?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Self-administration of subcutaneous interferon beta-1a (sc IFN β-1a) can be achieved with the RebiSmart® electromechanical autoinjector. This study investigated adherence to, and duration of persistence with, the newest version of the device (v1.6) among 2644 people receiving sc IFN β-1a for multiple sclerosis (MS).
Research design and methods
This retrospective, observational study utilized data from RebiSmart® devices, recorded on the MSdialog database, between January 2014 and November 2019. Adherence and persistence were evaluated over a 3-year period and assessed in relation to age, sex, injection type, and injection depth.
Results
The population of RebiSmart® users (N = 2644) comprised of 1826 (69.1%) females and mean age was 39 (range 16–83) years. Adherence to RebiSmart® use and data transfer to the MSdialog database was consistently high (mean 91.7%; range 86.8–92.6%), including across all variables (81.6–100%). Mean (±SD) persistence during the study period was 1.35 ± 1.06 years, with a maximum recorded persistence of 5.1 years. In multivariate analysis, the longest durations of persistence were observed among older individuals and males (p < 0.0001 and p = 0.0078, respectively).
Conclusions
People living with MS were highly adherent to use of the RebiSmart® device, with higher persistence generally observed for older and/or male individuals.
Plain Language Summary
It is important for people living with multiple sclerosis (MS) to take their medication regularly – and to keep doing so – in order to control their symptoms. Some people with MS receive a medication called interferon beta-1a (Rebif®) as a subcutaneous injection (given just under the skin), and the RebiSmart® electromechanical autoinjector was designed to help them to self-inject such medication. This study aimed to find out whether people were using the RebiSmart® device as often as they should be, and how long they continued to use it for. Information was taken from the MSdialog database, which recorded peoples' use of the RebiSmart® device between January 2014 and November 2019. Records for 2644 people using the device were analyzed. Results showed that the RebiSmart® device was used most of the time (around 91.7%). On average, people kept using the device for around a year and 4 months before stopping. This duration was generally longer for men compared with women, and longer for older people than younger people. These results increase our understanding of how people are using the RebiSmart® device to treat their MS symptoms.
1. Introduction
Multiple sclerosis (MS) is a chronic, inflammatory disease of the central nervous system that causes degenerative neurological and physical symptoms [Citation1]. Relapsing MS, characterized by periods of new or worsening symptoms separated by periods without disease progression, accounts for around 85% of all newly diagnosed cases [Citation2,Citation3].
For people living with MS, the extent to which they follow the prescribed treatment (adherence) and the period of consistent use of the prescribed treatment (persistence) can have important consequences for management of their disease. Indeed, higher adherence to disease-modifying treatment (DMT) for MS has been shown to be associated with a lower risk of relapse [Citation4,Citation5]. However, in common with other chronic conditions, previous studies have observed low rates of adherence with DMTs among people living with MS [Citation6], highlighting the need to monitor these rates and investigate potential factors affecting reduced adherence to, and persistence with, such treatment regimens [Citation7,Citation8]. One study, for example, found discontinuation to be more likely for those with a higher level of pre-treatment disability [Citation9], while another study found that treatment discontinuation was associated with factors such as an increased number of magnetic resonance imaging scans and relapses [Citation10].
