 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
This study aimed to confirm that the incremental dose/clicks system dispenses accurate doses for the Merck family of fertility pen injectors.
Research design and methods
Set doses (Vset) for three dose dial settings (minimum dose [Vmin], midpoint dose [Vmid] and maximum dose [Vmax] for the follitropin alfa, choriogonadotropin alfa [D2 classification: single use/variable dose], and follitropin alfa:lutropin-alfa 2:1 combination pen injectors) or a single Vset for the choriogonadotropin alfa (D1 classification: single use/single dose) were assessed. Last dose administered by the multi-dose device was assessed for the 900 IU, 450 IU, 300 IU and 150 IU follitropin alfa, and the 900:450 IU, 450:225 IU and 300:150 IU follitropin alfa:lutropin-alfa 2:1 combination pen presentations.
Results
Dose accuracy tests for Vmin, Vmid and Vmax for the follitropin alfa and the follitropin alfa:lutropin-alfa 2:1 combination pen injectors, and last dose administered, were within acceptable limits according to ISO 11,608-1:2012/2014. Dose accuracy tests for the single use/single dose device classification and the single use/variable dose device classification of the choriogonadotropin alfa pen injector were also within the acceptable limits, according to ISO 11608-1:2000/2014.
Conclusions
The Merck family of fertility pen injectors functions reliably and the incremental dose/clicks system dispenses accurate doses.
1. Introduction
Patients undergoing fertility treatments are regularly prescribed a combination of gonadotropins in the form of intra-muscularly or subcutaneously injected drugs as part of their medical treatment regimens [Citation1]. Historically, these injections were carried out using a syringe after reconstitution of a powder in a vial; however, pre-filled pen multi-dose injectors are now widely used, including the follitropin alfa pen injector 2.0 (recombinant human follicle stimulating hormone [r-hFSH]; GONAL-f®, Merck KGaA, Darmstadt, Germany) [Citation2–4], choriogonadotropin alfa pen injector 1.0 (recombinant human chorionic gonadotropin [r-hCG]; Ovidrel®, Merck KGaA, Darmstadt, Germany) [Citation5] and r-hFSH:lutropin alfa (r-hFSH:recombinant human luteinizing hormone [r-hLH] 2:1 combination pen injector 2.0; Pergoveris®, Merck KGaA, Darmstadt, Germany) [Citation6]. Each of the Merck family of gonadotropin pen injector devices features an incremental dose selector, with a standard volume of 0.02 mL delivered per click.
There are many factors contributing to the safety, efficacy and efficiency of fertility treatment delivery, and several key considerations should be taken into account during the selection and/or use of a particular gonadotropin injection device. These include the accuracy of dose delivery, ease of use, the potential for handling errors, patient preference, user acceptability and convenience [Citation7–12].
Needle-based injection systems (NIS) for medical use fall under International Organization for Standard (ISO) 11608–1, which was originally issued in 2000, with updated versions issued in 2012 and 2014 [Citation13]. These updates specify essential performance requirements and test methods for NIS intended to be used with needles and with replaceable or non-replaceable containers. The 2012 updates to ISO 11608–1 include additional dose accuracy pre-conditioning testing, such as dry heat storage, cold storage, and vibration. The dose accuracy acceptance criteria were also changed in the 2012 update. The dialing resolution now takes into account the absolute error for the lowest possible doses of multi-dose devices. Also, the dose accuracy limits are now determined from the drug labeling for single dose devices in which the entire volume is expelled. The changes implemented in 2014 did not have any impact on pen mechanical devices with integrated non-replaceable containers, such as Merck’s pre-filled multi-dose fertility pens.
Most health regulatory authorities require manufacturers to conduct the studies described in the ISO standard, which represent a benchmark for product reliability [Citation3], before the registration of a new or re-designed NIS. These studies include: design verification to ensure that the pen injectors meet all the technical requirements of the product design specification, ISO 11608–1, the FDA guidance [Citation14], and the European Pharmacopoeia 8.0; design validation to ensure that the user needs are fulfilled (e.g., human factors studies); and process validation to show that at the end of all production steps, including final assembly, the pen injector is working as intended according to the requirements of the product design specification and ISO 11608–1. Product validation results are usually reported, rather than design verification results, as the end-assembled product reflects the product in the market and is closest to the end-user.
Precise dosing is also an essential feature of NIS, as this ensures that patients receive the prescribed amount of medication, which should be individualized to optimize efficacy and safety [Citation2,Citation4–6] and minimize drug wastage [Citation3]. Dose accuracy tests are one such set of performance requirement tests specified in ISO 11608–1. Pen injector devices are designed to provide an accurate dose dial that is labeled in international units (IUs) or mass increments (μg), thereby avoiding the mixing and/or diluent measurements in mL that are required when using a standard syringe for single or multi-dose use [Citation15]. All three of the Merck family of fertility injector pens (r-hFSH pen injector 2.0 [Citation4], r-hCG pen injector 1.0 [Citation5] and r-hFSH:r-hLH 2:1 combination pen injector 2.0 [Citation6]) are filled by mass; therefore, increments/doses can be expressed as both μg and IUs.
