ABSTRACT
Introduction
The manufacture of modern dry powder inhalers (DPIs), starting with the Spinhaler (Fisons) in 1967, was only possible thanks to a series of technological developments in the 20th century, of which many started first around 1950. Not until then, it became possible to design and develop effective, cheap and mass-produced DPIs. The link between these technological developments and DPI development has never been presented and discussed before in reviews about the past and present of DPI technology.
Areas covered
The diversity of currently used DPIs with single dose, multiple-unit dose and multi-dose DPIs is discussed, including the benefits and drawbacks of this diversity for correct use and the efficacy of the therapy. No specific databases or search engines otherwise than PubMed and Google have been used.
Expert opinion
Considering the relatively poor efficacy regarding lung deposition of currently used DPIs, the high rates of incorrect inhaler use and inhalation errors and the poor adherence to the therapy with inhalers, much effort must be put in improving these shortcomings for future DPI designs. Delivered fine particle doses must be increased, correct inhaler handling must become more intuitive and simpler to perform, and the use of multiple inhalers must be avoided.
1. Introduction
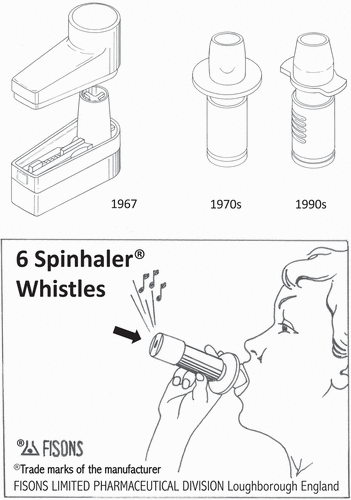
The introduction of the Spinhaler (Fisons) in 1967 to the market is more than 50 years ago. It is the first capsule inhaler that marks the beginning of the era of modern dry powder inhalers. Nevertheless, the Spinhaler is still used at present and, therefore, it is considered a contemporary device in this manuscript, similarly to several other comparable devices comprising the first generation of commercially successful dry powder inhalers. shows the design of an early prototype version of the Spinhaler (1967) in comparison with two later versions, as well as a Spinhaler from the 1970s in which already a feedback (whistle) signal to the patient is recommended for correct inhaler use. Currently, worldwide more than 100 dry powder inhalers (DPIs) have been fully developed and tested of which more than 40 have reached the market. Hundreds more that failed in reaching the patient are known from patents and a few devices have been withdrawn from the market for different reasons, of which the Pfizer Exubera for insulin is the most notable example.
Figure 1., Early prototype of the Spinhaler (Fisons), the first modern dry powder inhaler from 1967 (Museum of Plastics, Bournemouth, UK) compared with two later versions (1970 and 1990s) and a whistling version giving feedback about the inspiratory flow rate. The principle of operation of all versions is the same.
It may seem surprising that in spite of the proven interest in powder inhalation from the 1850s on, it lasted more than a century before successful devices could first be developed in the second half of the 20th century. There is a good reason for it however, and that is the lack of production means, including the technical knowledge, efficient machinery and suitable materials. This prevented mass production and reaching acceptable therapeutic efficiency in the administration of the powders to the lungs. The state-of-the-art technology before 1950 made inhalation also economically less interesting. Several scientific and technological developments needed to precede the design of the first modern capsule inhalers, of which the most important are discussed in the next paragraph. They are the enablers that make the design and development of modern powder inhalers possible. Most of these developments started only very briefly before the Spinhaler was introduced to the market, and some of them even in the second half of the 20th century, which is simultaneous to its development. At least six inventions and developments relating to dry powder inhalation can be mentioned. They are:
the design of efficient milling equipment suitable for controlling the drug particle size distribution,
the introduction of waste water regulations promoting the production of pharmaceutical grade lactose,
the scientific recognition of the existence of adhesive mixtures, for which the electron microscope was an unmissable attribute too,
the invention of hard gelatin (and later hydroxypropyl methylcellulose: HPMC) capsules as single-dose compartments and their improvements for inhalation,
design and development of appropriate filling equipment for these capsules, and
the invention and production of suitable plastics for inhaler device manufacture.
Not to mention that none of these developments would have been possible without the electrification of the industry in the beginning of the twentieth century. Brief histories of these developments that were necessary to establish the present of dry powder inhalation are summarized in the next paragraph.
2. The enablers for design, development, and production of DPIs
2.1. Milling equipment
One of the first prerequisites for manufacturing efficient DPIs is good milling equipment providing control over the size distribution of the powdered material. Most industrial mills currently used became first available at the end of the industrial revolution after better construction materials, such as Bessemer steel (1955), were invented. For instance, steam powered ball mills were already used from 1850 to 1900 on [Citation1,Citation2], but it required electricity and alloy steels to make them economically of interest for the variety of products they are nowadays used for, which shifts their widespread deployment to 1920 or later. Many size reduction techniques, including ball mills, use compressive forces, rather than relying on attrition from particle–particle collisions, however. This may result in powder caking that renders them unsuitable for many cohesive products when a controlled size reduction is required. First after fluid energy mills became available, powders with the desired fineness could be produced reproducibly. The first known design of a jet mill is from approx. 1880 [Citation2], but necessary advancements for the production of inhalation powders were first made in the 1920s and 1930s, when different principles for the utilization of high-velocity jets in milling processes were developed [Citation3–5]. Lynch and Rowland date the use of modern jet mills even to 1950 [Citation1]. Suitable micronization equipment was not the only necessity for a successful start of the dry powder inhalation era however.
2.2. Pharmaceutical grade lactose in low dose drug formulations for inhalation
The history of powder formulation for inhalation is also closely related to that of pharmaceutical grade lactose. Many formulations for inhalation require a diluent excipient because most drugs are administered in the microgram range. Micronized particles in such low doses cannot be metered in a reproducible way on a production scale in order to meet the requirements for an inhalation product. The excipient needs to be inert and have approval for inhalation by the Regulatory Authorities. Excipients in early dry powder inhalers (Abbott Aerohalor and Fisons Spinhaler) were limited to lactose and lactose is still one of the very few products accepted for inhalation by the FDA. Pharmaceutical grade lactose has to meet the identity, purity, and chemical stability specifications of the National Pharmacopoeias.
Lactose is made from whey, which is a by-product of cheese, butter, and casein production from milk. Technical lactose has been produced since approximately 1900 on, mainly for manufacturing penicillin and lactic acid, the latter having many applications in the food and chemical industries. The amount of whey produced by the dairy industry before the 1970s was much greater than what was needed for the production of some lactose and whey concentrate as supplementary food for cattle however. Most whey was discharged as industrial wastewater, giving a rather extreme contribution to the pollution of surface waters because of its high biochemical oxygen demand (BOD). The pollution became a threat to the organisms living in blue water and to the supply of drinking water. This resulted worldwide in different laws, treaties, and conventions to stop the pollution process, such as the 1948 Federal Water Pollution Control Act in the USA and the European Water Charter in 1968. Municipal sewage treatment plants were built rapidly in most industrialized countries from approx. 1950 on and around 1970 licensing systems for the disposal of waste water were introduced in most countries. They made the disposal of whey very expensive and it became lucrative to reduce its BOD by producing more lactose from it. European manufacturers started manufacturing pharmaceutical grade lactose around 1950, e.g. De Meijerij Veghel (DMV), currently DFE Pharma, the Netherlands (NL) in 1949 and Meggle, Germany in 1953. Only Sheffield Pharma (USA) was earlier to start already in 1940, whereas some others were much later (e.g. Borculo in 1985). To distinguish pharmaceutical grade lactose from edible lactose, it was initially simply referred to in literature as lactose BP (British Pharmacopoeia), lactose USP (United States Pharmacopoeia) or USP-NF (USP-National Formulary) [Citation6]. The Pharmacopoeia specifications do not include particle and powder physical properties however [Citation7], whereas inhalation lactose needs to be used in a size fraction that exhibits good flow properties for mass production. The size fraction also needs to facilitate good emptying of the dose (measuring) compartment [Citation8] and dispersion [Citation9] and its choice depends very much on the type of inhaler used [Citation10]. Into the 1980s much about the properties of lactose for tabletting and inhalation was still unknown however, and an abundance of studies was started to obtain a better understanding of the role of its physical properties (mainly the particle size distribution) on its processability into solid dosage forms (mainly tablets) and various properties of the end product. Currently, most suppliers offer a great variety of different size fractions of crystalline alpha monohydrate, either as standard product or as tailor made solution, for inhalation purpose and spray dried and (roller dried) beta anhydrous mainly for tabletting.
