1. Introduction
In recent years, immunotherapy has emerged as a groundbreaking avenue in cancer treatment. The landscape of immunotherapeutic approaches encompasses immune checkpoint inhibitors, antibody-based therapies, cell-based therapies, cytokines, cancer vaccines, and oncolytic viruses [Citation1]. Up to the present, the US Food and Drug Administration (FDA) has approved several immunotherapies, generating a new era for cancer treatment. Despite its remarkable success in treating solid cancers, immunotherapy faces significant challenges when it comes to addressing primary brain tumors, such as adult-type diffuse gliomas [Citation2,Citation3]. The predominant immunosuppressive microenvironment, coupled with intra and intertumoral heterogeneity, as well as the presence of the blood-brain barrier (BBB), collectively contribute to the limited success of cancer immunotherapy in brain cancers [Citation4]. Nevertheless, researchers continue to actively explore novel avenues for cancer immunotherapies in glioma due to the promising results. In addition to immune checkpoint inhibitors and CAR-T cell therapies, cytokines have emerged as promising therapeutics, leveraging their pro-inflammatory profile to activate the immune system. Immunostimulatory therapeutics, such as pro-inflammatory cytokines (e.g. tumor necrosis factor, IL-2, IL-1β, IL-17, IL-12), chemokines (e.g. IL-8, CC-chemokine ligand 2), and interferons (e.g. IFNα, IFNβ, IFNγ), play a pivotal role in stimulating immune responses and promoting immune cell migration [Citation5]. Cytokines stand out as highly effective targets for remodeling the inflammatory microenvironment in the context of cancer therapy. Despite their high potential, the development of cytokine-based therapeutics encounters challenges from short blood half-lives, high toxicity, and unfavorable tissue distribution [Citation6].
Recognizing these challenges, recent efforts in nanomedicine have been made to enhance the efficacy of cytokine-based therapy [Citation7]. This innovative approach seeks to improve the bioavailability of cytokines, enabling targeted delivery into the brain. Doing so, it opens doors for the precise targeting of specific immune cells, addressing the limitations associated with traditional cytokine therapies. This editorial aims to give an overview of the new advancements in developing cytokine-based nanotherapeutics and how those systems can improve the current treatment of brain cancers.
2. Developing cytokine-based nanotherapeutics
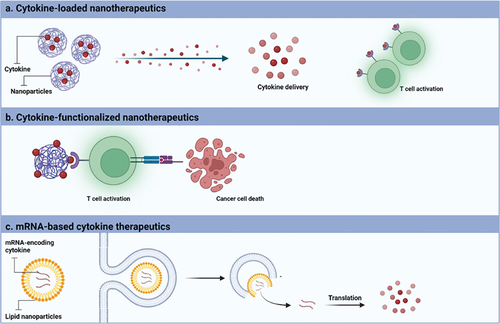
Cytokines, emerging as a novel class of biomacromolecules, have garnered significant attention among researchers for their potential in treating brain cancers through cancer immunotherapy. The targeted delivery of immune-activating cytokines, such as IL-2 and IFN-α, received approval from the Food and Drug Administration (FDA) for use in cancer immunotherapy for solid cancers dating back to the 1980s [Citation8]. Despite the promising outcomes observed in solid cancers with immunotherapies, their success in treating diffuse gliomas has been hampered by challenges such as an intensely immunosuppressive microenvironment, the presence of the blood-brain barrier (BBB), and associated toxicity concerns. In recent years, nanomaterials have emerged as valuable tools to enhance the pharmacokinetics, pharmacodynamics, and overall efficacy of cytokines. Cytokine-based nanotherapy for brain cancer can be approached in three distinctive ways: (i) utilizing cytokine-loaded nanotherapeutics, where cytokines are encapsulated into nanoparticles for the controlled release of cytokines and consequent activation of immune cells, (ii) anchoring cytokines to functionalize nanoparticles, enabling T cell activation through interaction with cytokine receptors on the surface of immune cells, and (iii) formulating mRNA-based cytokine therapeutics, where mRNA is engineered to encode cytokines, facilitating cytokine expression from targeted cells (). These innovative approaches hold the potential to address the unique challenges posed by brain cancers, providing avenues for improved treatment outcomes.