The multi-dose r-hFSH pen injector 2.0 [Citation4] has a pre-filled cartridge and is used by adult women receiving fertility treatments involving stimulation of follicular growth and development for ovulation induction and assisted reproductive technologies (ART) (). The r-hFSH pen injector 2.0 can be used by the women themselves, their partners or healthcare professionals. It is also indicated in some countries for use in conjunction with human chorionic gonadotropin (hCG) to stimulate spermatogenesis in adult men with hypogonadotropic hypogonadism. The r-hFSH pen injector 2.0, which is available in four dosing presentations (150 IU, 300 IU, 450 IU and 900 IU), enables delivery of multiple injections, allowing pen users to dial the desired dose in 12.5 IU (0.92 μg) increments. The dose is dialed before each injection, with a maximum individual dose of 150 IU for the 150 IU presentation, 300 IU for the 300 IU presentation, and 450 IU for the 450 IU and 900 IU presentations [Citation3].
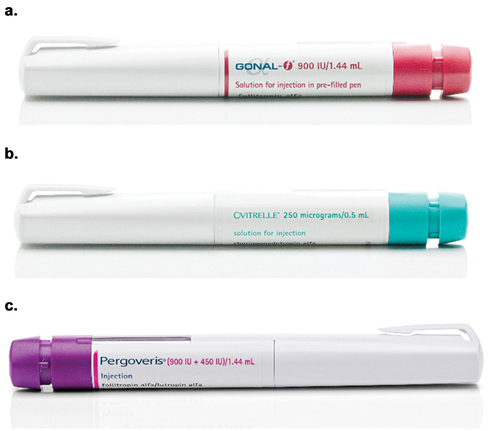
Figure 1. The Merck family of fertility pen injectors: the multi-dose r-hFSH pen injector 2.0 900 IU presentation (a), the r-hCG pen injector 1.0 (b) and the r-hFSH:r-hLH 2:1 combination pen injector 2.0 900 IU + 450 IU presentation (c). This figure is reprinted with permission of Merck KGaA, Darmstadt, Germany, who own the copyright of the images.
Previous product validation studies by Jeannerot et al. tested the dose accuracy of the three presentations of the r-hFSH pen injector 2.0 (300 IU, 450 IU and 900 IU) available at that time at the Bari manufacturing site according to the ISO 11608 version applicable at the time of testing (ISO 11608–1:2012/2014) [Citation3]. These studies showed that the r-hFSH pen injector 2.0 functions reliably, dispensing accurate doses under a range of cold, standard and warm atmospheres (ISO 11608–1:2012). It also delivered accurate doses subsequent to pen injector freefall, vibration, storage in either dry heat or the cold, and shipping pre-conditionings (ISO 11608–1:2014). These tests simulate the stresses that the device may be subjected to, for example being dropped (free-fall test), being stored in a fridge (cold storage) or its use in countries with hot climates (warm atmosphere). In addition, Christen et al. demonstrated the dose accuracy of the 300 IU, 450 IU and 900 IU presentations of an earlier version of the r-hFSH pen injector 2.0 under normal conditions at room temperature [Citation16].
The r-hCG pen 1.0 injector [Citation5] is used for ovulation triggering and luteinization in women with anovulation or oligo-ovulation and for final follicular development, ovulation triggering and luteinization following ovarian stimulation in women undergoing IVF treatment. This is a single dose, single use pen injector, delivering 250 μg r-hCG (approximately 6,500 IU/0.5 mL) ().
The r-hFSH:r-hLH 2:1 combination is indicated for the stimulation of follicular growth and development in adult women with severe FSH and LH deficiency. The r-hFSH:r-hLH 2:1 combination pen injector, available in three dosing presentations (900:450 IU, 450:225 IU and 300:150 IU) [Citation6], enables delivery of multiple injections, allowing pen users to dial the required dose in 12.5 IU:6.25 IU (0.92:0.28 μg) increments (). The dose is dialed before each injection, with a maximum dose of 300:150 IU for the 300:150 IU pen presentation and 450:225 IU for the 450:225 IU and 900:450 IU pen injector presentations.
Assessments of dose accuracy are very important, not only for health regulatory authorities, but also for healthcare providers and patients, to provide reassurance that the correct dose is being administered and wastage and treatment costs are minimized. The data previously published by Jeannerot et al. in 2016 and by Christen et al. in 2011 reported only the dose accuracy of the 300 IU, 450 IU and 900 IU presentations of the r-hFSH pen injector [Citation3,Citation16]. Since these data were published, the 150 IU pen injector has been developed, but dose accuracy studies for this new r-hFSH formulation have not yet been published. Furthermore, no data are publicly available on the dose accuracy of the r-hCG or r-hFSH:r-hLH pen injectors. Therefore, in the interest of full transparency, we have herein reported the results of the internal product validation studies carried out to further evaluate the dose accuracy of the latest presentations of the r-hFSH pen injector 2.0. These product validation studies, under standard atmospheric conditions, included the new 150 IU pen injector assembled at the manufacturing site in Bari, Italy, as well as the 300 IU, 450 IU and 900 IU pen presentations assembled at the manufacturing site in Aubonne, Switzerland. In addition, we report for the first time the assessments of dose accuracy for the r-hCG 1.0 pen injector and the r-hFSH:r-hLH 2:1 combination pen injector 2.0. These unpublished internal company data sets are reported herein to guarantee full transparency on the quality and reliability of the pen injectors, and the current article offers original and novel findings that build upon the earlier published reports. The motivation for publishing these data is to provide transparency and reassurance to the medico-scientific community, particularly the healthcare providers who prescribe these products, that the incremental dose/clicks systems are accurate in terms of equivalence of volume (dose) delivered, at standard atmospheric conditions, and confirm the dose accuracy product validation for the comprehensive family of Merck fertility pens using the most contemporary data.
