?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
Advanced technologies have made a significant impact on the development of drug delivery systems over the last few years and are remodeling the future of therapeutic care of patients. In particular, microparticles made from biocompatible polymers for pharmaceutical applications, where bioactive agents are continuously released from the particle as a function of time has been extensively studied. The electrohydrodynamic atomization technique, commonly known as electrospraying, has several key advantages compared to other techniques for making particles in the microscale such as improvement of dissolution rate of poorly water-soluble drugs, batch-scalability, reproducibility, effective encapsulation and simple setup configurations in microparticle production. Drug release characteristics are enhanced by using biodegradable polymer carriers, which sustain the release of encapsulated drugs. Moreover, multi-pharmacy or polypharmacy can be achieved by loading different drugs into multi-layered particles via electrospraying, and the controlled release of these ingredients has been made possible. Modern-day electrospraying can involve very advanced technological features such as simultaneous multi-flow electrohydrodynamics to deliver polypharmacy. In this invited editorial, we combine key reports on electrosprayed microparticles and their corresponding therapeutic applications. However, advancing this technology and particle characteristics to reliable dosage mass production and manufacturing for patient healthcare still requires significant research and development.
Several techniques have been developed for the encapsulation of therapeutic agents such as emulsion solvent evaporation/extraction, spray drying, electrospraying, coacervation, and microfluidics. Microparticles produced by these techniques have been extensively investigated for pharmaceutical applications, especially drug delivery. It is crucial to develop microparticles with better-controlled features including size, shape, surface properties, and component materials, which enables enhanced delivery of therapeutics [Citation1].
The most impressive and largely studied method is electrohydrodynamic atomization, also known as electrospraying. Electrospinning and electrohydrodynamic printing belong to the same family of process, and in general, electrospraying occurs with solutions have lower viscosities. The phenomenon of electrohydrodynamic atomization was described in 1600 when William Gilbert reported that a jet of liquid could be emitted from a droplet via an electrostatically charged chamber [Citation2]. It is used for producing microparticles for pharmaceutical applications due to several key advantages compared to other techniques such as the capability of spraying a wide range of materials, flexibility and versatility with various simple setup configurations for different applications, generation of smaller particles with better size distribution and much less agglomeration, and improved dissolution of poorly water-soluble drugs. Therefore, electrospraying is one of the most fascinating tools to be employed in the pharmaceutical field.
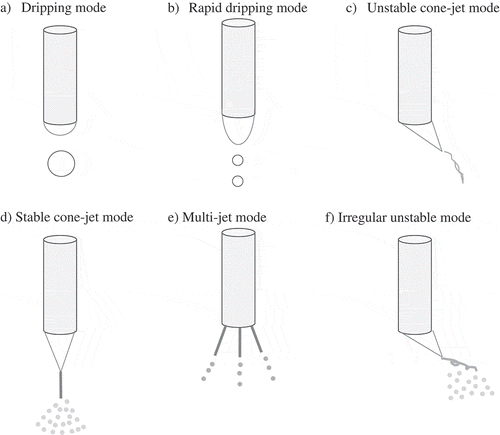
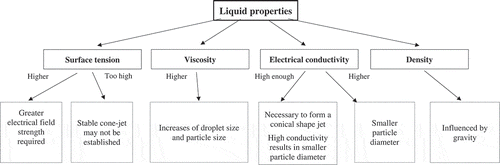
The basic setup for electrospraying consists of several components: a syringe pump, a metal nozzle connected to a high voltage power source, a grounded substrate as a collector and a monitor. In order to control better the process of particle forming and facilitate the generation of smaller particles with smoother surface morphology, the electrospraying setup should be isolated in a covered chamber [Citation3]. Basically, a stream of conductive liquid is pumped into a nozzle to which the high voltage (kV range) is applied during electrospraying to form microparticles. The principle of electrospraying is based upon the theory of charged droplets and this static charge causes an electrostatic force in the droplets [Citation4]. Only fragments of the solution are jetted at a low voltage, creating various modes such as dripping, rapid dripping, and unstable cone-jet mode. A sustained and a continuous jet can be obtained from the balance of several forces including surface tension, gravity and electric strengths on the liquid surface; these are stable cone-jet, multi-jet and irregular unstable jet modes (). The electrospray process is also influenced by the liquid properties including surface tension, viscosity, electrical conductivity and density as illustrated in .