Figure 1. Categories of cytokine-based nanotherapeutics for brain cancer treatment. (a) Cytokines are loaded into nanoparticles to allow a controlled release of cytokine in the targeted tissue allowing the T cell activation and consequent, cancer cell death. (b) Cytokine-functionalized nanotherapeutics allow the interaction with immune cells via cytokine receptors inducing the cancer cell death. (c) mRNA-based cytokine therapeutics are formulated to allow the intracellular delivery of mRNA-encoding cytokine and allow the cytokine translation into the cytoplasm and consequent increase of cytokine expression. Created with BioRender.com.
2.1. Cytokine-loaded nanotherapeutics
Nanomedicines offer a versatile platform for encapsulating cytokines, allowing precise modulation of their bioavailability and biodistribution profiles through controlled release and targeted delivery to specific organs or immune cells. Recently, proinflammatory cytokines such as IL-2, IL-12, IL-23, and TNF-α have been successfully encapsulated into polymeric and polypeptide nanoparticles to activate anti-cancer immune responses and enhance cancer immunotherapy outcomes [Citation9,Citation10]. In the context of brain cancers, immunostimulatory nanoparticles, specifically IL-12-loaded polymeric nanoparticles, have demonstrated the capability to increase the release of pro-inflammatory cytokines from both glioma cells and macrophages [Citation10]. Certainly, this strategy holds promise for utilization as vaccine adjuvants, given that the release of pro-inflammatory cytokines can potentially trigger immune system activation. Consequently, it is foreseeable that these nanosystems will play a role as vaccine adjuvants in the future. However, a significant challenge lies in effectively managing the in situ inflammation induced by the release of pro-inflammatory cytokines. Thus, it is imperative to concurrently assess safety alongside tumor efficacy to ensure the translational viability of these nanosystems.
2.2. Cytokine-functionalized nanotherapeutics
Since most of the cytokines act mostly via interaction with their cell surface receptors, cytokines can be functionalized on the nanoparticle surface to further interact with immune cells and enable their activation. For effective treatment of brain cancers, extensive research into the overexpressed receptors is crucial in developing targeted drug delivery systems. In the case of gliomas, IL13Rα2 is well-recognized for its overexpression on glioma cells in comparison to normal cells [Citation11]. For that reason, several studies have explored the conjugation of IL-13 peptides on the surface of drug delivery systems to achieve targeted treatment for gliomas [Citation12,Citation13]. Results showed an increase in cellular uptake, cellular internalization, and glioma localization for the conjugated nanoparticles. Additionally, recent research has shown that anchoring IL-2 on the surface of liposomes improved tumor infiltration by cytotoxic lymphocytes and increased cytokine production [Citation14]. While this study was conducted in a lung metastatic model and a subcutaneous model, the noteworthy outcomes, demonstrating equivalent immunostimulatory effects to free drugs, suggest a promising strategy that could potentially be applied to treat brain cancers. Although this innovative approach is relatively recent, it has the potential to enhance the efficacy of cytokine-based therapies through the active targeting of nanoparticles. It is important to emphasize that this strategy is expected to yield the greatest benefits following intratumoral administration via intracranial route or intrathecal injection. Consequently, combination therapy involving this approach is preferred for optimal results.
2.3. mRNA-loaded cytokine therapeutics
Another innovative approach involves utilizing mRNA technology to encode cytokines and delivering them intracellularly through nanoparticles. Given the inherent susceptibility of mRNA to rapid degradation by endonucleases upon systemic administration, it becomes imperative to formulate mRNA into a drug delivery nanosystem. Lipid nanoparticles (LNP) technology has proven effective in ensuring stable and intracellular delivery of mRNA. Apart from LNP, polymeric nanoparticles have been equally formulated to encapsulate mRNA molecules [Citation5].