2. Patients and methods
2.1. General methods
The focus of these studies was dose accuracy measurement testing carried out under standard atmospheric conditions (temperature: 23 ± 5°C; relative humidity: 50 ± 25%), as described in ISO 11608–1, for each of the Merck family of gonadotropin pen injectors and their presentations. These laboratory-based dose accuracy evaluations were performed at Haselmeier GmBH, Stuttgart, Germany, from 2015 to 2020, in accordance with the ISO version valid at the time of testing, following final assembly of the pen injectors at the manufacturing sites at Bari, Italy, or Aubonne, Switzerland.
Three dose dial settings were assessed for each pen, minimum dose (Vmin), midpoint dose (Vmid) and maximum dose (Vmax). The last dose administered by the multi-dose device was assessed for the 900 IU, 450 IU, 300 IU and 150 IU r-hFSH pen injector 2.0 presentations, and the 900:450 IU, 450:225 IU and 300:150 IU r-hFSH:r-hLH 2:1 combination pen injector 2.0 presentations. As required by the standard ISO 11608–1:2012/2014, the last dose accuracy was assessed on doses equal to 0.4 mL ±10%, i.e., on doses from 0.36 mL to 0.44 mL. Since the content of the 150 IU presentation for the r-hFSH pen injector 2.0 is 0.24 mL, which is lower than 0.4 mL, the last dose accuracy was determined on doses of 0.2 mL ±10%, i.e., on doses from 0.18 mL to 0.22 mL.
Pre-conditioning testing at cool and warm atmospheres, dry heat storage, cold storage, free-fall and vibration was also carried out for the 900 IU, 300 IU and 150 IU presentations of the r-hFSH pen injector 2.0, for the 900:450 IU and 300:150 IU presentations of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 and for the r-hCG pen injector 1.0 (Supplementary Table S1). Because the only difference between the 450 IU and 900 IU presentations of the r-hFSH pen injector 2.0 and the r-hFSH:r-hLH 2.0 pen injector is the fill volume of the cartridge assembled in the pen, these pre-conditioning tests were not performed on the 450 IU presentation of these devices.
Pen injectors containing either the drug (all r-hFSH:r-hLH 2:1 combination pen injector 2.0 presentations and the r-hCG pen injector 1.0) or placebo (all r-hFSH pen injector 2.0 presentations) were tested, as it has been demonstrated that the placebo used to represent r-hFSH (‘follitropin-alfa placebo’) has the same physical characteristics (density, viscosity and surface tension) as follitropin-alfa [Citation3].
2.2. Dose accuracy and last extractable dose of the r-hFSH pen injector 2.0 at standard atmospheric conditions (Study 1A, Aubonne, Switzerland and Study 1B, Bari, Italy)
A product validation study (Study 1A), was performed to confirm dose accuracy for the 900 IU, 450 IU and 300 IU presentations of the r-hFSH pen injector 2.0 after final assembly at the Merck Biopharma production site in Aubonne, Switzerland, according to the requirements of ISO 11608–1:2012, which was the version valid at the time of testing (August 2015). A subsequent assessment was done in the Verification and Validation Summary Report of Fertility pen injector 2.0 to show that the results were still relevant for ISO11608–1:2014. This study compliments a previously published product validation study on dose accuracy on the r-hFSH pen injector 2.0, after final assembly at Merck Biopharma production site in Bari, Italy [Citation3].
Another product validation study (Study 1B) was performed to confirm dose accuracy for the 150 IU presentation of the r-hFSH pen injector 2.0, after final assembly at Merck Biopharma production site in Bari, Italy, according to the requirements of ISO 11608–1:2014, which was the version valid at the time of testing (October 2017).
The four presentations of the r-hFSH pen injector 2.0 (900 IU, 450 IU, 300 IU and 150 IU) were tested, at a holding time of 5 seconds, using BBraun Pencylcap 29 G x 12 mm needles. Dose accuracy testing was performed on the four pen presentations for the three set doses (Vmin, Vmid and Vmax). Vmin for all the pen presentations was 12.5 IU. Vmid for the 900 IU and 450 IU presentations was 237.5 IU, Vmid for the 300 IU presentation was 162.5 IU, and Vmid for the 150 IU presentation was 87.5 IU. Vmax for the 900 IU and 450 IU presentations was 450 IU, Vmax for the 300 IU presentation was 300 IU, and Vmax for the 150 IU presentation was 150 IU (). Sixty measurements were carried out for each Vset for each pen presentation.
Table 1. r-hFSH pen injector 2.0, r-hFSH:r-hLH 2:1 combination pen injector 2.0 and r-hCG pen injector 1.0 target dose volume, and lower and upper specified limits, for each dial setting assessed (Studies 1–5).
In total, 410 devices were tested for the 900 IU presentation, 180 devices were tested for the 450 IU presentation, 742 devices were tested for the 300 IU presentation, and 180 devices were tested for the 150 IU presentation. Further details on the testing methodology have been reported by Jeannerot et al. [Citation3], including how dispensed volumes were measured gravimetrically using a calibrated balance.
