Bennett CA, et al. The optimum time to initiate habitual xylitol gum-chewing for obtaining long-term caries prevention. J Dent Res. 1999;78:797–803.
- Makinen KK, Bennett CA, Hujoel PP, et al. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995;74:1904–1913.
- Sadler MJ. Food, nutrients and food ingredients with authorised EU health claims. Vol. 1, Chapter 3. Cambridge: Woodhead Publishing Ltd.; 2014.
- Hayes C. Xylitol gum decreases the decayed, missing, and filled surfaces (DMFS) score over a 3-year period by an average of 1.9. J Evid Based Dent Pract. 2002;2:14–15.
- Zero D. Are sugar substitutes also anticariogenic? J Am Dent Assoc. 2008;139:9S–10S.
- Fontana M, González-Cabezas C. Are we ready for definitive clinical guidelines on xylitol/polyol use? Adv Dent Res. 2012;24:123–128.
- Honkala S, Runnel R, Saag M, et al. Effect of erythritol and xylitol on dental caries prevention in children. Caries Res. 2014;48:482–490.
- Sato S, Yoshinuma N, Ito K. The inhibitory effect of funoran and eucalyptus extract-containing chewing gum on plaque formation. J Oral Sci. 1998;40:115–117.
- Takahashi K, Fukazawa M, Motohira H, et al. A pilot study on antiplaque effects of mastic chewing gum in the oral cavity. J Periodontol. 2003;74:501–505.
- Nagata H, Inagaki Y, Tanaka M, et al. Effect of eucalyptus extract chewing gum on periodontal health: a double-masked, randomized trial. J Periodontol. 2008;79:1378–1385.
- Cheng L, Li J, He L, et al. Natural products and caries prevention. Caries Res. 2015;49:38–45.
- Olivier B, Serge AH, Catherine A, et al. Review of the nutritional benefits and risks related to intense sweeteners. Arch Public Health. 2015;73:41.
- EFSA completes full risk assessment on aspartame and concludes it is safe at current levels of exposure. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA); 2013. Available from: http://www.efsa.europa.eu/en/press/news/131210
- Winocur E, Littner D, Adams I, et al. Oral habits and their association with signs and symptoms of temporomandibular disorders in adolescents: a gender comparison. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:482–487.
- Durham J, Newton-John TR, Zakrzewska JM. Temporomandibular disorders. The BMJ. 2015;350:h1154.
- Marsh PD, Head DA, Devine DA. Ecological approaches to oral biofilms: control without killing. Caries Res. 2015;49:46–54.
- Zarco MF, Vess TJ, Ginsburg GS. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109–120.
- Wessel SW, van der Mei HC, Morando D, et al. Quantification and qualification of bacteria trapped in chewed gum. PLoS One. 2015;10:1–12.
- Jongsma MA, van der Mei HC, Atema-Smit J, et al. In vivo biofilm formation on stainless steel bonded retainers during different oral health-care regimens. Int J Oral Sci. 2015;6:1–7.
- Caglar E, Kavaloglu SC, Kuscu OO, et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Investig. 2007;11:425–429.
- Reid G, Younes JA, van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38.
- Twetman S. Are we ready for caries prevention through bacteriotherapy? Braz Oral Res. 2012;26(Suppl 1):64–70.