2. Pharmaceutical applications
Water solubility ratio of the drug is a crucial factor for drug effectiveness, especially for the oral route. The electrospraying process improves the dissolution of poorly water-soluble drugs. In literature data, the Edirisinghe Laboratory (www.edirisinghelab.com) demonstrated that microparticles containing poorly water-soluble drugs, biopharmaceutical classification system class II drug, such as progesterone [Citation3], celecoxib [Citation5], cisplatin [Citation6,Citation7] can be successfully produced by electrospraying. More importantly, different release profiles can be obtained due to the specialties and ratios of different polymers or polymer composites for controlling the release profile of drugs. For instance, progesterone-loaded poly(lactic-co-glycolic) acid (PLGA) particles were prepared in different copolymer ratios by electrospraying and the results indicated that a decrease in poly(lactic) acid:poly(glycolic) acid (PLA:PGA) ratio from 75:25 to 50:50 accelerated the release of progesterone [Citation3].
Electrospraying has also been successfully used for targeted therapeutic delivery systems by encapsulation of drugs into a suitable carrier. Through this method, not only the characteristics of drug release is predictable and can be controlled in a sustained, pulsed or prolonged manner, but also, the drug can be released at the diseased site to provide high and exclusive accumulation at the specific location. Moreover, this system can protect the drug from degradation and loss of bioactivity compared to other conventional dosage forms. Researchers have designed a smart multifunctional microcapsule of paclitaxel with titanium dioxide (TiO2) shell using the co-axial electrospray [Citation8]. Tetrabutyl titanium and Poly(vinylpyrrolidone) (PVP) were blended into a mixed solution of ethanol, DMF and acetic acid used for outer liquid. Paclitaxel, modified Fe3O4, and graphene quantum dots were dispersed in olive oil and used as the inner solution. The initial burst release of paclitaxel was suppressed by the TiO2 shell. The Fe3O4 inside the core shell functioned successfully for magnetic targeting. Moreover, ultrasound was employed to stimulate the release of paclitaxel, and the release behavior could be controlled by the length of repeatable ultrasound [Citation8].
Recently, successful processing and entrapment of therapeutic agents into a biodegradable polymer matrix for sustained release applications using electrospraying have been demonstrated in several studies. PLGA, poly(caprolactone) (PCL), PLA, and their derivatives are the most frequently used biodegradable polymers approved by The Food and Drug Administration. As shown in , there are many examples of drugs that were entrapped into these polymers by electrospraying. Researchers successfully encapsulated cisplatin into the PLGA matrix by electrospraying and the release profile of cisplatin was controlled by altering polymer concentration [Citation6]. In another study, two different configurations of electrospraying setups were used to produce cisplatin-loaded PLGA polymeric particles and to control the distribution of cisplatin within the particles. It was shown that core-shell structured particles had more sustained release compared to the uniform particles [Citation7].
Table 1. Drug loading and release characteristics of electrosprayed particles loaded with various bioactive agents.
In addition to the encapsulation of a single drug, electrospraying has also been used for delivering multiple drugs (polypharmacy) in that two or more therapeutic agents can be encapsulated into a polymer matrix or multi-shell structured particles. A co-axial capillary device incorporating three needles sharing the same vertical axis was used to produce triple-layered capsules composed of PLGA and poly(DL-lactic acid) (PDLLA) containing paclitaxel and doxorubicin. By simply changing the flow rate and polymer concentration at each layer, the particle size and shell thickness can be controlled. The study showed that drug burst release was reduced [Citation12]. In fact, such devices were invented in the last 10 years and have now led to the creation of an electrospraying device, which incorporates four co-axial needles, which can create for new levels of polypharmacy [Citation13].
3. Expert opinion
Micro/nanomaterials have great potential in the medicinal and pharmaceutical field due to mimicking the size range of biological molecules and entities. Polymer-based microparticles play a key role as vehicles in the controlled delivery of different forms and types of active substances, such as antidiabetic, anticancer, antihypertensive drugs, immunomodulatory agents, hormones, vitamins, nucleic acids, proteins, and antibodies. Polymers such as PLGA, PCL, PLA, etc., are approved by World Health Organization and Food and Drug Administration as substances that can be used in medicine and pharmacy. These biodegradable polymers have various specialties such as desirable processing characteristics, biocompatibility, and biodegradation at rates that can be arranged for the intended application. The release of medicines inside particles can be sustained over a long period, or cyclic over a long period, or burst release in a short time, or it can be released by environmental or other external effects. The crucial aim of controlling the drug release is improving the effectiveness of therapies, preventing both insufficient and overdosing intake. Moreover, controlled-delivery systems can maintain the drug levels within the desired range, decrease the frequency of dosage, ensuring better stability of the incorporated substances against degradation (e.g enzymatic), reduce toxicity, and increase patient compliance.