Data analysis test specifications for the r-hFSH pen injector 2.0 were carried out as published by Jeannerot et al. [Citation3] and summarized in .
2.3. Dose accuracy of the r-hCG pen injector 1.0 at standard atmospheric conditions (D1 device classification: single use/single dose device) (Study 2, Bari, Italy)
Study 2, carried out in March 2018, was a product validation study to confirm the dose accuracy of the r-hCG pen injector 1.0 as a D1 device classification (single use/single dose device as per label). This was conducted at the end of all production steps, including final assembly, at the Merck Biopharma production site in Bari, Italy, according to the requirements of ISO 11608–1:2000 and the product design specifications. Test methods were applied to the r-hCG pen injector 1.0, at a holding time of 5 seconds, using BBraun Omnican fine 29 G x 12 mm needles. As this is a test for single use/single dose classification, only a single Vset was assessed (defined as 25 increments [0.05 mL]) corresponding to 6,500 IU (250 µg). In total, 210 devices were tested at standard atmospheric conditions. Details of the test specifications are described in .
2.4. Dose accuracy of the r-hCG pen injector 1.0 at standard atmospheric conditions (D2 device classification: single use/variable dose device) (Study 3, Aubonne, Switzerland)
The purpose of Study 3 was to confirm the dose accuracy of the r-hCG pen injector 1.0 as a D2 (single use/variable dose) device classification. This was conducted in April 2019, at the end of all production steps, including final assembly, at the Merck Biopharma production site in Aubonne, Switzerland, according to requirements of ISO 11608–1:2014 and the product design specifications. These tests were carried out as part of a health authority request for a specific off-label indication for use of the r-hCG pen injector 1.0 classified as D2 device under ISO 11608–1:2014, to determine a temporary recommendation for the use of r-hCG in male infertility. The test methods were applied to the r-hCG pen injector 1.0 at a holding time of 5 seconds, using BBraun Omnican fine 29 G x 12 mm needles.
Vmin was defined as 1 increment (260 IU/10 μg), Vmid was defined as 13 increments (3,380 IU/130 μg) and Vmax was defined as 25 increments (6,500 IU/250 μg). Dose accuracy tests were performed on 180 devices (60 for Vmin, 60 for Vmid, and 60 for Vmax) under standard atmospheric conditions. Details of the test specifications are reported in .
2.5. Dose and last extractable dose accuracy of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 at standard atmospheric conditions (Study 4, Bari, Italy)
Study 4 was a product validation study to confirm the dose accuracy of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 after final assembly at the Merck Biopharma production site in Bari, Italy. Study 4 was conducted in March 2016 according to the requirements of ISO 11608–1:2012, which was the version valid at the time of testing. An assessment was also done in the Verification and Validation Summary Report of Fertility pens 2.0 to show that the results are still relevant per ISO 11608–1:2014.
Dose accuracy testing was performed on three pen presentations (900 IU:450 IU, 450 IU:225 IU, and 300 IU:150 IU) for the three Vset values at a holding time of 5 seconds, using BBraun Pencylcap 29 G x 12 mm needles. Vmin for all the pen presentations was 12.5 IU. Vmid for the 900:450 IU and 450:225 IU presentations was 237.5:118.75 IU, and Vmid for the 300:150 IU presentation was 162.5:81.25 IU. Vmax for the 900:450 IU and 450:225 IU presentations was 450:225 IU, and Vmax for the 300:150 IU presentation was 300:150 IU. Sixty measurements were carried out for each Vset for each pen presentation. In total, 410 devices were tested for the 900:450 IU presentation, 180 for the 450:225 IU presentation and 742 for the 300:150 IU presentation.
Data analysis test specifications for the r-hFSH:r-hLH 2:1 combination pen injector 2.0 were carried out as published by Jeannerot et al. [Citation3] and are reported in .
2.6. Dose and last extractable dose accuracy of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 at standard atmospheric conditions (Study 5, Aubonne, Switzerland)
Study 5 was a product validation study to confirm the dose accuracy of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 after final assembly at the Merck Biopharma production site in Aubonne, Switzerland, according to the requirements of ISO 11608–1:2014.
Dose accuracy testing was performed on three r-hFSH:r-hLH 2:1 combination pen injector 2.0 presentations (900:450 IU, 450:225 IU and 300:150 IU) for the three Vsets at holding time of 5 seconds, using BBraun Pencylcap 29 G x 12 mm needles. The test volumes were the same as those detailed for testing at the Merck Biopharma production site in Bari, Italy (see Section 2.5).
Data analysis test specifications for the r-hFSH:r-hLH 2:1 combination pen injector 2.0 were carried out as published by Jeannerot et al. [Citation3] and are reported in .
2.7. Statistics and tolerance limit calculations and acceptance criteria
The tests and methodology described in the current article were performed according to those requested by regulatory authorities for medical devices. The tests of dose accuracy followed the internationally harmonized standard ISO 11608. The test and sampling methods, pre-conditioning criteria and other aspects of testing specified in this ISO are intended to verify the design of the pen injector at a high confidence level, even going beyond dose accuracy. The tests and methodology used in the article consider all requirements for functional consistency.
