ABSTRACT
Introduction: Apaziquone (also known as EO9 and QapzolaTM) is a prodrug that is activated to DNA damaging species by oxidoreductases (particularly NQO1) and has the ability to kill aerobic and/or hypoxic cancer cells.
Areas covered: Whilst its poor pharmacokinetic properties contributed to its failure in phase II clinical trials when administered intravenously, these properties were ideal for loco-regional therapies. Apaziquone demonstrated good anti-cancer activity against non-muscle invasive bladder cancer (NMIBC) when administered intravesically to marker lesions and was well tolerated with no systemic side effects. However, phase III clinical trials did not reach statistical significance for the primary endpoint of 2-year recurrence in apaziquone over placebo although improvements were observed. Post-hoc analysis of the combined study data did indicate a significant benefit for patients treated with apaziquone, especially when the instillation of apaziquone was given 30 min or more after surgery. A further phase III study is ongoing to test the hypotheses generated in the unsuccessful phase III studies conducted to date.
Expert opinion: Because of its specific pharmacological properties, Apaziquone is excellently suited for local therapy such as NMIBC. Future studies should include proper biomarkers.
1. Introduction
Apaziquone, originally known as EO9 (3-hydroxy-5-aziridinyl-1-methyl-2-(1H-indole-4,7-dione)prop-β-en-α-ol), was first synthesized at the University of Amsterdam in 1987 [Citation1]. It was developed as one of a series of derivatives of mitomycin C in a program of work sponsored by the Dutch Cancer Society. Its preclinical evaluation was coordinated by the New Drug Development Office of the European Organisation for the Research and Treatment of Cancer (EORTC) in Amsterdam and involved a number of laboratories belonging to the Screening and Pharmacology Group and Pharmacology and Molecular Mechanism groups across Europe [Citation2]. Apaziquone belongs to a class of anticancer drugs known as bioreductive drugs which are prodrugs designed to be activated by enzymes (oxidoreductases) present within the tumor [Citation3]. Depending on the enzymology of individual tumors, it also has the ability to target hypoxic cells and in this context, it also functions as a hypoxia-activated prodrug [Citation3,Citation4]. Apaziquone has had a chequered history in that it failed to show antitumor activity in the clinic following intravenous administration. One key reason for its failure was poor drug delivery to tumors caused by its poor systemic pharmacokinetic properties in conjunction with poor penetration through avascular tissue [Citation5]. Paradoxically, these poor pharmacological properties are beneficial for locoregional therapy and the rationale for testing apaziquone against non-muscle invasive bladder cancer (NMIBC) was generated. The pharmacology of apaziquone has been extensively reviewed elsewhere [Citation6] and the purpose of this article is to (i) to summarize the clinical studies where apaziquone was administered intravenously with the aim of putting its history into an appropriate context and (ii) to focus on the pharmacology, toxicity, and clinical activity of apaziquone against NMIBC.
2. Overview of NMIBC and available therapies
Bladder cancer is the ninth most common cancer worldwide with 429,800 new cases diagnosed in 2012 [Citation7,Citation8]. In the USA, it is the fourth most common cancer in males with an estimated 76,960 new cases of bladder cancer (both sexes) diagnosed in 2016 and 16,390 deaths [Citation9]. Similarly, it is the fourth most common cancer in Europe with an estimated 151,252 cases in 2012 (both sexes) and 52,411 deaths [Citation10]. The majority of these cases (70–75%) present as NMIBC that are defined as papillary tumors (low and high grade) confined to the mucosa and invading the lamina propria (stage Ta and T1 respectively) and includes high grade, flat tumors confined to the mucosa (carcinoma in situ [CIS] or Tis) [Citation11]. Treatment of NMIBC (including high-risk G3 and CIS) typically involves surgical removal by transurethral resection of bladder tumors (TURBT), but NMIBC has a tendency to recur after TURBT alone. Adjuvant intravesical instillation of chemotherapy or immunotherapy has been shown to reduce tumor recurrence rates and prevent disease progression [Citation12]. Commonly used chemotherapeutic drugs for G1/G2 and Ta/T1 tumors include mitomycin C, valrubicin, epirubicin, cisplatin, and doxorubicin and European Association of Urology (EAU) and American Urological Association guidelines recommend single, immediate post-TURBT intravesical instillation for low- and intermediate-risk NMIBC [Citation13,Citation14]. Meta-analysis data have shown that this treatment reduces recurrence rates by 11.7–13.0% compared to TURBT alone [Citation15–Citation17]. In the USA, four agents have been approved by the FDA for use in NMIBC; ThioTEPA in 1959, Bacillus Calmette-Guérin (BCG) Tice in 1989, BCG Connaught in1990, and valrubicin in 1998 [Citation18]. ThioTEPA is no longer widely used because of toxicity issues and mitomycin C is commonly used off-label as an intravesical treatment for NMIBC. The use of mitomycin C is also associated with rare but severe toxicities [Citation19–Citation22]. Intravesical immunotherapy with BCG has been widely used since the 1970s and is frontline treatment for patients presenting with high-risk lesions (G3 and CIS) and is not administered immediately after surgery [Citation23,Citation24]. Apaziquone has been evaluated against low-risk (G1/G2 and Ta/T1) tumors.