2.3. Adhesive mixtures
The mixing of free-flowing lactose as diluent with small amounts of micronized drug is not a simple routine procedure. There is traditionally a great concern about the homogeneity and stability of mixtures with great differences in the particle size distributions between the constituents. Usually, such mixtures are prone to segregation, particularly when the particles differ in density and shape too [Citation11,Citation12]. However, Coulson and Matrai discovered with the electron microscope that very fine particles show a tendency of adhering onto the larger (carrier or host) particles in a rather homogeneous distribution over their surface [Citation13]. This renders the mixture a greater stability than that of random mixtures in which there is no noticeable adhesion between the particles. This fundamentally different concept of mixing was considered new by Hersey (1975) and he named it ‘ordered mixing’ [Citation14]. Often, ordered mixtures have a higher degree of homogeneity than to be predicted with classic theories on random mixing [Citation15]. In a long-lasting debate in literature about the correct nomenclature for this new mixing concept, also the name ‘interactive mixture’ has been introduced [Citation16] until Staniforth (1987) proposed to re-term this type of blend in ‘adhesive mixture’ [Citation17]. He argued that all matter interacts, but it depends on other forces whether this will result in adhesion or not. Currently, all three names are still used in literature. Staniforth also observed that mixing is a dynamic process in which the state of adherence of fines to the carrier crystals (adhesive mixture) can exist next to the state of fines being agglomerated into soft, mostly spherical pellets, in a variable ratio between both states during the mixing process. The existence of such agglomerates can have a great effect on the homogeneity of the blend. He named this dynamic concept ‘total mixing’ [Citation18]. This finding has made clear that adhesive mixture preparation is a delicate process in which various parameters are critical and have to be selected and controlled carefully. They determine the spatial distribution of fines over the carrier surface, the degree to which the fines are present in the mixture as agglomerates and the extent to which the fines are firmly pressed onto the carrier surface [Citation19]. In spite of a plethora of studies on adhesive mixtures, the mechanisms and variables involved are still only partly understood. Yet, adhesive mixtures are currently the most widely used type of formulation for low-dose inhalation drugs. Very important for the research on adhesive mixtures was the availability of scanning electron microscopes (SEMs). It was the invention of the electromagnetic lens by Busch in 1927 that made the development of SEMs possible [Citation20]. Several experimental concepts were constructed and tested before the first true SEM was described and developed in 1942. Signal processing to improve the micrographs (1956) and advanced secondary electron detection (1960) followed the experimental phase [Citation21]. As a result of all these improvements, a first commercially available SEM was launched in 1965 by Cambridge Scientific Instruments. Currently, SEM can be combined with techniques for element analysis, like Energy Dispersive X-Ray Spectroscopy (EDXS, also known as EDX and EDS) and Anti-Stokes Raman Scattering (CARS), that enable for instance to map drug distributions over the carrier particles in adhesive mixtures [Citation22].
2.4. Gelatine and hydroxypropyl methylcellulose (HPMC) capsules
Hard gelatin capsules, also a necessity for the first generation of DPIs, were conceived in the first half of the 19th century. Different inventors were involved in the development and improvement of capsule manufacture in the period between 1830 and 1850 [Citation23]. From this period different patents are known of which the one that was granted to James Murdoch in 1847 gives a detailed description of the basic principle of manufacture as it is still used today [Citation24]. Since 1931 machines have been developed to produce simultaneously bodies and caps, and capsules as oral dosage form have become available in a wide range of different sizes for powder quantities ranging from approximately 80 to 1650 mg. However, when Fisons developed the Spinhaler for the Intal formulation with 20 mg sodium cromoglycate (SC) in capsule no. 2 in the late 1960s as the first of a series of capsule-based DPIs from other companies, they faced several challenges using such capsules for inhalation [Citation25,Citation26]. The oral capsules in use were not suitable for puncturing with needles and the capsule filling machines were designed for measuring powder weights of about 200–300 mg into capsules no. 2 (weighing 61 mg itself), which is more than ten times higher than the Intal dose (20 mg). The available capsule filling machines neither had the required production capacity. In addition, the cohesiveness and poor flowability of the micronized SC increased the difficulties in achieving a consistent dose measuring and a good capsule emptying during inhalation. Adding lactose in a size fraction from 70 to 100 μm in an equal amount as the drug was found to yield the necessary improvement in flow and fluidization properties [Citation6] and the problem of capsule puncturing was solved by Eli Lilly (Qualicaps). They changed the composition of the gelatine blends, which resulted in holes with a small flap that stayed open after the needles were retracted instead of the flaps breaking off [Citation26]. Because gelatine has a high moisture content (approx. 13–16%) and the material becomes brittle when losing moisture (as from exposure to air with a low relative humidity, or from water transfer to the drug formulation), currently hydroxypropyl methylcellulose (HPMC or hypromellose) is a better alternative for gelatine [Citation27]. HPMC capsules (with 4 to 6,5% moisture) have a lower triboelectrification potential too and they came on the market around 1990 [Citation26], although the first patent is already known from 1950 [Citation27]. Increasing the weight and shape uniformity of the capsules and reducing their weight and the residual lubricant content were other developments needed to improve the capsule properties for inhalation, particularly after companies like Boehringer Ingelheim reduced the capsule fill weight for tiotropium bromide to only 5.5 mg. This is only 11.5% of the (gelatine) capsule (no. 3) weight (48 ± 3 mg), which causes a variation in capsule weight to overshadow completely the spread in metered powder mass. A comparative evaluation study with gelatine and HPMC capsules on capsule properties relevant to inhalation under different environmental conditions is known from Pinto et al. [Citation28].
2.5. Capsule filling technique
When the Spinhaler was developed in the 1960s only two types of capsule-filling machines were available: those using the capsule body as measuring volume (e.g. Tevopharm Cap III semi-automatic machine) and those using a separate dosator with an adjustable volume. After having produced small batches of the Intal capsules first with modified CAP III machines, using an additional plate with small holes for pre-filling of 40 mg doses before they were transferred to the capsule bodies, Fisons decided to adapt their production (dosator) machines to improve the metering consistency and increase the production capacity. They started a research project with Nottingham University and MG2 and concluded that the formation of a powder arch in the dosator tube is necessary to prevent powder from falling from the tube when this is moved from the powder hopper to the capsule [Citation29,Citation30]. The arch formation appeared to depend on the cohesiveness and bulk density of the powder as well as on the friction between the powder and the inner wall of the dosator tube. First with the use of mini-dosators, applying a minimal amount of powder compression, filling capsules at the required speed became possible. From the period Fisons conducted their research to the present day, significant advancement has been made in filling technique in general, as well as that specifically for inhalation capsules by companies like Harro Höfliger, MG2, Zanasi and Bosch. One of the first fully automated capsule filling machines was presented to the marked in 1957/1958 by Höfliger + Karg (acquired by Bosch in 1970, now Syntegon since 2020). Having an auger principle, it filled capsules to the rim and in 1959 they replaced this principle by a dosing disc/tamping pin system to enable fill weight variation. It is still in use for modern GKF filling machines made by Bosch/Syntegon. The dosator system was invented by Zanasi in 1957 and applied in their LZ57 capsule filling machine but both dosator and dosing disc have never found acceptance for the production of DPI capsules because of the powder compaction that jeopardizes dispersion during inhalation.
2.6. Plastics
Arguably the most important development as prerequisite for the rapid expansion and diversification of dry powder inhalers in the past 50 years is the invention of plastics. Although some plastics were already discovered, or developed in the 1800s (e.g. Parkesine, Celluloid, Galalith and mineral filled Shellac), or the early 1900s (e.g. polyoxybenzylmethyl glycoanhydride, better known as Bakelite), they were not suitable for (mass) production of dry powder inhalers. Most plastics currently used for DPIs were first discovered or developed in the period between 1940 and 1960, including acrylonitrile-butadiene-styrene (ABS: 1948), which is the construction material used for the Spinhaler prototype in 1967, polypropylene (PP: 1951), polyoxy-methylene (POM: 1951) and polycarbonate (PC: 1953). Most multidose reservoir inhalers are assemblies of parts produced from different plastics to meet specific requirements regarding powder protection (e.g. from moisture uptake), stiffness against deformation, friction between moving parts (e.g. of the dose measuring mechanism), high wear resistance (e.g. to guarantee a constant switch point for safety valves) and minimal tribocharge effects.