The tolerance limit factor (k) is a statistical figure based on the number of samples under test, the confidence level (γ) and the probability content (p). It is used to calculate the tolerance limits of dose accuracy using the formulas () and
), where
is the average value of the tested samples
is the standard deviation of the tested population
k is the tolerance limit factor, which depends on the number of samples under test, the confidence level (γ) and the probability content (p)
As stated in ISO 11608–1, () shall be higher than the lower specification limit (LSL) of dose accuracy defined for the pen and
) shall be lower than the upper specification limit (USL) of dose accuracy defined for the pen. ktar is the target k value, which is defined in ISO 11608–1 (for example, ktar = 2.670 for N = 60 samples, with a probability content of 97.5% at a 95% confidence level). kact is the k value calculated from the actual dose accuracy measurements performed on the pens. It is described as the lower of the two values defined by ([USL − X]/standard deviation [SD]) and (X − LSL]/SD); whereby X = the mean value of the collected volume. Having (
) ≥ LSL, (
) ≤ USL, and kact ≥ ktar ensures, at γ % confidence level that at least, p % of the population of samples under test falls within the tolerance limits. In summary, the pen population satisfies the requirements for accuracy when, for each Vset (i.e Vmin, Vmid and Vmax), the following criteria are fulfilled:
3. Results
3.1. Dose accuracy testing at standard atmospheric conditions for all pen presentations
Dose accuracy tests were performed for Vmin, Vmid and Vmax on all presentations of the r-hFSH pen injector 2.0, the r-hCG pen injector 1.0, and the r-hFSH:r-hLH 2:1 combination pen injector 2.0. Assessment of the last dose administered by the multi-dose device and cartridge was performed for the 150 IU, 300 IU, 450 IU and 900 IU presentations of the r-hFSH pen injector 2.0, and the 300:150 IU, 450:225 IU, and 900:450 IU presentations of the r-hFSH:r-hLH 2:1 combination pen injector 2.0. The results of all dose accuracy tests were within the acceptable limits defined in ISO 11608–1 and are summarized below (Supplementary Table S2).
3.2. Dose accuracy of the r-hFSH pen injector 2.0 at standard atmospheric conditions (Study 1A, Aubonne, Switzerland and Study 1B, Bari, Italy)
All pen injectors passed visual inspection. All doses dispensed from the 150 IU, 300 IU, 450 IU, and 900 IU presentations of the r-hFSH pen injector 2.0 were within the acceptance limits defined in ISO 11608–1:2012/2014, under standard atmospheric conditions ( and ). kact was greater than ktar for all doses for all presentations. The mean deviation from the actual dose was low for all doses investigated. Last extractable dose accuracy results (last dose error) for each injector pen presentation are presented in Supplementary Table S3.
Figure 2. Summary diagram of Vmin, Vmid, Vmax for r-hFSH pen injector 2.0 (a) 150 IU*, (b) 300 IU†, (c) 450 IU†, (d) 900 IU† (Study 1A, aubonne, Switzerland and Study 1B, Bari, Italy). The orange lines/dots represent the upper specification limits, and the gray lines/dots represent the lower specification limits. Error bars are calculated as ktar*SD. *Final assembly at the Merck Biopharma production plant at Bari, Italy. †Final assembly at the Merck Biopharma production plant at aubonne, Switzerland.
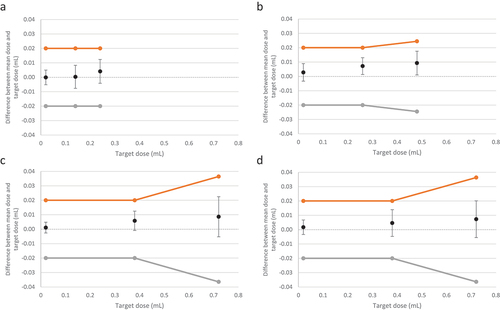
Table 2. The r-hFSH pen injector 2.0 mean actual doses and deviations from target dose (Study 1A, Aubonne, Switzerland and Study 1B, Bari, Italy).
3.3. Dose accuracy of the r-hCG pen 1.0 injector under standard atmospheric conditions (D1 device classification: single use/single dose device) (Study 2, Bari, Italy)
The devices used for the single use/single dose device classification (D1) of the r-hCG pen injector 1.0 passed visual inspection. Dose accuracy tests showed that the Vset was within the acceptable limits according to ISO 11608–1: 2000 under standard atmospheric conditions (; Supplementary Figure S1). kact was greater than ktar, and the mean deviation from the actual dose was low.
Table 3. Mean actual doses and deviations from target dose for r-hCG pen 1.0 injector (Study 2, Bari, Italy [D1 device classification] and Study 3, aubonne, Switzerland [D2 device classification]).
3.4. Dose accuracy of the r-hCG pen injector 1.0 at standard atmospheric conditions (D2 device classification: single use/variable dose device) (Study 3, Aubonne, Switzerland)
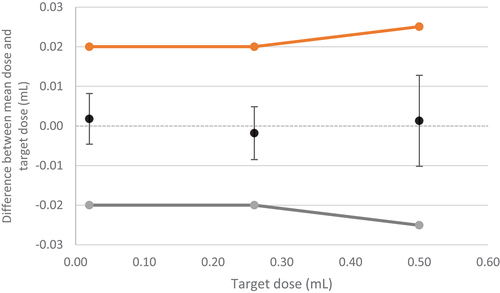
The devices used for the single use/variable dose device classification (D2) of the r-hCG pen injector 1.0 passed visual inspection. Dose accuracy tests showed that the Vmin, Vmid and Vmax were within the acceptable limits defined in ISO 11608–1:2014, under standard atmospheric conditions ( and ). kact was greater than ktar, and the mean deviation from the actual dose was low.