Despite adjuvant intravesical therapy, recurrence rates remain high (30–70%) and EAU and AUA guidelines recommend frequent cystoscopic and cytologic surveillance that continues for the lifetime of the patient. Recurrence of the disease together with the cost of intensive surveillance strategies contributes to the high cost of treating this disease [Citation25]. Some authors have stated that bladder cancer has the highest lifetime treatment costs per patient of all cancers due to the high recurrence rate and sustained invasive monitoring requirements [Citation26]. There is therefore a strong pharmacoeconomic need for new therapies that can delay recurrence rates in NMIBC. In addition, the relative lack of approved treatment options for NMIBC and the fact that no new therapies have been approved for NMIBC in the twenty-first century graphically illustrates the need to develop new drugs for the management of NMIBC [Citation18]. Finally, NMIBC is a nonlethal disease and as most patients are old with other comorbid conditions such as cardiovascular and pulmonary diseases [Citation27,Citation28], the safety of drugs used after TURBT is also of paramount importance. The toxicity profile of novel therapeutics is also therefore an important consideration in the management of these patients.
3. Introduction to apaziquone
Apaziquone was originally developed at the University of Amsterdam in a research project sponsored by the Dutch Cancer Society (). The original patent was published in 1987 (WO87/06227) and maintained by the University of Amsterdam until Kyowa Hakko licensed the compound in 1994 [Citation29]. Kyowa Hakko released the license in 1997/98 and the patent was maintained by the NDDO Oncology BV and INC Research. Spectrum Pharmaceuticals (Irvine, California) in-licensed exclusive worldwide rights to EO9 (currently branded as Qapzola) from INC Research in 2001. Formulations designed specifically for the intravesical administration of apaziquone were developed [Citation30,Citation31] and patented in 2007 (WO2007092964). These formulations led to greater stability of apaziquone in human urine [Citation32]. In 2008, Spectrum Pharmaceuticals and Allergan signed an exclusive collaboration for the development and commercialization of EO9 (apaziquone) but Spectrum Pharmaceuticals subsequently regained exclusive rights to apaziquone in 2013.
3.1. Chemistry
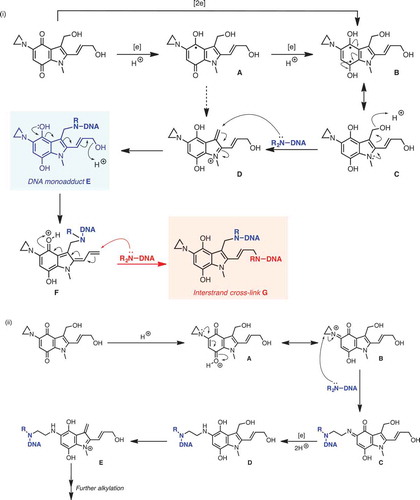
The original synthesis of apaziquone (EO9) was reported in 1987 [Citation1] and since then various methodologies have been described [Citation33–Citation35] including large-scale synthesis methods [Citation36,Citation37]. Whilst apaziquone is structurally related to mitomycin C, its mechanism of action is very different particularly with respect to NQO1 activation (see below in Section 3.2). The chemical structure of EO9 is presented in . It is an indolequinone that has three active centers (i) an aziridinyl group at C5, (ii) a vinylic group at C2, and (iii) the hydroxymethyl group at C3. The mechanism(s) of action are presented in and and these include the generation of reactive oxygen species via redox cycling [Citation38] () and alkylation reactions via (i) enzymatic reduction and (ii) proton assisted aziridine ring opening ().