Subcutaneous interferon beta-1a (sc IFN β-1a; Rebif®, Merck Europe, B.V., Amsterdam, the Netherlands) is a well-established, first-line DMT used in the treatment of relapsing MS with cumulative exposure of 1,908,836 patient-years to 30 September 2022 (Merck, data on file). Treatment is typically self-administered at a recommended dose of 44 μg three times weekly (tiw) [Citation11], over long-term treatment periods. The RebiSmart® electromechanical autoinjector (Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA) was developed to help people living with MS to self-administer such treatment, and studies show that it is associated with improved adherence and decreased rates of relapse and hospitalizations [Citation12–16]. The RebiSmart® device can be adjusted by the patient to suit their own preferences (such as injection depth, from 4 mm to 10 mm), with around 95% of users describing the device as ‘very easy’ or ‘easy’ to use [Citation17]. The device has evolved over time to meet the changing needs of people living with MS, as part of a thorough and continuous evaluation of whether the device is optimized for patient benefit. The current version (1.6), for example, was updated to incorporate a number of design and usability improvements identified in usability studies, such as adding on-screen instructions and reminders and providing more detailed instructions for cartridge storage, use, and disposal (Merck, data on file). Against this background, the current study evaluated whether this newest version of the RebiSmart® device allows for a high level of patient adherence and a prolonged duration of persistence with self-administered sc IFN β-1a over the normal usage duration for the device (i.e. 3 years). Further to this, the study evaluated whether adherence and persistence are influenced by factors such as age, sex, injection type (titrated or untitrated, with titrated dosing being recommended upon treatment commencement to minimize the risk of adverse events), and injection depth.
2. Patients and methods
2.1. Design and data source
This was a retrospective, observational study of people living with relapsing MS who were prescribed the RebiSmart® autoinjector for self-administration of sc IFN β-1a tiw. Those included in the study were from Argentina, Austria, Belgium, Canada, Chile, Colombia, England and Wales, Finland, France, Germany, Greece, Hungary, Israel, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland, and Taiwan.
Anonymized data on adherence and duration of persistence were taken from the MSdialog database between January 2014 and November 2019, to align with use of the newest version of the device (version 1.6). Those with mean monthly adherence <20% in the first months of use, signifying erratic usage, were excluded from further analysis.
2.2. Ethics statement
The study was purely observational and included only anonymized information contained in the MSdialog database. Signed informed consent was obtained from all participants prior to enrollment in the database, as a prerequisite for data collection and the creation of general reports based on anonymized data.
2.3. Endpoints
The primary endpoints were adherence to (%), and duration of persistence (years) with, the RebiSmart® device over the normal usage duration of one device (i.e. 3 years). Adherence was defined as the proportion of injections prescribed to a patient that were recorded as having been injected, per month. Adherence was recorded weekly, and monthly adherence was the mean average of weekly measurements.
Persistence was defined as the duration between first and last recorded use of the RebiSmart® autoinjector, in years. The first valid injection was recorded when the patient had ≥20% monthly adherence, while the last valid injection was recorded when the two subsequent months had 0% adherence. Factors assessed, in relation to adherence and persistence, were participant age, sex, injection type (titrated or untitrated), and injection depth (4–6 mm, 8 mm, or 10 mm). Regarding injection type, the recommended titration posology for sc IFN β-1a is that patients should be started at a dose of 8.8 µg tiw for 2 weeks and thereafter titrated to the target dose of 44 µg tiw (22 µg tiw can be used if patients cannot tolerate the higher dose).
2.4. Statistical analysis
Given the exploratory objective of the study, no adjustment for multiplicity was done. Adherence was summarized descriptively, and mean adherence was plotted over time. The proportions of participants with adherence >80% was modeled using a generalized estimating equation. Age (categorical), sex, injection type, and injection depth were included in the model to estimate odds ratios together with p values.
Persistence was summarized descriptively and plotted using the Kaplan–Meier method. A proportional hazard regression model (including the same factors listed above) was used to estimate hazard ratios and corresponding p values.
3. Results
3.1. Patients
Overall, 2644 RebiSmart® users were evaluated in the study. The study population comprised 1826 females (69.1%) and mean age was 39 (range 16–83) years. The largest number of records from RebiSmart® users were from Italy (29.1%), followed by Chile (12.2%), the Netherlands (10.1%), Canada (8.4%), Colombia (8.2%), and Portugal (6.1%). Initial injection depth at treatment commencement was most commonly 10 mm (59.2% [n = 1564]) or 8 mm (28.4% [n = 751]), and the first injection was an untitrated dose in 68.1% (n = 1801). In total, 99 participants were excluded from the study due to erratic usage of RebiSmart® with mean monthly adherence <20%. Data on numbers of participants who were treatment naïve or had prior experience with the RebiSmart® device were unavailable from the MSdialog database. Patient characteristics are shown in .