3. Dry powder inhalation: present inhalers
The introduction of DPIs has significantly reduced the use of nebulizers and metered dose inhalers (MDIs). It is remarkable, however, that in its country of birth (UK), DPIs in 2017 still contributed less than 30% to total respiratory retail units versus 70% for MDIs [Citation31]. In nearly all other European countries the DPI-share of the market is higher than the MDI-share with Sweden as extreme: 85% for DPIs versus only 13% for MDIs. Interestingly, MDI and DPI together have almost decimated liquids for nebulization in Europe, with an exception for Italy where nebulization liquids still contribute 44% to retail sales. The fast conquering of a significant part of the inhalation market confirms that DPIs have certain advantages over MDIs and nebulizers [Citation32]. It is, however, disappointing that at present device and formulation studies are mostly still performed separately instead of integrating them in order to strive for the best possible combination. It is also disappointing that formulation studies continue to outnumber device improvements massively considering that there is still plenty of room for DPI performance improvement, as will be discussed hereafter. In fact, the number of formulation studies is so overwhelming that it is impossible to summarize them all. Moreover, most of them will remain in the experimental phase. Only a few examples have found their way to production and they will be presented tablewise in paragraph 3.8. Also several device developments can be found in recent patents and literature that may never become available to the patient. Therefore, the present of dry powder inhalation is limited to presenting the different categories of marketed capsule, multiple unit-dose, multi-dose reservoir and active DPIs only, the latter utilizing external energy for dispersion of the drug formulation. A category of miscellaneous developments is added in the paragraphs 3.5–3.7 to address some special DPI developments.
3.1. First generation capsule inhalers
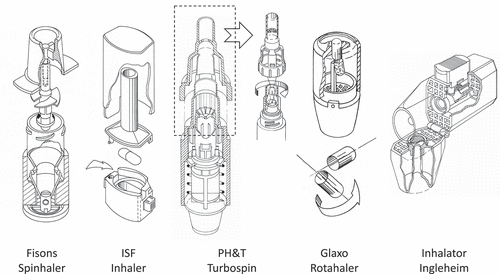
Within a few years after the introduction of the Spinhaler, several other capsule inhalers followed (). They all have basically the same working principle and they can be considered the category ‘first generation dry powder inhalers.’ All examples shown in are still on the market and the difference between them is in the way in which the capsules are pierced (or opened) and forced to move by the air stream during inhalation to discharge the powder formulation. Many different studies have been performed on the rate and efficiency of capsule emptying [Citation6]. the powder dispersion in capsule inhalers [Citation33,Citation34] and the effect of design modifications on their performance [Citation35,Citation36]. A lot has been written also about the specific benefits [Citation37,Citation38] and shortcomings with accompanying risks of capsule inhalers [Citation39] and these pros and cons need no further mentioning in this manuscript.
3.2. Multiple unit-dose inhalers
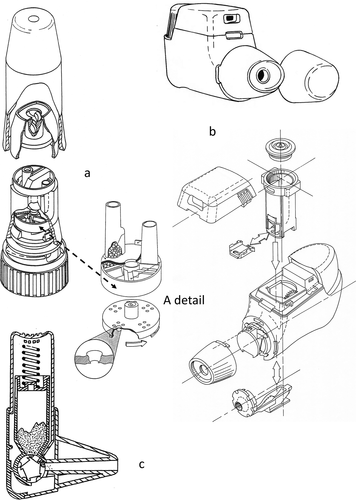
Around 1990, the first multi-dose inhalers were launched on the market in which multiple unit-dose (blister) inhalers () can be distinguished from multi-dose reservoir inhalers (). Among the first devices carrying more than one dose were the Astra Turbuhaler [Citation40] and Glaxo Diskhaler. An important advantage of the multiple unit-dose type is that they carry pre-metered doses in blisters, making the accuracy of dose metering independent of inhaler handling by the patient. A disadvantage is the limited number of doses in the device, only four to eight for the Diskhaler ()). For this device, the blisters need to be pierced with a rather big pin to make a sufficiently high flow rate for blister emptying possible. This creates flaps of the lidding foil hanging in the blister and shielding part of the powder from being entrained by the air stream. The Glaxo Diskus or Accuhaler (launched in 1988), contains a long strip with 60 blisters for which the lidding foil is peeled off in front of the mouthpiece opening ()). Emptied blisters and the lidding foil are coiled up separately and this requires a rather complex blister transport system [Citation41]. More recently, the GSK Ellipta ()) was introduced and this inhaler has one or two coiled blister strips for fixed-dose (double and triple) combination therapies [Citation42].
Figure 3. GSK multiple unit-dose (blister) inhalers with Diskhaler (a), Diskus or Accuhaler (b) and Ellipta (c).
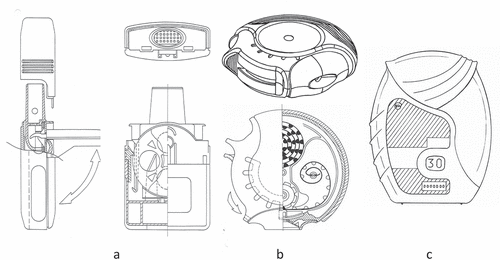
Figure 4. GSK originator and generic Advair Diskus multiple unit-dose inhalers with Mylan Wixala Inhub (a), Sandoz AirFluSal Forspiro (b), Glenmark Salflutin and Stalpex (c) and Neutec Airmaster (d).
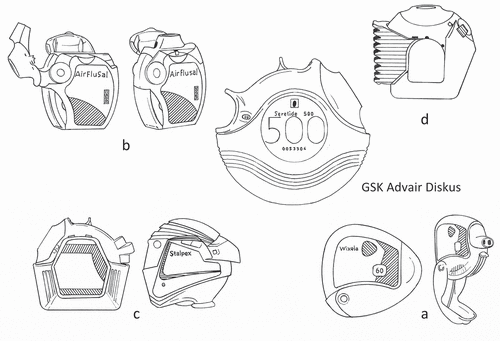
One of the latest developments in multiple unit-dose inhalers is the expiration of GSK’s patents for Advair (2010) and the Diskus (2016). This has resulted in the development of a series of generic devices for Advair (fluticasone dipropionate with salmeterol) with basically the same design regarding their working principle as the Diskus (). However, in the operation procedures there are several small, but essential differences between the devices, whereas most of them also look different. This is objectionable, as it confuses the patient when they are switched over from the originator Diskus. This likely results in more handling errors.
3.3. Multi-dose reservoir inhalers
At the end of the 20th century, also the first multi-dose reservoir inhalers were launched. They have a reservoir for the powder formulation, containing up to 200 doses, and a metering cavity to separate single doses from the bulk. In contrast with most capsule and blister inhalers, many of them have a more efficient dispersion principle yielding higher fine particle fractions already at a lower flow rate [Citation43]. For accurate-dose metering into the cavity, which is by the force of gravity and requires good flow properties of the powder formulation, the inhaler needs to be held in the correct position. Metering cavities are in rotatable disks (e.g. AstraZeneca Turbuhaler: )), in movable slides (e.g. Meda Novolizer: )), or in rotatable cylinders (e.g. Orion Easyhaler: )). They are the forerunners of many other devices of more or less the same reservoir type that are currently on the market (e.g. Leiras Taifun, Chiesi NEXThaler, TEVA Spiromax, Merck, Sharpe & Dohme Twisthaler, etc.). The working principles and performances of these devices have well been described extensively before [Citation43]. Nearly all devices contain adhesive mixture formulations for the drug, except for the Turbuhaler and Twisthaler. They carry soft pellets with small lactose amounts [Citation40,Citation44].
3.4. DPIs utilizing external energy
Disappointingly, after the introduction of the aforementioned two categories of multi-dose DPIs, not many innovative concepts have been developed that were successful on the market. Developments include those of devices making use of external energy for the powder dispersion. The idea is to make inhaler performance independent of the inspiratory effort and basically, three types of energy are, or have been used:
compressed air, e.g. Exubera (Pfizer), Aspirair (Vectura), ResQhaler (Aespira Ttd.),
electrical energy, e.g. Spiros (Dura), Microdose DPI (Microdose Therapeutx), Taper DPI (3 MTM), and
thermal energy, Adasuve Staccato One Breath TechnologyTM (Alexza) for the delivery of loxapine and apomorphine
OccorisTM Technology (Team Consulting) and Inspiromatic (Inspiro Medical) are also active inhalers, but they are still in the development and testing phase, respectively, [Citation45,Citation46]. The TEVA Spiromax, formerly named Airmax (Norton Healthcare), uses compressed air for accurate dose metering only [Citation47]. It is, however, a persistent misconception that patient-independent aerosol properties yield a more constant therapy [Citation48]. This is only true if the inhaled flow rate is always the same too, meaning that it cannot be varied by the patient. At a higher flow rate, constant aerosol properties result in a substantially higher oropharyngeal deposition and also in a shift of deposition toward larger airways in the tracheobronchial tree [Citation49]. A finer aerosol and/or a higher fine particle dose at a higher flow rate compensates, at least partly, for this effect. Limiting the flow rate within a relatively narrow range is also advantageous to lung deposition, as even for small particles the peripheral deposition decreases at a higher velocity in the respiratory tract [Citation50]. The benefit of using external energy is, therefore, particularly guaranteeing sufficient flow rate for dose entrainment and dispersion and not for achieving a more patient independent lung deposition. So far, all externally energized DPIs have not been very successful on the market for different reasons, including the Spiros and Exubera. They are expensive, vulnerable to battery failure, have reduced portability and/or limited applicability [Citation51,Citation52].