Figure 1. Redox reactions and generation of reactive oxygen species following the reduction of apaziquone by one electron oxidoreductases. The quinone nucleus of apaziquone is reduced by NAD(P)H dependent one electron oxidoreductases such as cytochrome P450 reductase to a semiquinone radical which redox cycles back to the parent quinone in the presence of oxygen generating superoxide anions. Hydrogen peroxide is generated via superoxide dismutase (SOP) mediated reactions and this in the presence of trace metals can lead to the formation of hydroxyl radicals and subsequent damage to cellular macromolecules.
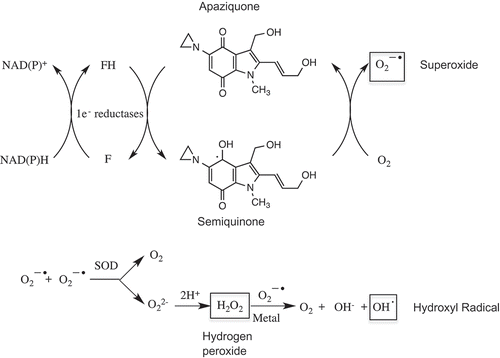
Figure 2. Mechanisms for DNA alkylation by apaziquone: (i) Pathways involving one- and two-electron reduction. Proton-coupled single-electron reduction of apaziquione generates stablised radical A, which can be further reduced (via B) to key intermediate C; C can be alkylated via cation D, formed by acid-catalysed dehydration, to give DNA monoadduct E. can undergo a second dehydration, giving dienyl cation F, which can be trapped in a second DNA alkyation event, giving interstrand cross-linked species G. (ii) Proton-accelerated mechanism. The conjugation of the aziridinyl lone-pair (acting as a vinylogous amide) into the quinone ring allows for an addition proton-accelerated DNA alkylation mechanism. Thus, protonation leads to cation A, which is in resonance with B; ring-opening of the reactive aziridinium moiety of B by DNA leads to aza-quinone species C, which can then undergo reduction to D. Acid-catalysed dehydration of D gives methylene indolium cation E, which can function as an active alkylating agent.
3.2. Mechanism of action and pharmacodynamics
The mechanism of action of apaziquone is complex and has been extensively reviewed elsewhere [Citation6]. Briefly, the pharmacodynamics properties of apaziquone can be segregated into (i) factors that influence the bioreductive activation process and (ii) how cells respond to the type and extent of DNA damage induced following bioreductive activation. Several factors influence the bioreductive activation process and cell kill is determined by a complex interaction between the expression of one and two electron oxidoreductases in cells, the presence or absence of oxygen, and extracellular pH (pHe). Several oxidoreductases are capable of reducing apaziquone but the major ones are the two-electron reductase NAD(P)H:quinone oxidoreductase 1 (NQO1, also known as DT-diaphorase) and a series of one-electron reductases including cytochrome P450 reductase [Citation6]. Reduction of apaziquone by purified NQO1 and cytochrome P450 reductase leads to free radical generation and DNA-damaging species in cell-free assays [Citation38–Citation40] ( and ). The types of DNA damage observed in cell-free assays include interstrand cross-links, mono-adducts, and strand breaks [Citation39,Citation41–Citation43]. With regards to the induction of DNA cross-links, there are some discrepancies in the literature with some studies reporting cross-linking [Citation41,Citation42], whereas others report no cross-link induction following reduction of apaziquone by NQO1 in cell-free assays [Citation43]. Species-dependent differences (rat vs. human NQO1) may account for these differences but similar conflicting results were reported following the analysis of DNA interstrand cross-link induction in human cancer cells using alkaline elution or comet assay techniques [Citation41,Citation44]. Whilst the extent of DNA interstrand cross-link induction maybe uncertain, it is clear that apaziquone is reduced by one- and/or two-electron reductases to species that are capable of damaging DNA by free radical generation and alkylation.