Table 1. Baseline characteristics and first intake characteristics at index for all patients providing RebiSmart® usage data.
3.2. Adherence
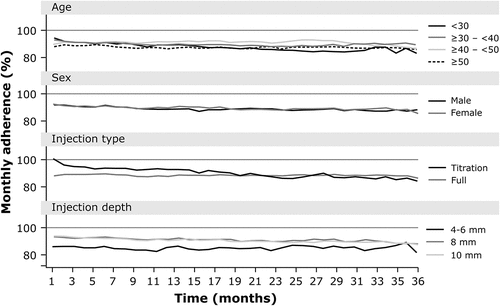
Mean adherence to use of the RebiSmart® device was 91.7% overall, ranging from 86.8% to 92.6% over the 3-year period ( and ). Mean adherence was similar across age groups, exceeding 90%, and between males (91.5%) and females (91.8%). Regarding injection-related aspects, mean adherence was higher for those who initiated on titrated dosing (95.1%) compared with untitrated dosing (90.1%); odds ratio 1.39, p = 0.0037 (multivariate analysis; and ). A trend was observed for higher levels of treatment adherence in the first 18 months in those receiving a titrated dose. Mean adherence was also higher for those using a 10 mm injection depth (92.7%) compared to those using a 4–6 mm injection depth (86.4%); odds ratio 0.53, p < 0.0001 (multivariate analysis; and ). Findings for univariate analysis of adherence data are shown in Supplementary Table S1.
Table 2. Average adherence to, and duration of persistence with, the RebiSmart® device by category.
Table 3. Multivariate analyses of the association of sex, age, injection type, and injection depth with rates of adherence to, and duration of persistence with, the RebiSmart® device.
3.3. Persistence
Mean (±SD) duration of persistence during the study period was 1.35 ± 1.06 years, with a maximum of 5.1 years (). A total of 9.1% of the original study population used the RebiSmart® device for the normal usage duration of 3 years.
Figure 2. Persistence probabilities over 3 years, according to A) Age, B) Sex, C) First injection type, and D) First injection depth.
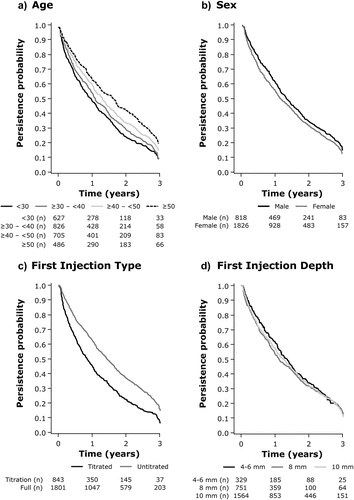
Older individuals had a longer duration of persistence than younger age groups ( and ). For example, mean (±SD) persistence was 1.60 ± 1.13 years in those aged ≥50 years compared with 1.13 ± 0.95 years in those aged <30 years (). Maximum persistence by age cohort followed a similar trend: 5.0, 5.1, 4.6, and 4.1 years, respectively, in descending age cohorts. In multivariate analysis, persistence was significantly higher in both the ≥50 years and ≥40–<50 age groups compared with those aged <30 years (hazard ratio: 0.6725 and 0.7679, respectively; both <0.0001) (). Male participants showed a longer duration of persistence (1.44 ± 1.06 years) than females (1.31 ± 1.06 years) (); hazard ratio, 0.8841 (p = 0.0078; ). Those using the untitrated injection type also had a greater duration of persistence (1.49 ± 1.09 years) than those using a titrated dose (1.06 ± 0.94 years (); hazard ratio, 1.4712 (p < 0.0001; )). However, durations of persistence were similar for all injection depths (1.35 ± 0.97 years for 4–6 mm, 1.28 ± 1.06 years for 8 mm, 1.39 ± 1.08 years for 10 mm, ), with no significant differences in multivariate analyses (). Findings for univariate analysis of persistence data are shown in Supplementary Table S1.