Recent advancements have witnessed the application of mRNA molecules encoding various cytokines for the treatment of brain cancers. These mRNA formulations, either employed as standalone treatments or in combination, have demonstrated efficacy in promoting anticancer immunity. For instance, calcium carbonate nanoparticles loaded with IL-12 mRNA exhibited a potent antitumor effect, fostering a synergistic immunotherapy by inducing necroptosis-mediated immune responses in a glioblastoma model [Citation15]. Clinical trials involving regulatable IL-12 gene therapy have reported encouraging outcomes, demonstrating limited toxicity and a promising antitumor immune response, particularly when administered intracranially [Citation16]. These findings underscore the potential of mRNA technology encoding cytokines, coupled with nanoparticle delivery systems, in revolutionizing therapeutic strategies for immune response modulation in the context of brain cancers.
Given the promising results of mRNA-encoding cytokines in the context of glioblastoma and several other solid cancers, researchers are enhancing treatment strategies by formulating cocktails of mRNAs that encode combinations of proinflammatory cytokines. A recent advancement involves the local administration of a cocktail mixture comprising mRNA-encoding IL-12, interferon-α (IFN-α), granulocyte macrophage colony-stimulating factor, and IL-15 sushi domain in diverse preclinical tumor models [Citation17]. This approach demonstrated notable enhancements, including increased intratumoral IFN-γ induction, systemic antigen-specific T cell expansion, and the elicitation of an immune response. Anticipating future developments, we envision the next generation of treatments involving the encapsulation of these cocktails of mRNAs into nanomedicines. These nanomedicines will activate multiple signaling pathways simultaneously, paving the way for more comprehensive and effective therapeutic interventions. This next generation of therapeutics aims to capitalize on the success of intracellular mRNA delivery, thereby circumventing in vivo degradation. Preliminary findings suggest that intratumoral delivery of IL-12 and IL-27 mRNAs loaded into lipid nanoparticle (LNP) formulations facilitates robust infiltration of natural killer (NK) cells and CD8+ T cells into solid tumors [Citation18]. We anticipate that upcoming investigations will delve into the application of these strategies in the context of brain tumors, where the complexity of treatments are notably heightened.
3. Expert opinion
Despite the notorious benefits of cytokines to treat cancer, clinical trials are still limited due to the several severe associated toxicities. The development of cytokine-based nanotherapeutics to treat brain cancers is a promising field for cancer immunotherapy since it allows to (i) potent the anti-tumor immunity, (b) change the tumor microenvironment phenotype and (c) repress the tumor growth without reporting systemic toxicity. Notably, nanotherapeutics have demonstrated sustained tumor inhibition alongside the activation of recruited effector cells, resulting in the secretion of anti-tumor signaling molecules and subsequent cancer cell death. However, a major challenge in the development of cytokine-based nanotherapeutics lies in limited delivery, attributed to the presence of the blood-brain barrier (BBB) and the high fabrication costs associated with such nanodelivery systems. Consequently, advocating for local delivery of cytokine-based nanotherapeutics is highly recommended for treating brain cancers, offering a potential expansion of current immunotherapy landscapes. This delivery platform is anticipated to be a focal point in future research, possibly in combination with other therapeutic modalities such as existing checkpoint inhibitors or cell-based therapies. Recent studies have highlighted the advantages of combining IL-12 delivery with CAR-T therapy, resulting in enhanced effectiveness of CAR-T cells and complete eradication of glioblastoma in mouse models [Citation19]. Recognizing the benefits of cytokine delivery, researchers have modified CAR-T cells to secrete IL-12, resulting in a stronger pro-inflammatory response, improved T cell activation, and modulation of the tumor microenvironment. Despite challenges such as manufacturing control, neurotoxicity and cytokine release syndrome are associated with CAR-T therapy [Citation20]. Delivering simultaneously cytokine-loaded nanotherapeutics might overcome the previous limitations and enhance CAR-T therapy protocols. Additionally, it holds promise in facilitating the infiltration and activation of NK and T cells, shifting M2-like macrophage phenotypes toward M1-like, and inducing ischemic tumor necrosis.