In addition to the presence of oxidoreductases, the oxygenation state of cells has a significant bearing on the pharmacodynamic response. In the presence of oxygen, apaziquone is reduced predominantly by the two-electron reductase NQO1 and several studies have demonstrated that IC50 values are typically inversely proportional to NQO1 activity [Citation45–Citation48]. In the absence of oxygen however, significant potentiation of apaziquone activity is only seen in cells that have low or no NQO1 activity [Citation47,Citation49], particularly those that harbor the C609T polymorphic variant of NQO1 [Citation49,Citation50]. In cells with low or no NQO1, one-electron reductases assume a prominent role under these conditions, reduction of apaziquone is oxygen sensitive with the semiquinone rapidly redox cycling back to the parent compound resulting in the production of reactive oxygen species (). When oxygen is low or absent, the half-life of the semiquinone increases providing more time to either alkylate DNA directly or be reduced further to produce the hydroquinone [Citation51]. The experimental evidence in vitro clearly indicates that the activity of apaziquone is strongly dependent upon NQO1 activity and cause cell death in an oxygen independent manner but in cells that are devoid of NQO1 activity, apaziquone is able to selectively target hypoxic cells. The response of tumors in vivo could however not be predicted based on NQO1 activity [Citation52], but it is likely that poor systemic pharmacokinetics and penetration through avascular tissue compromised drug delivery resulting in suboptimal levels of apaziquone in tumors [Citation5]. This suboptimal accumulation of apaziquone in tumors is possibly sufficient to exert a radiosensitizing effect. The ability to target hypoxic cells suggests that combination therapy with radiotherapy could be effective and this was indeed demonstrated in three syngeneic rat lung cancer models and a rhabdomyosarcoma in vivo () [Citation53], but this strategy was never considered as a clinical strategy.
Table 1. In vivo antitumor activity of apaziquone (EO9) in combination with radiation in experimental rat tumors. The tumors were two squamous cell lung carcinomas (L17 and L42), a lung adenocarcinoma (L27), and a rhabdomyosarcoma (BA1112). All tumors were grown subcutaneously and EO9 was administered intraperitoneally (daily × 5 at 0.4 mg/kg). When EO9 was combined with 5 daily doses of 4Gy, additive or synergistic effects were observed. This data were originally reported by Kal et al [Citation53].
The activity of apaziquone is strongly influenced by pHe. Under mild acidic extracellular conditions (pH 6.0), the cytotoxic potency of indolequinones containing aziridine ring functionality (including apaziquone) is significantly enhanced in vitro [Citation44,Citation54]. The activity of apaziquone remains inversely proportional to NQO1 activity under acidic pHe conditions, but potency is significantly enhanced [Citation55]. In contrast to mitomycin C where reducing the pH increases substrate specificity and reduction by NQO1 [Citation56], the substrate specificity of apaziquone for NQO1 is not affected by pH [Citation55]. Whilst the cytotoxic potency of apaziquone is enhanced under acidic conditions, its chemical stability is decreased resulting in enhanced formation of EO5A in the absence of cells [Citation54]. These apparently paradoxical properties can be explained by proton-assisted aziridine ring opening and enhanced nucleophilic attack (), the outcome of which would vary depending on the type of nucleophile (e.g. water or DNA) present in the system.
In summary, the pharmacodynamic properties of apaziquone are determined by a complex ‘cocktail’ of parameters including (i) the presence or absence of NQO1, (ii) the presence of one-electron reductases such as cytochrome P450 reductase, (iii) the presence or absence of oxygen, (iv) acidic pHe, and (v) repair of DNA damage. The preclinical data suggests that apaziquone should (i) target the aerobic and hypoxic fraction of NQO1 rich tumors equally efficiently, (ii) target the hypoxic fraction of tumors that have low NQO1 activity, particularly those that harbor the inactivating NQO1 single nucleotide polymorphic variant, and (iii) target cells that reside in an environment where pHe is mildly acidic. Most of these parameters are related to the bioreductive activation process but as stated above, the second important parameter that determines response is how the cell responds to the damage induced in terms of detoxification and repair. As apaziquone can induce strand breaks via the induction of ROS, antioxidant defense mechanisms could modulate activity and antioxidants such as catalase and Tempol have been shown to reduce DNA damage and apaziquone cytotoxicity [Citation57,Citation58]. Despite the fact that apaziquone has been studied for 30 years, comparatively little is known about the role DNA repair plays in determining response and further studies are required to address this issue.