Figure 3. Summary diagram of Vmin, Vmid, Vmax for the r-hCG pen injector 1.0 (D2 device classification: single use/variable dose device) (Study 3, Aubonne, Switzerland). The orange lines/dots represent the upper specification limits, and the gray lines/dots represent the lower specification limits. Error bars are calculated as ktar*SD. Device tested at the Merck Biopharma production plant at Bari, Italy.
3.5. Dose and last extractable dose accuracy of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 under standard atmospheric conditions (Study 4, Bari, Italy)
All pen injectors tested at the production site in Bari, Italy, passed visual inspection. Dose accuracy tests for Vmin, Vmid and Vmax for the 900:450 IU, 450:225 IU and 300:150 IU presentations of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 were within the acceptance limits defined in ISO 11608–1:2012 under standard atmospheric conditions ( and ). kact was greater than ktar for all doses for all presentations. The mean deviation from the actual dose was low for all doses investigated. Last extractable dose accuracy results (last dose error) for each injector pen presentation are presented in Supplementary Table S4.
Figure 4. Summary diagram of Vmin, Vmid, Vmax for the r-hFSH:r-hLH 2:1 combination pen injector 2.0 (A) 900:450 IU, (B) 450:225 IU, (C) 300:150 IU (Study 4, Bari, Italy). The orange lines/dots represent the upper specification limits, and the gray lines/dots represent the lower specification limits. Error bars are calculated as ktar*SD. Devices tested at the Merck Biopharma production plant at Bari, Italy.
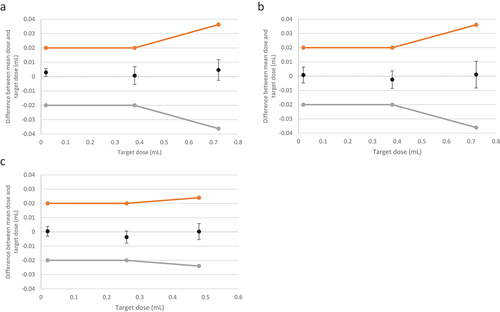
Table 4. Mean actual doses and deviations from target dose for r-hFSH:r-hLH 2:1 combination pen injector 2.0 (Study 4, Bari, Italy).
3.6. Dose and last extractable dose accuracy of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 at standard atmospheric conditions (Study 5, Aubonne, Switzerland)
All pen injectors tested at the production site in Aubonne, Switzerland, passed visual inspection. Dose accuracy tests for Vmin, Vmid and Vmax for the 900:450 IU, 450:225 IU, and 300:150 IU presentations of the r-hFSH:r-hLH 2:1 combination pen injector 2.0 were within the acceptance limits defined in ISO 11608–1:2014, under standard atmospheric conditions (; Supplementary Figure S2). kact was greater than ktar for all doses for all presentations. The mean deviation from the actual dose was low for all doses investigated. Last extractable dose accuracy (dose error) results for each injector pen presentation are presented in Supplementary Table S5.
Table 5. Mean actual doses and deviations from target dose for r-hFSH:r-hLH 2:1 combination pen injector 2.0 (Study 5, Aubonne, Switzerland).
3.7. Results summary
Irrespective of the fertility pen, the variant, or the manufacturing site, dose accuracy results show that kact value is always significantly above the targeted k value defined by the standard. Therefore, this kact value provides a high level of confidence with regards to dose accuracy and shows that the performance of the pen is higher than the standard requirements.
4. Discussion
The aim of this paper was to report the results of five studies on the dose accuracy of the Merck family of fertility pen injectors, comprising the r-hFSH pen injector 2.0 (900 IU, 450 IU, 300 IU, and 150 IU presentations), the r-hCG pen injector 1.0 (6,500 IU presentation), and the r-hFSH:r-hLH 2:1 combination pen injector 2.0 (900:450 IU, 450:225 IU, and 300:150 IU presentations). The tests and methodology described in the current article were performed according to those requested by regulatory authorities for medical devices. The tests of dose accuracy followed the internationally harmonized standard ISO 11608. The test and sampling methods, pre-conditioning criteria and other aspects of testing specified in this ISO are intended to verify the design of the pen injector at a high confidence level, even going beyond dose accuracy. The tests and methodology used in the article consider all requirements for functional consistency.
As reported herein, all three fertility pen injectors functioned reliably, and the incremental dose/clicks system dispensed accurate doses. All presentations of each pen injector passed the acceptance criteria specified within the ISO standard, and the last extractable dose supported the claimed volume label for all pen injectors tested. The results of these five studies provide users with confidence that they can accurately administer the prescribed dose in locations outside of a clinic environment (i.e. at home or at work), thereby minimizing wastage and reducing treatment costs. Furthermore, the convenience of not attending clinics, enabled by the use of multi-dose, multi-use pens, will hopefully be beneficial to patients and also reduce the burden on patients and healthcare professionals, both of which are more important than ever in light of the disruption to fertility services due to the COVID-19 pandemic.