3.5. Miscellaneous developments
Around the millennium change several developments were started in various directions to improve dry powder inhaler technique and pulmonary therapy in general. They cannot all be described and, therefore, only some highlights are addressed. A frequently neglected aspect in reviews on the history of DPIs is the capsule filling process that went through a series of spectacular advancements to meet the requirements for high-speed low-dose drug metering. In addition to aforementioned principles, a vacuum drum system has successfully been developed and applied for the production of low-dose insulin blisters for the Exubera-inhaler by Harro Hoefliger. It allows for reproducible filling of powder amounts as small as 1 mg, depending on the powder properties [Citation53]. More highly sophisticated filling techniques are in development and these low-dose metering principles require an accurate in-line fill weight check. This has become possible by the development of high-speed pre-weight controllers measuring individual capsules before and after filling with powder up to 100.000 capsules per hour. Currently, not only capsules, but also other single-, as well as multi-dose compartments with different volumes and designs and made of different construction materials can be filled and sealed. Thanks to these innovations, inhaler production lines have since the 1980s rapidly evolved in complex, GMP compliant and fully computer-controlled production robots, including parts-assembling and inhaler-packaging steps. This put packaging companies in a leading position in the complex interplay with inhaler designers, plastic molders and the pharmaceutical companies.
3.6. Alternatives for lactose-based inhalation products
One of the weak points of dry powder inhalation is the use of lactose-based adhesive mixtures for low-dose drugs in DPIs that have no distinct effective dispersion principle. Currently adhesive mixtures yield FPFs of maximally 40 to 50% of the label claim, even in the presence of magnesium stearate, with an average value across all currently available DPIs of only approximately 30%. This leaves considerable room for improvement. Several alternative carrier materials have been tested, mostly sugars (e.g. mannitol) with different result depending (among other variables) on the type of drug and the inhaler used. Moreover, most alternative carrier materials investigated so far are not (yet) approved by the FDA for inhalation. A different approach is changing the classic DPI concept from passive into active by using external energy for dispersion or to omit the use of carrier for the drug. Several examples are known for this approach and they vary from drug particles attached to a coiled dimpled tape that are liberated and dispersed into the air stream by vibratory action during inhalation (Taper DPI, 3 M [Citation54]:) to drug particles that are rapidly heated on a heat pad during inhalation. The heating vaporizes the drug that subsequently condenses into particles in the cold inhaled air stream (Staccato System, Alexza). In contrast, the Technosphere platform (MannKind) uses self-assembling fumaryl diketopiperazine (FDKP) particles as drug carrier that are small enough (MMAD is 2–2.5 μm) to be inhaled. This too makes separation of drug and carrier particles during inhalation otiose [Citation55]. Not having to separate drug particles from the surface of larger carrier particles in adhesive mixtures may potentially lead to higher FPFs. Unfortunately, in vitro and in vivo deposition studies with these concepts are scarce or even completely lacking. Studies as with Technosphere insulin [Citation55] and Staccato loxapine [Citation56] focussed on pharmacokinetics, efficacy, safety and tolerability aspects instead of on lung deposition. Scraper DPIs, like Jethaler and MAGhale have a different dose measuring principle. They have the drug-excipient blend compressed in a ring-shaped tablet adjacent to a ceramic scraper disk that is connected to a spring-loaded mechanical drive. During inhalation the scraper action is started with a knob to remove part of the ring-shaped tablet as fine particles and disperse them into to the inhaled air stream. It has been shown for the Jethaler that the consistency of delivered dose and fine particle dose is low due to considerable variation in the mass and particle size scraped from the tablet [Citation57]. For the MAGhaler it has been reported that between 70 and 80% of the delivered dose is deposited in the oropharynx due to the fact that separation of drug and excipient is very incomplete with this concept too [Citation58]. Currently, both devices are no longer available.
Future replacement of lactose may also be desirable for another reason. Currently, there is an increasing interest in lactose and lactic acid for other applications. Lactose can be used as a sweetening, stabilizing and moisture conservation ingredient in food products. Lactic acid, among many other applications, can be used as acidulant in fruit juices and beverages and as preservation and flavoring agent in pharmaceutical and cosmetic products [Citation59]. Particularly, the demand for lactic acid as monomer in the preparation of poly lactic acid (PLA) is exponentially growing, with estimated annual growth rates between 15 and 18% [Citation60]. PLA is a ‘green plastic,’ environmentally friendly and very suitable for 3D printing and this construction technology is expected to grow exponentially too in the next 10 years. Although many raw materials can be used for lactic acid manufacturing, like waste paper sludge, corn starch, corn stover and other agricultural waste products, there is currently lack of clarity about which of these materials and which production process is most cost competitive and has highest agricultural sustainability [Citation60–63]. Therefore, whey may remain an important raw material for the production of this highly valuable hydroxycarboxylic acid and this may put pressure on the price and availability of lactose in the future. Also, because in some countries (e.g. the Netherlands, one of the largest pharmaceutical grade lactose manufacturers) there are discussions going on to reduce the livestock drastically because of multiple adverse environmental and health effects [Citation64].
3.7. Special dry powder inhaler devices
At present, various special inhaler types are being introduced. The interest in inhaled antibiotics, which are mostly in the range of several tens to hundreds of milligrams, has resulted in the development of high dose DPIs (e.g. Pharmaxis Orbital, PureIMS CyclopsTM and Hovione 8Shot). Simultaneously, a strong interest in pulmonary vaccination has been developed [Citation65] which resulted in several single-dose vaccine inhalers (e.g. Manta SoloTM, Perlen PerlamedTM BLISTair, Hovione TwinCaps, Iconovo ICOone). Vaccine inhalers, and also high-dose inhalers for hygroscopic drugs, need to be disposable and this makes them suitable also for other applications, like rescue medication (e.g. in case of off episodes in Parkinson’s disease), suppression of mental and psychotic disorders and the administration of anti-viral products [Citation66]. The TwinCaps is the first of mentioned devices having market approval (Japan) for Inavir, an influenza anti-viral drug. In the past two decades, several inhaler patents expired and this resulted in the development of generic devices with the same drug, or drug combination as has already been mentioned for the Advair Diskus. Also, for Boehringer’s Spiriva tiotropium capsules in the HandiHaler several generic alternatives have been developed, e.g. Cipla Tiova (rotacaps, Glenmark Tavulus, Mylan NeumoHaler and TEVA Zonda. Generic DPIs are generally cheaper than originator products, but not all are well appreciated by patients however, as has repeatedly been reported for the salmeterol/fluticasone Elpenhaler [Citation67–69]. This can result in poor inhaler technique and non-adherence to the therapy that is at the cost of the efficiency of the therapy.
3.8. Particle engineering and simulation studies
In addition to aforementioned DPI device innovations, a plethora of formulation and particle engineering studies can be found in literature. They are meant to improve the powder dispersion [Citation70,Citation71] increase lung deposition [Citation55,Citation72], enhance drug absorption [Citation73], increase the stability of the formulation [Citation74], prevent water uptake [Citation71] or hinder clearance by macrophages [Citation75]. Different solutions are possible to achieve either of the aforementioned effects but many formulations are unlikely to reach the patient. They will not be discussed, but a few of the most noteworthy examples are shown in . Currently, also various other ‘micronisation’ techniques than fluid energy milling for the drug are used [Citation43]. They include spray-drying, freeze-drying, spray-freeze-drying, precipitation and super-critical fluid technology. These techniques enable the aforementioned incorporation of stabilizers (with sugar glass technology) and dispersion enhancers (e.g. L-leucine). Spray drying is particularly suitable for high-dose drugs that do not need dilution with large quantities of excipients (e.g. inhaled antibiotics) for reproducible dose measuring. They can be delivered to the lungs as pure drug (no excipients), or as engineered particles with specific properties. A well-known example is PulmoSphereTM tobramycin (TOBI) [Citation33,Citation70]. Different techniques are also available for carrier manufacture or modification and drug-excipient mixing. Computation Fluid Dynamics (CFD) and Discrete Element Method (DEM) simulation studies are other developments finding rapidly application in the inhalation therapy. They vary from studying and optimizing inhaler performance [Citation76,Citation77], to the prediction of lung deposition [Citation78–80]. For more detailed information, the reader is directed to Kassinos et al. [Citation81]and for functional respiratory imaging in combination with CFD to the publications of the Fluidda group [Citation82].