This avenue of research presents an opportunity to advance the field and develop more effective and targeted brain cancer immunotherapies as a single therapy or in combination therapy with other immunotherapeutic approaches.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. doi: 10.1038/s41577-020-0306-5
- Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20(9):1100–1109. doi: 10.1038/s41590-019-0433-y
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106
- Yu MW, Quail DF. Immunotherapy for Glioblastoma: Current progress and challenges. Front Immunol. 2021;12:676301. doi: 10.3389/fimmu.2021.676301
- Deckers J, Anbergen T, Hokke AM, et al. Engineering cytokine therapeutics. Nat Rev Bioeng. 2023;1(4):286–303. doi: 10.1038/s44222-023-00030-y
- Baldo BA. Side effects of cytokines approved for therapy. Drug Saf. 2014;37(11):921–943. doi: 10.1007/s40264-014-0226-z
- Riley RS, June CH, Langer R, et al. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z
- Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15. doi: 10.1038/s41416-018-0328-y
- Wang X-S, Zheng Z-S, Zheng M-F, et al. IL-2-loaded polypeptide nanoparticles for enhanced anti-cancer immunotherapy. Chin J Polym Sci. 2023;41(7):1059–1068. doi: 10.1007/s10118-023-2898-2
- Sousa F, Lee H, Almeida M, et al. Immunostimulatory nanoparticles delivering cytokines as a novel cancer nanoadjuvant to empower glioblastoma immunotherapy. Drug Deliv Transl Res. 2023. doi: 10.1007/s13346-023-01509-2
- Bhardwaj R, Suzuki A, Leland P, et al. Identification of a novel role of IL-13Rα2 in human glioblastoma multiforme: interleukin-13 mediates signal transduction through AP-1 pathway. J Transl Med. 2018;16(1):369. doi: 10.1186/s12967-018-1746-6
- Liang R, Wu C, Liu S, et al. Targeting interleukin-13 receptor α2 (IL-13Rα2) for glioblastoma therapy with surface functionalized nanocarriers. Drug Deliv. 2022;29(1):1620–1630. doi: 10.1080/10717544.2022.2075986
- Gao H, Yang Z, Zhang S, et al. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep. 2013;3(1):2534. doi: 10.1038/srep02534
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9(1):6. doi: 10.1038/s41467-017-02251-3
- Zhao P, Tian Y, Lu Y, et al. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. J Nanobiotechnol. 2022;20(1): doi: 10.1186/s12951-022-01731-z
- Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci, Trans Med. 2019;11(505):eaaw5680. doi: 10.1126/scitranslmed.aaw5680
- Hotz C, Wagenaar TR, Gieseke F, et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci Transl Med. 2021;13(610):eabc7804. doi: 10.1126/scitranslmed.abc7804
- Liu JQ, Zhang C, Zhang X, et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J Control Release. 2022;345:306–313. doi: 10.1016/j.jconrel.2022.03.021
- Ghasemi A, Martinez-Usatorre A, Li L, et al. Cytokine-armed dendritic cell progenitors for antigen-agnostic cancer immunotherapy. Nat Cancer. 2024;5(2):240–261. doi: 10.1038/s43018-023-00668-y
- Genoud V, Migliorini D. Novel pathophysiological insights into CAR-T cell associated neurotoxicity. Front Neurol. 2023;14:1108297. doi: 10.3389/fneur.2023.1108297