Jeannerot et al. investigated the dose accuracy of the 300 IU, 450 IU, and 900 IU presentations of the r-hFSH pen injector 2.0, after final assembly at the Merck Biopharma production site in Bari, Italy [Citation3]. The r-hFSH pen injector 2.0 was demonstrated to dispense accurate doses under a range of various conditions, including: standard, cool and warm atmospheres; after free fall, preconditioning with vibration, dry heat and cold storage and regardless of the dispense force of the pen injector. Dosing accuracy was also maintained following packaging, transportation, and simulated storage for 2 years. In addition, the Pencylcap needles maintained their integrity throughout these processes [Citation3]. Furthermore, in an earlier study, the dose accuracy of the 300 IU, 450 IU and 900 IU presentations of a previous version of the r-hFSH pen injector 2.0 was confirmed under normal conditions at room temperature [Citation16]. Our results presented here are consistent with these previous product validation studies of the r-hFSH pen injector 2.0, and show that this device can administer accurate doses regardless of the use of different versions of the pen presentations, different manufacturing sites or different ISO standards. Our decision to publish this company data stems from our commitment to guarantee full transparency on the quality and reliability of the pen injectors for the benefit and reassurance of the final users (i.e. patients and clinicians). As such, the current article offers original and novel findings based on the most up-to-date data on the 150 IU dose of the r-hFSH pen injector 2.0, the dose accuracy of the r-hCG and r-hFSH:r-hLH pen injectors, and the dose accuracy (D2 device classification; single-use/variable dose device) of the r-hCG pen injector at min, medium and max increments. These new data build upon the earlier published reports and provide a high level of reassurance that the volume delivered by each of the three pens corresponds to the drug dose in IU or μg, in compliance with the relevant ISO standards. The r-hFSH pen injector 2.0 has been well accepted by the majority of infertile patients and infertility nurses, as it was perceived as easy to teach, easy to use and easy to learn. Notably, 94% of patients preferred the pen to a reconstitution and conventional needle and syringe method [Citation7]. Additionally, simulated-use studies confirmed that the r-hFSH pen injector 2.0 was easy to use by both patients and healthcare professionals [Citation12,Citation17]. Ninety-two per cent of women deemed it easy to learn to fit the needle, and 90% of women with infertility agreed that they would recommend the pen injector to their friends and family if they required IVF treatment [Citation12]. Similarly, 93% of the nurses found the redesigned fertility pen injector easy to use and believed it would be easy to teach. While more than 80% found each of the steps easy, most found the steps easier to teach than expected, and 97% would recommend the redesigned fertility pen injector to a colleague [Citation17]. The usability of the r-hCG pen injector 1.0 was evaluated in a study by Saunders et al. [Citation18]. The r-hCG pen injector 1.0 was rated favorably by women with infertility and specialist nurses for its ease-of-use; furthermore, the reported risk of dosing errors was not higher with the r-hCG pen injector 1.0 compared with the existing r-hCG prefilled syringe [Citation18].
The r-hFSH alfa originator pen injector 2.0 was rated significantly higher than a single-use biosimilar r-hFSH alfa pen injector, a reusable biosimilar r-hFSH pen injector with cartridge, and the reusable r-hFSH delta pen injector with cartridge in terms of overall handling, general feeling on safe handling, dosage selection and correction, performing the injection, and overall preference. Rates of total handling errors were significantly lower with the r-hFSH alfa originator pen injector 2.0 pen injector than with the other three pen injectors (p < 0.001 for all comparisons vs. r-hFSH pen injector 2.0) [Citation9]. While a more recent study reported that the single-use pen injector was associated with fewer critical handling errors compared with the multi-use r-hFSH pen injectors, it should be acknowledged that there are fewer steps during which critical handling errors can be made with single-use devices compared with multi-use pens [Citation19].
The five studies reported here highlight the substantial amount of testing that has been conducted on the Merck family of fertility pens, including new presentations, with the dose accuracy tested under standard atmospheric conditions. However, it should be noted that these assessments were carried out by laboratory scientists, who are not the intended users of the pen injectors, and the injections were not delivered into human tissue but tested in a laboratory environment; therefore, although these conditions meet the international standards, they do not provide information on the real-world use of the pen injectors by patients.
5. Conclusion
The data from the validation studies performed according to the relevant ISO standards that were valid at the time of testing demonstrated that the r-hFSH pen injector 2.0, the r-hFSH:r-hLH 2:1 combination pen injector 2.0, and the r-hCG pen injector 1.0 performed as per the state-of-the-art specifications under all the required conditions. The incremental dose/clicks systems were accurate in terms of equivalence of volume (dose) delivered for the complete Merck family of fertility pen injectors, providing re-assurance to healthcare providers and patients alike on dose delivery, flexibility of gonadotropin administration, and reduction in drug wastage and treatment costs. The results reported here complement previous reports of the Merck r-hFSH multi-dose pen injectors that showed these devices to function reliably under the range of different conditions studied [Citation3,Citation16]. Furthermore, the contemporary data reported in the present study builds on and reinforces previously published evidence showing that the Merck family of fertility pens were user-friendly, had lower error and mishandling rates than other pen injectors, and they were perceived as being easy to teach, easy to learn, and were well-accepted by intended users [Citation7,Citation9,Citation12,Citation17,Citation18].