Table 1. Some formulation and particle engineering innovations in dry powder inhalation.
3.9. The carbon footprint of dry powder inhalation
A final comment should be made on the environmental aspect of inhalation. A greater future interest in DPIs may depend on the fate of MDIs. Their hydroxy fluoroalkane (HFA) propellants are greenhouse gases (GHGs) that are harmful to the atmosphere and discussions have already been started about whether they need to be replaced again [Citation92]. This may not appear to be equally successful for all drug formulations and, therefore, DPIs may become a better alternative for these drugs. DPIs in most studies also result in lower error frequency percentages compared to MDIs [Citation93–96], but similar as for MDIs, DPIs are subjected to a critical examination regarding their carbon footprint, which is generally expressed in their CO2-equivalent (CO2-eq) for production and usage. In reality, the environmental degradation of inhalation has two different aspects however: littering and a contribution to the greenhouse effect. The problem of littering can technically be solved by introducing a packaging deposit-refund system for empty inhalers [Citation97]. Collecting used DPIs makes also (at least partial) recycling possible, particularly for inhalers that consist of only one type of plastic and recycling can easily halve the carbon footprint of a polycarbonate inhaler. The carbon footprint of plastic inhalers depends further very much on where and how they are manufactured, including the raw materials. Plastics can be produced in a much more environmentally friendly manner by excluding coal (e.g. for energy generation) from the production process [Citation98]. Possible reductions in GHG for inhalers expressed in kilograms CO2 emission may seem impressive [Citation99], particularly for MDIs, but they have to be seen in perspective. Fluorinated gases contribute only approximately 3% to all GHG emissions [Citation100] of which the share of HFAs in MDIs is only 3% too. Of all fluorinated gases produced, 97% is used otherwise, mostly as refrigerants [Citation101], meaning that the GHG emission of HFAs released from MDIs is only 0.09% of total GHG. A greater disadvantage of HFA-134a and HFA-227 is their relatively long lifetime of 14 and 34.2 years respectively [Citation102] which causes them to accumulate in the atmosphere, whereas CO2 is part of the short-term carbon cycle [Citation103]. Similarly, all plastics contribute only 3.8% to global GHG emissions [Citation98,Citation104,Citation105] of which around 40% is used for packaging and only 17% for medical applications [Citation106], including a small fraction for plastic inhalers. This makes inhaler plastic also responsible for less than 0.1% of the global plastic impact. It does not mean that possibilities to reduce the carbon footprint of DPIs should be ignored without consideration, but it must be acknowledged that mass reductions in material, using bio-based plastics or making design adjustments in favor of a lower carbon footprint can harm the efficiency of drug delivery with the device. Whatever decision is made in this respect, the significance of its effect should be weighed against the type and extent of its influence on DPI performance and acceptance by the patient. The use of bio-based plastics should also be judged on cost competitiveness [Citation63], agricultural impacts such as eutrophication and acidification and competition with food production too [Citation61]. Also their end of life management is still unclear, particularly because about three-quarter of all bio-based plastic used worldwide is not biodegradable [Citation97]. Additionally, complex inhalers may require different plastic types for good performance of specific functions (e.g. valve switching, sliding or rotating dose measuring compartments, etc.). This can include fossil-based components in a bio-based DPI body making littering still highly unwanted and recycling difficult. More importantly, changing the plastic mass and plastic type can affect the DPI robustness and performance in many different ways. When the inhaler parts become very light and less rigid, the risks of deformation and improper fitting of the parts increase. This not only gives the impression of a poor-quality device, it can also result in leakage of false air. In high-dose inhalers powder retention is generally in the mg-range, which is the reason why they are better disposed after a single use. When the DPI is used again, all following inhalations are negatively influenced by these powder residues, particularly when the powder absorbs moisture from the air. Powder residues are also likely to spread into their direct environment, such as pockets and handbags. This is annoying for the patient. Such effects influence a patient’s satisfaction with the DPI negatively and this is known to be at the cost of a good compliance with the instructions for correct inhaler use and the adherence to the therapy [Citation107]. Therefore, the influence of a DPI modification, even when it has a rather insignificant immediate effect on the efficacy of dose delivery to the lung, may have a negative impact on correct use the long term. Poor adherence and incorrect inhaler use result in a worse clinical outcome, often accompanied with a higher number of hospital visits, and higher costs [Citation108].
4. Conclusions
The development of DPIs forged ahead in the second half of the 20th century after various enablers in the form of appropriate production technology and suitable materials became available for their mass production. In addition, new ‘adhesive’ (initially referred to as: ‘ordered’ or ‘interactive’) and ‘total’ mixing concepts as extensions to the up till then prevailing ‘random’ mixing theory were developed. They provided the scientific basis for a great number of studies into a better understanding of the properties of blends consisting of small mass amounts of micronized drug and much larger (carrier) excipient particles. Dry powder inhalation diversified quickly into three different directions of capsule, multiple unit-dose and multi-dose reservoir inhalers within a very short period of less than 20 years and only a few innovations have been made since. Future developments should not focus solely on increasing the delivered fine particle dose to the lungs, but also on finding ways to motivate patients to use their DPIs correctly and consistently more often in order to increase the adherence to the therapy.
5. Expert opinion
After more than 4000 years inhalation history (see part 1), a cynical person might ask: ‘what advancements have we made? We started in ancient times with putting herbs on a hot stone to evaporate their volatile components for inhalation and one of the most innovative inhalers to date, the Alexza Staccato system for loxapine, still uses the same principle.’ Obviously, such a comment does not do right to the great technical achievements made to improve this principle. Nevertheless, it has to be acknowledged that in spite of great advancements in particle engineering (), dispersion of most inhalation powders into suitable aerosols leaves much to be desired ()) and that real innovation in inhaler devices remains scarce. Particularly, the lack of effective dispersion principles performing well at a moderate flow rate of 40–60 L/min (corresponding with approx. 4 kPa) for adhesive mixtures is disturbing. Future devices should no longer be designed primarily as dispensers, but also become good dispersers of the drug formulations [Citation10,Citation109]. Not developing new formulations for new pulmonary drugs, but getting these drugs effectively in the lungs is the greatest challenge. Poor dispersion is not the only shortcoming of dry powder inhalation however. As for MDIs and nebulizers, the compliance with the instructions for correct use of the inhaler and the adherence to the therapy need further to be improved to make powder inhalation more efficient. Estimated rates of poor compliance and poor adherence are different between nebulizers, MDIs and DPIs, and for DPIs they have the orders of magnitude of 60% ()) and 50% ()) respectively. The high error frequency and poor adherence are a weakness of pulmonary drug administration in general and a great social and health-economic burden in a period of increasing prevalence of obstructive lung diseases and restrictions in health care spending [Citation110]. They may also be of great direct risk for the patient in case of one-off administrations. For example, pulmonary vaccination has to be good or the patient is insufficiently protected against infection. In case of deadly diseases this can be lethal and also rescue medications require adequate inhalation technique for immediate relief.
Figure 6. Orders of magnitude for the dose fractions available for total lung deposition at a moderate flow rate (A: black and shaded sectors), the averaged rates of correct inhaler technique (B, black sector) and of good adherence to the therapy (C, black sector). The figure shows the improvements that are (still) possible for all three aspects of inhalation, in spite of the increase in lung deposition achieved in the period from 1990 on by approximately a factor four (6A). Rates for correct inhaler use and good adherence have been found to depend particularly on patient’s age, the type of inhaler and the definitions used for incorrect inhalation technique and non-adherence scores [Citation93,Citation110–113]. In most studies, critical error frequencies are higher for MDIs than for DPIs [Citation93]. The white (in A, B and C) and gray sectors (in B and C) represent the room for improvement of DPI therapy.