4. Discussion
Adherence to, and persistence with, treatment are important factors in the management of people living with MS, impacting disease-related and quality-of-life outcomes. Alongside prescribers, manufacturers have an important role to play in terms of supporting people living with MS to achieve high rates of treatment adherence and persistence, especially with the use of adjunctive devices. Whilst these factors can be difficult to monitor, the RebiSmart® electromechanical autoinjector – when paired with data transfer to the MSdialog registry – allows for simple data collection and analysis. In the current study, involving over 2500 RebiSmart® users, rates of monthly adherence with the RebiSmart® device were 86% or above over the normal usage duration of 3 years. This indicates that participants were typically complying with their prescribed dosage regimen, in accordance with previous studies on the RebiSmart® device [Citation12–15,Citation18]. A notable finding was that increased adherence was observed in those using a greater injection depth, which has not been previously studied in such a large population of RebiSmart® users. Such findings contrast with those of Moccia and colleagues [Citation19] who found that adherence to RebiSmart® was not associated with injection depth; but the study was performed in a much smaller population and categorical analysis of injection depth was not evaluated. A previous study also revealed that more patients using the RebiSmart® device had a preference for the 10 mm injection depth over the 8, 6, or 4 mm depths [Citation20], supporting our findings. The most likely explanation is that deeper injection may be less painful, and further studies are warranted to better understand the reasons for the greater overall adherence and preference for the 10 mm injection depth with the RebiSmart® device.
Results from this study of the RebiSmart® device indicated that persistence tended to wane over time. At 2 years after commencing treatment, more than half of participants had experienced at least a 2-month gap in use of the RebiSmart® device and MSdialog database. This may imply that these individuals were still using the RebiSmart® device but without sharing their data to the MSdialog system, or that they had changed/ceased treatment. While corresponding data for each of these categories were not available from the MSdialog database, a real-world study found that of 5956 people receiving treatment with sc IFN β-1a, 36.9% discontinued treatment at 1 year increasing to 55.8% at 3 years [Citation10]. Patients who discontinued sc IFN β-1a in the latter study had worse adherence or were taking additional medication at follow-up versus the overall population.
Participants used RebiSmart® and shared injection data for a longer total duration if they were male and/or in older age groups. There are many reasons why users might experience a treatment gap of 2 months or more (which would be registered as a discontinuation in this study), such as increased disease activity. Younger people living with MS are more likely to experience inflammatory disease activity, which might trigger a change in therapy. Indeed, previous studies have found that relapse rates, neurologist visits, and frequency of magnetic resonance imaging are generally higher in younger individuals [Citation21,Citation22]. The results of our study were supported by the findings of Allignol et al., who observed that treatment discontinuations were higher in younger vs older participants who may persist with their current treatment for longer durations due to reduced disease activity, rather than necessarily being an indication of successful treatment [Citation22]. Moreover, younger and female individuals are more likely to require treatment gaps as a result of pregnancy or attempting to become pregnant, before the recent change in the EU SmPC [Citation11]. Discontinuations in this analysis were greater overall (around 30–50% annually) compared with a previous analysis of the MSBase registry (Australia) in 2013. In the 2013 analysis, annual probability of discontinuation was 21.6% overall and 19.9% for those treated with sc IFN β-1a 44 μg [Citation23]. This difference might reflect the greater choice of therapies now available to people living with MS and, anecdotally, a lower tolerance of disease activity by physicians and, therefore, a more rapid escalation to other therapies.