Author contributions
All authors were involved in the conception and design, or analysis and interpretation of the data; the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Declaration of interest
M Lispi and T D’Hooghe are employees of Merck Healthcare KGaA, Darmstadt, Germany. S Longobardi is an employee of Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany. At the time of the study, E Cottell was also an employee of Merck Healthcare KGaA, Darmstadt, Germany. D Michalet, TD Araujo and R Gleixner are employees of Ares Trading SA, Aubonne, Switzerland, an affiliate of Merck KGaA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A peer reviewer on this manuscript have received an honorarium from Expert Opinion on Drug Delivery for their review work. Another peer reviewer on this manuscript has disclosed that they were an unpaid, invited, consulting author on efficacy for one of the Merck studies. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Availability of data and materials
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck KGaA’s Data Sharing Policy. All requests should be submitted in writing to Merck KGaA’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavor to gain agreement to share data in response to requests.
Dose accuracy supplementary material_150124.docx
Download MS Word (60.4 KB)Acknowledgments
Medical writing assistance was provided by Steven Goodrick and Helen Brereton, inScience Communications, Springer Healthcare Ltd, London, UK, and funded by Merck Healthcare KGaA, Darmstadt, Germany.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17425247.2024.2311127
Additional information
Funding
References
- Lunenfeld B, Bilger W, Longobardi S, et al. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol. 2019;10:429.
- FDA. Highlights of prescribing information for gonal-f RFF redi-ject 2013 [cited 2022 Jan 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021684s036lbl.pdf
- Jeannerot F, Cusin A, Schertz J. Dose accuracy of the redesigned follitropin alfa pen injector for infertility treatment. Expert Opin Drug Deliv. 2016;13(12):1661–1669.
- EMA. GONAL-f: summary of product characteristics. The Netherlands: European Medicines Agency. 2023 [cited 2023 Oct 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/gonal-f-epar-product-information_en.pdf
- EMA. Ovitrelle: summary of product characteristics. The Netherlands: European Medicines Agency; 2021 [cited 2022 Jan 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/ovitrelle-epar-product-information_en.pdf
- EMA. Pergoveris: summary of product characteristics. The Netherlands: European Medicines Agency; 2021 [cited 2022 Jan 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/pergoveris-epar-product-information_en.pdf
- Abbotts C, Salgado-Braga C, Audibert-Gros C. A redesigned follitropin alfa pen injector for infertility: results of a market research study. Patient Prefer Adherence. 2011;5:315–331. doi:10.2147/PPA.S21421
- Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–29. doi: 10.1016/j.clinthera.2006.10.006
- Longobardi S, Seidler A, Martins J, et al. An evaluation of the use and handling errors of currently available recombinant human follicle-stimulating hormone pen injectors by women with infertility and fertility nurses. Expert Opin Drug Deliv. 2019;16(9):1003–1014.
- Dallagiovanna C, Mensi L, Di Gesaro L, et al. Satisfaction and usability of the recombinant chorionic gonadotropin prefilled pen: a survey in Italy. Drugs Context. 2022;11:1–4. doi: 10.7573/dic.2021-10-3
- Choi BC, Zhou C, Ye H, et al. A comparative, observational study evaluating dosing characteristics and ovarian response using the recombinant human follicle-stimulating hormone pen injector with small-dose dial in assisted reproductive technologies treatment in Asia: IMPROVE study. Reprod Biol Endocrinol. 2022;20(1):15. doi: 10.1186/s12958-021-00882-2
- Schertz J, Worton H. Patient evaluation of the redesigned follitropin alfa pen injector. Expert Opin Drug Deliv. 2017;14(4):473–81. doi: 10.1080/17425247.2017.1289174.
- ISO. ISO 11608-1: needle-based injection systems for medical use — requirements and test methods — part 1: needle-based injection systems 2014 [cited 2022 Jan 18]. Available from: www.iso.org/standard/65021.html
- FDA. Technical considerations for pen, jet, and related injectors intended for use with drugs and biological products. 2020 [cited 2022 Jan 18]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-pen-jet-and-related-injectors-intended-use-drugs-and-biological-products
- Mahony MC, Patterson P, Hayward B, et al. Human factors engineering and design validation for the redesigned follitropin alfa pen injection device. Expert Opin Drug Deliv. 2015;12(5):715–25. doi: 10.1517/17425247.2015.1033395.
- Christen M, Schertz JC, Arriagada P, et al. The redesigned follitropin alpha pen injector for infertility treatment. Expert Opin Drug Deliv. 2011;8(6):833–839. doi:10.1517/17425247.2011.581658
- Schertz J, Worton H. Nurse evaluation of the redesigned fertility pen injector: a questionnaire-based observational survey. Expert Opin Drug Deliv. 2018;15(5):435–42. doi:10.1080/17425247.2018.1450386
- Saunders H, Schertz JC, Hecker C, et al. The recombinant human chorionic gonadotropin prefilled pen: results of patient and nurse human factors usability testing. Expert Opin Drug Deliv. 2012;9(8):893–900. doi:10.1517/17425247.2012.698607
- Saunders H, Bjargestad Lamp L, Donat H, et al. Risk of dosing errors in ART treatment: user experience of single- vs multi-use follitropin alfa pens. Expert Opin Drug Deliv. 2021;18(5):643–54. doi:10.1080/17425247.2021.1863944