![Figure 6. Orders of magnitude for the dose fractions available for total lung deposition at a moderate flow rate (A: black and shaded sectors), the averaged rates of correct inhaler technique (B, black sector) and of good adherence to the therapy (C, black sector). The figure shows the improvements that are (still) possible for all three aspects of inhalation, in spite of the increase in lung deposition achieved in the period from 1990 on by approximately a factor four (6A). Rates for correct inhaler use and good adherence have been found to depend particularly on patient’s age, the type of inhaler and the definitions used for incorrect inhalation technique and non-adherence scores [Citation93,Citation110–113]. In most studies, critical error frequencies are higher for MDIs than for DPIs [Citation93]. The white (in A, B and C) and gray sectors (in B and C) represent the room for improvement of DPI therapy.](/cms/asset/932c1ce6-15e3-4a41-8367-d39379c28c8e/iedd_a_2112570_f0006_b.gif)
Uncertain is what inhaler design improvements are likely to contribute to a more correct use and a better adherence to the therapy, as these undesirable features are often rather the result of an incorrect attitude toward the therapy [Citation114,Citation115], or being poorly instructed and coached [Citation116], than a consequence of device design, although poor satisfaction with the device may result in not using it [Citation107]. From previous investigations into inhaler errors, only a few aspects can be concluded that can be related to inhaler design. Crystyn et al. [Citation93] observed a trend toward higher error rates with a larger number of handling steps. It has also been reported that age and/or disease-related motor function impairment and cognitive disabilities can make certain handling steps or inhalation difficult to perform correctly [Citation117,Citation118]. Some devices that require fine motor skills for multiple handling steps score indeed significantly and consistently worse in comparative ease of preparation and usability studies [Citation69]. It is, therefore, of utmost importance that inhalers have simple operating procedures and that they can instinctively be used correctly. It seems logical to assume that convenience of administration, satisfaction with the technique and adherence to the therapy are positively related to each other. This would make the adherence also dependent on the inhaler design. Although many studies can be found about adherence to medical treatments, studies about the relationship between inhaler type (or design) and adherence are scarce. Early observations on the use of TOBI Podhaler suggested improved patient adherence relative to TOBI nebulization [Citation119]. Additionally, patient’s self-reported treatment satisfaction and convenience from using the Podhaler were at that time already reported by Konstan et al. [Citation120]. This makes a positive relationship between satisfaction, convenience and adherence likely indeed. However, the reduction in patient burden from using a powder inhaler relative to nebulization is rather extreme (from 31.5 to 6.5 minutes on a daily basis [Citation119];), whereas the difference in adherence between wet and dry administration of TOBI was found to be only very moderate [Citation121]. Generally, the adherence to nebulization in cystic fibrosis is around 50% for adult patients, depending on how adherence is defined [Citation122–124]. This seems to indicate that adherence is difficult to explain in terms of convenience and satisfaction only. Patient preference for a particular device may also be a determinant for the preparedness to use it, and preference includes more aspects than convenience, ease of use and administration time. Preference is also influenced by inhaler design (shape and size), color, hand- and mouthfeel, portability, etc., which can be summarized as ‘patient satisfaction with the device.’ In this respect, also inhaler resistance must be mentioned. The resistance is not an independent variable however. Changing its value can have serious consequences for the inhaler performance, the aerosol deposition in the lungs and the patient’s ability to operate the DPI correctly. There exist many studies on inhaler preference but they are often a rather unreliable source of information for DPI design [Citation125]. It has been concluded that nearly 80% of such studies are sponsored by the pharmaceutical industry and of these sponsored studies, in more than 80% of the cases, the device of the sponsor was selected as most preferable. Therefore, more research into the real reasons for poor adherence and the effect of DPI design thereon is urgently needed. The development and introduction of smart inhalers, also referred to as connected inhalers, may be helpful in making this clear and improving patients’ attitude toward their inhalation treatment.
Article highlights
The present of dry powder inhalation started in the late 1960s with the invention of the capsule-based Fisons Spinhaler
In a very short period of 20 years DPIs diversified into three different categories of capsule, multiple unit-dose and multi-dose reservoir inhalers and nearly all developed concepts from this pioneer period (approx. 1970–1990) are currently still in use
Similar as from MDIs and nebulizers, but for different reasons, only a part of the dose from DPIs reaches the site of action. Hence, dry powder inhalation still has a great potential for improvement
The compliance with the instructions for correct DPI use and good adherence to the therapy should challenge designers of future DPIs to make incorrect use more difficult and inhaler performance more robust
The environmental impact of inhalation is currently under discussion, but device designers should watch out for making wrong decisions in DPI design to reduce their rather insignificant carbon footprint
Declaration of interest
The employer (University of Groningen, the Netherlands) of AH de Boer, P Hagedoorn and F Grasmeijer receives royalties from the sales of the Genuair and CyclopsTM dry powder inhalers. The employer (PureIMS, Roden, the Netherlands) of F Grasmeijer is the manufacturer of the CyclopsTM dry powder inhaler. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Dr. Karlheinz Seyfang and Dr. Marco Laackmann from Harro Hoefliger, Germany for their valuable information and interesting discussions about DPI (capsule and cartridge) filling and assembling.
Additional information
Funding
References
- Lynch AJ, Rowley CA. The history of grinding. Society for Mining, Metallurgy and Exploration Inc (SME). Littleton. Colorado; 2005.
- Gommeren HJC. Study of a closed-circuit jet mill plant using on-line particle size measurements. Delft The Netherlands: Delft University Press; 1997.
- American Pulverizing Corporation. Art of pulverizing US16-8717. 1926.
- American Pulverizing Corporation. Method of pulverizing minerals and similar materials US1948609. 1934.
- International Pulverizing Corporation. Method and apparatus for providing material in finely divided form. 1936. US2032827.
- Bell JH, Hartley PS, Cox JSG. Dry powder aerosols I: a new powder inhalation device. J Pharm Sci. 1971;60(10):1559–1564.
- Gamble JF, Chin W-S, Gray V. Investigation into the degree of variability in the solid-state properties of common pharmaceutical excipients – anhydrous lactose. AAPS PharmSciTech. 2010;11(4):1552–1557.
- Steckel H, Eskandar F, Borowski M, et al., Selecting lactose for capsule-based dry powder inhaler. PharmTech.com 2004: Cited Jan 2022. Available from: http://www.pharmtech.com/selecting-lactose-capsule-based-dry-powder-inhaler
- Guenette E, Barrett A, Kraus D, et al. Understanding the effect of lactose particle size on the properties of DPI formulations using experimental design. J Pharm Sci. 2009;380:80–88.
- de Boer AH, Hagedoorn P, Gjaltema D, et al. Air classifier technology (ACT) in dry powder inhalation part 4: performance of air classifier technology in the Novolizer multi-dose dry powder inhaler. Int J Pharm. 2006;310(1–2):81–89.
- Travers DN, White RC. The mixing of micronized sodium bicarbonate with sucrose crystals. J Pharm Pharac. 1971;23:261S.
- Boehringer Ingelheim Pharma. Method for producing powdery formulations. 2002.
- Coulson JM, Maitra NK. The mixing of solid particles. Ind Chem Manuf. 1950;26:55–60.
- Hersey JA. Ordered mixing: a new concept in powder mixing practice. Powd Technol. 1975;11(1):41–44.
- Lacey PMC. The mixing of solid particles. Trans Inst Chem Eng. 1943;21:53–59.
- Thiel WJ, Lai F, Hersey JA. Comments on ‘suggestions on the nomenclature of powder mixtures. Powd Technol. 1981;28(1):117–118.
- Staniforth JN. Order out of chaos. J Pharm Pharmacol. 1987;39(5):329–334.
- Staniforth JN. Total mixing.Int J Pharm Technol Prod Manuf. 1981;2:7–12
- Grasmeijer F, Grasmeijer N, Hagedoorn P. Recent advances in the fundamental understanding of adhesive mixtures for inhalation. Curr Pharm Design. 2015;21(40):5900–5914.
- Freundlich MM. Origin of the electron microscope. Sci. 1963;142:185–188.
- Bogner A, Jouneau P-H, Thollet G, et al. A history of scanning electron microscopy developments: towards ‘wet-STEM’ imaging. Micron. 2007;38(4):390–401.
- Fussel AL, Grasmeijer F, Frijlink HF, et al. CARS microscopy as a tool for studying the distribution of micronised drugs in adhesive mixtures for inhalation. J Raman Spectroscop. 2014;45(7):495–500.
- Stegemann S. Hard gelatin capsules today – and tomorrow. 2002. cited Jan 2002. Available from: https://cpsl-web.s3.amazonaws.com/kc/library/hard-gelatin-capsules-today-and-tomorrow.pdf
- Murdoch J. Capsules for protecting medicinal and other preparations. London, patent 11,937. 1847
- Jones BE. A history of DPI capsule filling. Inhalation. 2009;3:20–23.
- Jones BE. Pulmonary delivery & dry powder inhalers: advances in hard-capsule technology. ONdrugdelivery 2010; 16-18, cited Mar 2022. Available from: https://www.ondrugdelivery.com/wp-content/uploads/2018/11/Nov2010.pdf
- Al-Tabakha MM. HPMC capsules: current status and future prospects. J Pharm Pharmaceut Sci. 2010;13:128–142.