A greater duration of persistence was observed in those using the untitrated dose compared with titrated dosing. However, those starting with a titrated dose are more likely to be newly initiating sc IFN β-1a, whereas users of untitrated dosing are more likely to have been using sc IFN β-1a for some time prior to the start of the evaluation period. Prior users may have been more likely to have already established that sc IFN β-1a is effective in controlling their relapses, providing a high likelihood of persistence. However, a limitation of this study is that information about previous therapies or whether participants were using sc IFN β-1a prior to January 2014 was not available from the MSdialog database. Other limitations of the study are that the data are correlative and do not indicate the reasons for treatment discontinuation/interruption or lack of adherence. Treatment interruptions of 2 months or more would have been recorded as a cease in persistence, and do not take into account those returning to sc IFN β-1a treatment after a break. This could have artificially lowered findings for duration of persistence. We also have to consider that side effects of local injection (e.g. pain) and injection anxiety – both of which may impact adherence and persistence – were not evaluated in the present study. That said, a previous study of users of RebiSmart® found that, by week 24 of treatment, 70% of participants were less fearful of injection compared with when they started and there was no relationship with adherence [Citation13]. This may be related to the fact that the device employs a concealed needle, which cannot be seen by the patient (either before or after injection), while the range of comfort settings allows for adjustment of needle speed and injection depth, for example, to minimize the potential for injection-site pain. Further studies would clearly be beneficial to provide a more in-depth analysis of these features and the potential impact on adherence and persistence with RebiSmart®, and how the device compares to other assistive devices used for MS therapy. For example, an online survey of patient (and MS nurse) preferences and perceptions regarding an upgraded version of the device noted that the overall reaction to the updated device was positive, and that it was rated higher than other self-injecting devices on all tested features [Citation24].
5. Conclusions
The RebiSmart® electromechanical autoinjector was generally associated with high rates of adherence, consistent with previous observations. The present study adds to these findings in terms of how long patients remain using the current RebiSmart® device and MSdialog system; indeed, a greater duration of persistence was observed for males and with increasing age of the user. Regarding injection-related aspects, a trend was also observed for greater adherence among those using deeper injections and with titrated dosing, the latter also being associated with a greater duration of persistence. Together, these results increase current understanding of use of the RebiSmart® device among people receiving sc IFN β-1a for MS.
Declaration of interest
F Piras and L Arnaud are employees of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA. E Henninger is an employee of Evi-Science, Geneva, Switzerland and provides statistical consultancy to Merck. M Keiser is a former employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA; current affiliation: EMD Serono, Inc., Rockland, MA, U.S.A, an affiliate of Merck KGaA. A Seitzinger is an employee of Merck Healthcare KGaA, Darmstadt, Germany. D Jack is an employee of Merck Serono Ltd., Feltham, UK, an affiliate of Merck KGaA. QL Masne is an employee of Merck Santé S.A.S., Lyon, France, an affiliate of Merck KGaA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Delivery for their review work, but have no other relevant financial relationships to disclose.
Author contributions
REB_RebiSmart_MDdialog_Manuscript_Suppl._Table_1.docx
Download MS Word (14 KB)Acknowledgments
Medical writing assistance was provided by Ruth Butler-Ryan of inScience Communications, Springer Healthcare Ltd., UK, and funded by Merck Healthcare KGaA, Darmstadt, Germany.
Data availability statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17425247.2023.2221432.
Additional information
Funding
References
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002 Apr 6;359(9313):1221–1231. doi: 10.1016/S0140-6736(02)08220-X
- McKay KA, Kwan V, Duggan T, et al. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. BioMed Res Int. 2015;2015:817238.
- The Multiple Sclerosis International Federation. Atlas of MS. 3rd Edition; Sep 2020. cited 2023 May. Available from: https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf
- Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89–100. doi: 10.2165/11533330-000000000-00000
- Sauri-Suarez S, Quinones-Aguilar S, Contreras-Marin A, et al. Adherence to self-administering interferon-β1a using RebiSmart® device in Mexican patients with relapsing multiple sclerosis. PLoS ONE. 2020;15(4):e0230959. doi: 10.1371/journal.pone.0230959
- Mardan J, Hussain MA, Allan M, et al. Objective medication adherence and persistence in people with multiple sclerosis: a systematic review, meta-analysis, and meta-regression. J Manag Care Spec Pharm. 2021;27(9):1273–1295. doi: 10.18553/jmcp.2021.27.9.1273
- Wong J, Gomes T, Mamdani M, et al. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci. 2011 May;38(3):429–433.