The biggest challenge facing the adaptation of electrosprayed microparticles in drug delivery at a commercial level are mass production and reliability of dosage. The former requires the creation of innovative engineering and this is in progress [Citation13] where therapeutic product comprising layered-electrosprayed microparticles may bring relief to patients suffering from chronic conditions, e.g. urinary tract infections (UTI), which cost the NHS in the UK a vast amount of expenditure. Current oral and other treatment strategies used in these instances may not be so effective and in the longer term can lead to the microbes developing resistance to the antibiotics. The reliability of the therapeutics mass produced will also depend on modern but simple and economical engineering, i.e. will each drug microcapsule offer the same dosage and release characteristics, this will depend on controlling the size distribution of the particles precisely.
Overall, electrohydrodynamic routes are in competition with other new technologies being developed, such as gyration [Citation14] and microfluidic methods [Citation15] to manufacture products. Therefore, rapid investment is necessary to take a perfectly viable laboratory-scale technique to a genuine industrial manufacturing route.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Otto DP, Otto A, de Villiers MM. Differences in physicochemical properties to consider in the design, evaluation and choice between microparticles and nanoparticles for drug delivery. Expert Opin Drug Deliv. 2015;12(5):763–777.
- Gilbert W, Magnete D (1600), 1967.
- Zhang Y, Shams T, Harker AH, et al. Effect of copolymer composition on particle morphology and release behavior in vitro using progesterone. Mater Des. 2018;159:57–67.
- Peltonen L, Valo H, Kolakovic R, et al. Electrospraying, spray drying and related techniques for production and formulation of drug nanoparticles. Expert Opin Drug Deliv. 2010;7(6):705–719.
- Bohr A, Kristensen J, Dyas M, et al. Release profile and characteristics of electrosprayed particles for oral delivery of a practically insoluble drug. J R Soc Interface. 2012;9(75):2437–2449.
- Parhizkar M, Reardon PJ, Knowles JC, et al. Electrohydrodynamic encapsulation of cisplatin in poly (lactic-co-glycolic acid) nanoparticles for controlled drug delivery. Nanomedicine. 2016;12(7):1919–1929.
- Reardon PJ, Parhizkar M, Harker AH, et al. Electrohydrodynamic fabrication of core-shell PLGA nanoparticles with controlled release of cisplatin for enhanced cancer treatment. Int J Nanomedicine. 2017;12:3913–3926.
- Jing Y, Zhu Y, Yang X, et al. Ultrasound-triggered smart drug release from multifunctional core-shell capsules one-step fabricated by coaxial electrospray method. Langmuir. 2011;27(3):1175–1180.
- Zhang S, Kawakami K, Yamamoto M, et al. Coaxial electrospray formulations for improving oral absorption of a poorly water-soluble drug. Mol Pharm. 2011;8(3):807–813.
- Mangrio FA, Dwivedi P, Han S, et al. Characteristics of artemether-loaded poly(lactic-co-glycolic) acid microparticles fabricated by coaxial electrospray: validation of enhanced encapsulation efficiency and bioavailability. Mol Pharm. 2017;14(12):4725–4733.
- Zhang C, Yao Z-C, Ding Q, et al. Tri-needle coaxial electrospray engineering of magnetic polymer yolk–shell particles possessing dual-imaging modality, multiagent compartments, and trigger release potential. ACS Appl Mater Interfaces. 2017;9(25):21485–21495.
- Kim W, Kim SS. Synthesis of biodegradable triple-layered capsules using a triaxial electrospray method. Polymer. 2011;52(15):3325–3336.
- Labbaf S, Ghanbar H, Stride E, et al. Preparation of multilayered polymeric structures using a novel four-needle coaxial electrohydrodynamic device. Macromol Rapid Commun. 2014;35(6):618–623.
- Heseltine PL, Ahmed J, Edirisinghe M. Developments in pressurized gyration for the mass production of polymeric fibers. Macromol Mater Eng. 2018;303(9):1800218.
- Gultekinoglu M, Jiang X, Bayram C, et al. Honeycomb-like PLGA-b-PEG structure creation with T-Junction microdroplets. Langmuir. 2018;34(27):7989–7997.