- Pinto JT, Wutscher T, Stankovic-Brandl M, et al. Evaluation of the physico-mechanical properties and electrostatic charging behavior of different capsule types for inhalation under distinct environmental conditions. AAPS PharmSciTech. 2020;21(128):1–10.
- Jolliffe IG, Newton JM, Cooper D. The design and use of an instrumented mG2 capsule filling machine simulator. J Pharm Pharmacol. 1982;34(4):230–235.
- Jolliffe IG, Newton JM. Practical implications of theoretical consideration of capsule filling by the dosator nozzle system. J Pharm Pharmacol. 1982;34(5):293–298.
- Janson C, Henderson R, Löfdahl M, et al. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax. 2020;75(1):82–84.
- Ramadan WH, Sarkis AT. Patterns of use of dry powder inhalers versus pressurized metered-dose inhalers devices in adult patients with chronic obstructive pulmonary disease or asthma: an observational comparative study. Chronic Respir Dis. 2017;14(3):309–320
- Duddu SP, Sisk SA, Walter YH, et al. Improved lung delivery from a passive dry powder inhaler using an engineered PulmoSpher powder. Pharm Res. 2002;19(5):689–695.
- Coates MS, Chan H-K, Fletcher DF, et al. Influence of air flow on the performance of a dry powder inhaler using computational and experimental analyses. Pharm Res. 2005;22(9):1445–1453.
- Coates MS, Chan H-K, Fletcher DF, et al. Influence of mouthpiece geometry on the aerosol delivery performance of a dry powder inhaler. Pharm Res. 2007;24(8):1450–1456.
- Schur J, Lee S, Adams W, et al. Effect of device design on the in vitro performance and comparability for capsule-based dry powder inhalers. AAPS J. 1012;14(4):667–676.
- Lavorini F, Pistolesi M, Usmani OS. Recent advances in capsule-based dry powder inhaler technology. Multidiscip Resp Med. 2017;12(11):2–7.
- Buttini F, Quarta E, Allegrini C, et al. Understanding the importance of capsules in dry powder inhalers. Pharmaceutics. 2021;13(11):1936.
- de Boer AH, Hagedoorn P, Hoppentocht M, et al. Dry powder inhalation: past, present and future. Expert Opin Drug Deliv. 2016;14(4):499–512.
- Wetterlin K. Turbuhaler: a new powder inhaler for administration of drugs to the airways. Pharm Res. 1988;5(8):506–508.
- Chrystyn H. The DiskusTM: a review of its position among dry powder inhaler devices. Int J Clin Pract. 2007;61(6):1022–1036.
- Grant AC, Walker R, Hamilton M, et al. The ®ELLIPTA Dry powder inhaler: design, functionality, in vitro dosing performance and critical task compliance by patients and caregivers. J Aerosol Med Pulm Drug Deliv. 2015;28(6):474–485.
- de Boer AH, Thalberg K. Dry powder inhalers (DPIs). In: Kassinos S, Bäckman P, Conway J, et al., editors. Inhaled medicines. London: Elsevier Academic Press; 2021:99–146.
- Vaidya S, Ziegler D, Tanase A-M, et al. Pharmacokinetics of mometasone furoate delivered via two dry powder inhalers. Pulm Pharmacol Ther. 2021;70:102019.
- Cazzola M, Cavalli F, Usmani OS, et al. Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. Exp Opin Drug Deliv. 2020;17(5):635–646.
- Steuer G, Prais D, Mussaffi H, et al. Inspiromatic-safety and efficacy study of a new generation dry powder inhaler in asthmatic children. Pediatr Pulmonol. 2018;53(10):1348–1355.
- Hirst PH, Newman SP, Clark DA, et al. Lung deposition of budesonide from the novel dry powder inhaler AirmaxTM. Resp Med. 2002;96(6):389–396.
- Dolovich M, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032–1045.
- DeHaan WH, Finlay WH. Predicting extrathoracic deposition from dry powder inhalers. Aerosol Sci. 2004;35(3):309–331.
- Usmani OS, Biddisombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of β2-agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497–1504.
- Heinemann L. The failure of Exubera: are we beating a dead horse? J Diabetes Sci Technol. 2008;2(3):518–529.
- Xiroudaki A, Schoubben A, Giovagnoli S, et al. Dry powder inhalers in the digitalization era: current status and future perspectives. Pharmaceutics. 2021;13(9):1455.
- Eskandar F, Lejeune M, Edge S. Low powder mass filling of dry powder inhalation formulations. Drug Developm Ind Pharm. 2011;37(1):24–32
- Sitz R. Current innovations in dry powder inhalers. ONdrugdelivery, 2010, https://www.ondrugdelivery.com/wp-content/uploads/2018/11/Nov2010.pdf Cited Apr 2022
- Angelo R, Rousseau Grant M, et al. Technosphere insulin: defining the role of Technosphere particles at the cellular level. J Diabetes Sci Technol. 2009;3(3):545–554.
- Valdes J, Shipley T, Rey JA. Loxapine inhalation powder (Adasuve). A new and innovative formulation of an antipsychotic treatment for agitation. P&t. 2014;39:621–623.
- de Boer AH, Gjaltema D, Hagedoorn P, et al. Comparative in vitro performance evaluation of the Novopulmon 200 Novolizer and Budesonid-Ratiopharm Jethaler: two novel budesonide dty powder inhalers. Pharmazie. 2004;59(9):692–699.
- Newman S, Malik S, Hirst P, et al. Lung deposition of salbutamol in healthy human subjects from the MAGhaler dry powder inhaler. Resp Med. 2002;96(12):1026–1032.
- Krishna BS, Nikhilesh GSS, Tarun B, et al. Industrial production of lactic acid and its applications. Int J Biotech Res. 2018;1. 43–54.
- Komesu A, Rocha de Oliveira JA, da Silva Martins LH, et al. Lactic acid production to purification: a review. BioResources. 2017;12(2):4364–4383.
- van den Oever M, Molenveld K, van der Zee M, et al. Bio-based and biodegradable plastics- facts and figures. Wageningen, Food & Biobased Research, report 1722
- Farooqi S, Ram S, Shehzadi A, et al. Lactic acid production using various raw materials and microorganisms. Annals Life Sci. 2019;5. 1–8.
- Wellenreuther C, Wolf A, Zander N. Cost competitiveness of sustainable bioplastic feedstocks – a Monte Carlo analysis for polylactic acid. 2022;Cleaner Eng Technol. 6:100411.
- Post PM, Hogerwerf L, Bokkers EAM, et al. Effects of Dutch livestock production on human health and the environment. Sci Total Environ. 2020; 737: 139702.
- Tonnis WT, Lexmond AJ, Frijlink HW, et al. Devices and formulations for pulmonary vaccination. Expert Opin Drug Deliv. 2013;10(10):1383–1397.
- de Boer AH, Hagedoorn P. The role of disposable inhalers in pulmonary drug delivery. Exp Opin Drug Deliv. 2015;12(1):143–157.
- van der Palen J, van der Valk P, Goosens M, et al. A randomised cross-over trial investigating the ease of use and preference of two dry powder inhalers in patients with asthma and chronic obstructive pulmonary disease. Exp Opin Drug Deliv. 2013;10(9):1171–1178.
- Zervas E, Samitas K, Gaga M. Assessment of satisfaction with different dry powder inhalation devices in Greek patients with COPD and asthma: the ANASA study. Int J COPD. 2016;11:1845–1855.
- Schreiber J, Sonnenburg T, Luecke E. Inhaler devices in asthma and COPD patients – a prospective cross-sectional study on inhaler preferences and error rates. BMC Pulm Med. 2020;20(1):222.
- Dellamary LA, Tarara TE, Smith DJ, et al. Hollow porous particles in metered dose inhalers. Pharm Res. 2000;17(2):168–174.
- Schur J, Price R, Lewis D, et al. From single excipients to dual excipient platforms in dry powder inhaler products. Int J Pharm. 2016;514(2):374–383.
- Buttini F, Brambilla G, Copelli D, et al. Effect of flow rate on in vitroaerodynamic performance of ®NEXThaler in comparison with ®Diskus and turbohaler® dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2016;29(2):167–178.
- Sørli JB, Sivans KB, Da Silva E, et al. Bile salt enhancers for inhalation: correlation between in vitro and in vivo lung effects. Int J Pharm. 2018;550(1–2):114–122.
- Shetty N, Cipolla D, Park H, et al. Physical stability of dry powder inhaler formulations. Expert Opin Drug Deliv. 2020;17(1):77–96.
- Edwards DA, Hanes J, Caponetti G. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868–1872.