- Hansen K, Schussel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS ONE. 2015;10(7):e0133279. doi: 10.1371/journal.pone.0133279
- Moccia M, Palladino R, Carotenuto A, et al. Predictors of long-term interferon discontinuation in newly diagnosed relapsing multiple sclerosis. Mult Scler Relat Disord. 2016;10:90–96.
- Sabido-Espin M, Munschauer R. Reasons for discontinuation of subcutaneous interferon beta-1a three times a week among patients with multiple sclerosis: a real-world cohort study. BMC neurol. 2017 Mar 23;17(1):57. doi: 10.1186/s12883-017-0831-4
- Merck Europe B.V. Rebif (Summary of Product Characteristics). Amsterdam, The Netherlands; 2021. https://www.ema.europa.eu/documents/product-information/rebif-epar-product-information_en.pdf
- Bayas A, Ouallet JC, Kallmann B, et al. Adherence to, and effectiveness of, subcutaneous interferon beta-1a administered by RebiSmart® in patients with relapsing multiple sclerosis: results of the 1-year, observational SMART study. Expert Opin Drug Deliv. 2015 Aug;12(8):1239–1250.
- Devonshire VA, Feinstein A, Moriarty P. Adherence to interferon beta-1a therapy using an electronic self-injector in multiple sclerosis: a multicentre, single-arm, observational, phase IV study. BMC Res Notes. 2016;9:148.
- Edo Solsona MD, Monte Boquet E, Casanova Estruch B, et al. Impact of adherence on subcutaneous interferon beta-1a effectiveness administered by RebiSmart® in patients with multiple sclerosis. Patient Prefer Adherence. 2017;11:415–421.
- Lugaresi A, De Robertis F, Clerico M, et al. Long-term adherence of patients with relapsing-remitting multiple sclerosis to subcutaneous self-injections of interferon beta-1a using an electronic device: the RIVER study. Expert Opin Drug Deliv. 2016;13(7):931–935. doi: 10.1517/17425247.2016.1148029
- Rieckmann P, Ziemssen T, Penner IK, et al. Adherence to subcutaneous interferon beta-1a in multiple sclerosis patients receiving periodic feedback on drug use by discussion of readouts of their RebiSmart® injector: results of the prospective cohort study REBIFLECT. Adv Ther. 2022;39(6):2749–2760. doi: 10.1007/s12325-022-02100-w
- Devonshire V, Arbizu T, Borre B, et al. Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb study. BMC neurol. 2010;10(1):28. doi: 10.1186/1471-2377-10-28
- Lugaresi A, Florio C, Brescia-Morra V, et al. Patient adherence to and tolerability of self-administered interferon beta-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC neurol. 2012;12:7.
- Moccia M, Palladino R, Russo C, et al. How many injections did you miss last month? A simple question to predict interferon beta-1a adherence in multiple sclerosis. Expert Opin Drug Deliv. 2015;12(12):1829–1835. doi: 10.1517/17425247.2015.1078789
- Willis H, Webster J, Larkin AM, et al. An observational, retrospective, UK and Ireland audit of patient adherence to subcutaneous interferon beta-1a injections using the RebiSmart® injection device. Patient Prefer Adherence. 2014;8:843–851.
- Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368–1374. doi: 10.1136/jnnp.2008.145805
- Allignol A, Boutmy E, Sabido Espin M, et al. Effectiveness, healthcare resource utilization and adherence to subcutaneous interferon beta-1a according to age in patients with multiple sclerosis: a cohort study using a US claims database. Front Neurol. 2021;12:676585.
- Kalincik T, Spelman T, Trojano M, et al. Persistence on therapy and propensity matched outcome comparison of two subcutaneous interferon beta 1a dosages for multiple sclerosis. PLoS ONE. 2013;8(5):e63480. doi: 10.1371/journal.pone.0063480
- Colten S, Verdun Di Cantogno E, Jack D. Uncovering nurses’ and patients’ preferences and perceptions of Rebismart® 3.0 autoinjector versus other assistive devices in multiple sclerosis. Mult Scler Relat Disord. 2023;71(104259):10–11.