- Coates MS, Fletcher DF, Chan H-K, et al. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics Part 1: grid structure and mouthpiece length. J Pharm Sci. 2004;93(11):2863–2876.
- de Boer AH, Hagedoorn P, Woolhouse R, et al. Computational fluid dynamics (CFD) assisted performance evaluation of the TwincerTM disposable high-dose dry powder inhaler. J Pharm Pharmacol. 2012;64(9):1316–1325.
- Walenga RL, Worth Longest P. Current inhalers deliver very small doses to the lower tracheobronchial airways: assessment of healthy and constricted lungs. J Pharm Sci. 2016;105(1):147–159.
- Koullapis P, Olsson B, Kassinos SC, et al. Multiscale in silico lung modelling strategies for aerosol inhalation therapy and drug delivery. Curr Opin Biomed Eng. 2019;11:130–136.
- Worth Longest P, Bass K, Dutta R, et al. Use of computational fluid dynamics deposition modelling in respiratory drug delivery. Expert Opin Drug Deliv. 2019;16(1):7–26.
- Kassinos S, Bäckman P, Conway J, et al., eds. Inhaled medicines. Optimizing development through integration of in silico, in vitro and in vivo approaches. London: Elzevier Academic Press; 2021.
- van Holsbeke C, De Backer J, Vos W, et al. Use of functional respiratory imaging to characterize the effect of inhalation profile and particle size in lung deposition of inhaled corticosteroid/long-acting β2-agonists delivered via a pressurized metered-dose inhaler. Ther Adv Respir Dis. 2018;12:1–15.
- Newhouse MT, Hirst PH, Dudda SP. Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest. 2003;124(1):360–366.
- Geller DE, Weers J, Heuerding S. Development of an inhaler dry-powder formulation of tobramycin using pulmospheretm technology. J Aerosol Med Pulm Drug Deliv. 2011;24(4):175–182.
- Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022.
- Staniforth JN. Carrier particles for use in dry powder inhalers. 2010;8(920):781.
- Begat P, Price R, Harris H, et al. The influence of force control agents on the cohesive-adhesive balance in dry powder inhaler formulations. Kona. 2005;23(1):109–121.
- Tian G, Worth Longest P, Li X, et al. Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. J Aerosol Med Pulm Drug Deliv. 2013;26(5):248–265.
- Corradi M, Chrystyn H, Corsio BG, et al. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclomethasone and formoterol to treat large and small airways in asthma. Exp Opin Drug Deliv. 2014;11(9):1497–1506.
- Dinh K, Myers DJ, Glazer M, et al. In vitro aerosol characterization of Staccato loxapine. Int J Pharm. 2011;403(1–2):101–108.
- Spyker DA, Cassella JV, Stoltz RR, et al. Inhaled loxapine and intramuscular lorazepam in healthy volunteers: a randomized placebo-controlled drug-drug interaction study. Pharm Res Per. 2015;3:e00194.
- Pritchard JN. The climate is changing for metered-dose inhalers and action is needed.Drug Design Dev Ther 2020; 14:3043–3055.
- Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. Prim Care Respir Med. 2017;27(1):22.
- Castel-Branco MM, Fontes A, Figueiredo IV. Identification of inhaler technique errors with a routine procedure in Portuguese community pharmacy. Pract Pharm. 2017;15:1072.
- Pessôa CL, Mattos MJ, Alho AR, et al. Most frequent errors in inhalation technique of patients with asthma treated at a tertiary care hospital. Einstein (São Paulo). 2019;17(2):eAO4397.
- Ngo CQ, Phan DM, Vu GV, et al. Inhaler technique and adherence to inhaled medications among patients with acute exacerbation of chronic obstructive pulmonary disease in Vietnam. Int J Environ Res Public Health. 2019;16(2):185.
- Verrips A, Hoogendoorn S, Jansema-Hoekstra, et al. The circular economy of plastics in the Netherlands. In: So W, Chow C, Lee J, editors. Environmental Sustainability and education for waste management. Singapore: Springer; 2019 https://org/10.1007/978-981-13-9173-6_4.
- Zheng J, Suh S. Strategies to reduce the global carbon footprint of plastics. Nat Climate Change. 2019;9(5):374–378.
- Wilkinson AJ, Braggins R, Steinbach I, et al. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open. 2019;9(10):e028763.
- United States Environmental Protection Agency (EPA) report ‘overview of greenhouse gases. 2022, Cited Apr 2022. Avilable from: https://www.epa.gov/ghgemissions/overview-greenhouse-gases
- Jason C, Henderson R, Löfdahl M, et al. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax. 2020;75(1):82–84.
- Solvay (Daikin) Solkane HFA-227 and HFA-134A datasheet cited Apr 2022. Avilable from: https://www.daikinchem.de/sites/default/files/pdf/Propellants/Daikin%20Propellants%20-%20Solkane%20227,%20134a%20pharma.pdf
- Carton W, Lund JF, Dooley K. Undoing equivalence: rethinking carbon accounting for just carbon removal. Front Clim. 2021. DOI:10.3389/fclim.2021.664130.
- Voulvoulis N, Kirkman R, Giakoumis T, et al. Examining material evidence, the carbon fingerprint, 2019, cited Apr 2022. Avilable from: www.imperial.ac.uk/media/imperial-college/faculty-of-natural-sciences/centre-for-environmental-policy/public/Veolia-Plastic-Whitepaper.pdf
- Shen M, Huang W, Chen M, et al. (Micro)plastic crisis: un-ignorable contribution to global greenhouse gas emissions and climate change. J Cleaner Prod. 2020;254:120138.
- Irfan M, Ahmad M, Fareed Z, et al. On the indirect environmental outcomes of COVID-19: short0term revival with futuristic long-term implications, 2021
- Mäkelä MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293.
- Parisini I, Cheng SJ, Symons DD, et al. Potential of a cyclone prototype spacer to improve in vitro dry powder delivery. Pharm Res. 2014;31(5):1133–1145.
- Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018; 19(1). 10.1186/s12931-017-0710-y
- Capanoglu M, Misirlioglu ED, Toyran M, et al. Evaluation of inhaler technique, adherence to the therapy and their effect on disease control among children with asthma using metered dose or dry powder inhalers. J Asthma. 2015;52(8):838–845.
- Sriram KB, Percival M. Suboptimal inhaler medication adherence and incorrect technique are common among chronic obstructive pulmonary disease patients. Chronic Respir Dis. 2016;13(1):13–22
- Suppli Ulrik C, Backer V, Søes-Petersen, et al. The patient’s perspective: adherence or non-adherence to asthma controller therapy? J Asthma. 2006;43(9):701–704.
- Bosley CM, Fosbury JA, Cochrane GM. The psychological factors associated with poor compliance with treatment in asthma. Eur Respir J. 1995;8(6):899–904.
- Lycett H, Wildman E, Raebel EM, et al. Treatment perceptions in patients with asthma: synthesis of factors influencing adherence. Respir Med. 2018; 141: 180–189.
- Haughney J, Barnes G, Partridge M, et al. The living & breathing study: a study of patients’ views of asthma and its treatment. Primary Care Respir J. 2004;13(1):28–35.
- Lexmond AJ, Hagedoorn P, Frijlink HW, et al. Prerequisites for a dry powder inhaler for children with cystic fibrosis. Plos One. 2017;12(8):e0183130.
- Luinstra M, Isufi V, de Jong L, et al. Learning from Parkinson’s patients: usability of the Cyclops dry powder inhaler. Int J Pharm. 2019;567:118493.
- Weers J. Inhaled antimicrobial therapy – Barriers to effective treatment.Advanced Drug Deliv Rev. 2015; 85:24–43.
- Konstan MW, Flume PA, Kappler M, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: the Eager trial. J Cyst Fibros. 2011;10(1):54–61.
- Hamed K, Conti V, Tian H, et al. Adherence to tobramycin inhaled powder vs inhaled solution in patients with cystic fibrosis: analysis if US insurance claims data. Patient Preference Adherence. 2017;11:831–838.
- Latchford G, Duff A, Quinn J, et al. Adherence to nebulised antibiotics in cystic fibrosis. Patient Edu Couns. 2009;75(1):141–144.
- Howell IB, Tugwell A, Bhaskaran D, et al. Adherence to nebulised therapies in people with cystic fibrosis starting Elexacaftor/Tezacaftor/Ivacaftor (Kaftrio). Thorax. 2021;76(Suppl 2):A40.
- CHMP’s assessment report for paediatric studies submitted according to Article 46 of the Regulation (EC) No 1901/2006 TOBI Podhaler. 533035. EMA. 2016.
- Anderson P. Patient preference for and satisfaction with inhaler devices. Eur Respir Rev. 2005;14(96):109–116.