3.3. Pharmacokinetics and metabolism
Full details of preclinical and clinical pharmacokinetics following intravenous administration have been reviewed in detail elsewhere [Citation6]. The suboptimal pharmacokinetic profile of apaziquone is illustrated by the results of phase I studies where apaziquone is rapidly cleared from the systemic circulation with half-lives ranging from 0.8 to 19 min [Citation59]. Furthermore, the predominant metabolite found in blood and urine following intravenous administration was the inactive, aziridine ring open metabolite EO5A [Citation54,Citation59]. Following intravesical administration, two clinical studies have investigated the pharmacokinetic and safety of apaziquone [Citation60,Citation61]. In the first of these studies [Citation61], apaziquone was administered 2 weeks after TURBT and for the first six patients entered into the trial, each received an escalating dose of apaziquone with one dose per week (for a total of 6 weeks). At all doses administered, no detectable levels of apaziquone or EO5A were found in the blood. At the end of the 1-h intravesical instillation, over 60% of the administered dose of apaziquone (but not EO5A) was recovered from urine, the concentration being proportional to the dose administered [Citation61]. Overall exposure parameters within the bladder considerably exceeded plasma AUC values following intravenous administration indicating that therapeutically relevant concentrations of drug were delivered to tumors in the bladder [Citation6,Citation61]. The second study was conducted in a total of 20 patients with NMIBC and a single 4 mg/40 ml dose of apaziquone was administered intravesically within 6 h of TURBT [Citation60]. No apaziquone or EO5A were detected in the blood of patients during and after the instillation. In acidified urine, a degradation product known as EO-9-Cl has been reported [Citation31], but the presence of this product was not observed in clinical studies. Pharmacokinetic studies following the intravesical administration of apaziquone to NMIBC patients are therefore limited but in both studies, high levels of apaziquone (4 mg/40 ml) were delivered into the bladder but no detectable levels of drug or known metabolites reached the systemic circulation.
3.4. Clinical efficacy
A number of clinical trials were conducted following the intravenous administration of apaziquone and details of these are reviewed elsewhere [Citation6]. This review will focus only on the clinical trials conducted to date following the intravesical administration of apaziquone to NMIBC patients. In a phase I/II pilot study, 12 patients with low-risk NMIBC were administered apaziquone intravesically (once a week for 6 weeks; 0.5–16 mg/40 ml) and antitumor effects were determined against marker lesions left in the bladder at TURBT [Citation61]. A total of eight complete responses as defined by both visual loss of the marker lesion () and pathological confirmation of no tumor at the marker lesion site was reported. Biomarker studies were conducted in the phase I/II study using immunohistochemical analysis of NQO1 and Glut-1, the latter being an endogenous marker of hypoxia [Citation61]. Whilst a broad range of NQO1 and Glut-1 protein expression was detected in tumors, there was no correlation between the response of the marker lesion and biomarker expression. These studies were only conducted on 12 patients and no further biomarker studies were performed in subsequent clinical trials. Because of the low number of patients, it is advised to include extensive biomarker studies (focusing on NQO1 and other hypoxia biomarkers) in future studies. Specific PET imaging probes might also help to determine rate of hypoxia in tumors.
Figure 3. The presence of a marker lesion prior to (A) and after (B) a 6 week course of apaziquone. The results were first presented in the review article by Phillips et al, reproduced with permission [Citation6].
![Figure 3. The presence of a marker lesion prior to (A) and after (B) a 6 week course of apaziquone. The results were first presented in the review article by Phillips et al, reproduced with permission [Citation6].](/cms/asset/7c698142-d47a-4489-9303-378a16d693d8/iemt_a_1341490_f0003_oc.jpg)
Using an identical marker lesion study design, 31 out of a total of 46 patients with multiple pTa or pT1 tumors achieved a complete response in phase II trials at a dose of 4 mg/40 ml [Citation62]. Marker lesion studies have been conducted for many other cytotoxic and immune response modifiers, but the complete response rate achieved by apaziquone (67%) was the highest reported complete response rate [Citation63]. Two-year follow-up studies demonstrated that long-term results were good in comparison to other ablative studies with a recurrence-free rate of 49.5% at 2 years [Citation64]. Similar results were reported using patients enrolled on the phase I/II clinical pilot study [Citation65]. Phase II studies of intravesically administered apaziquone to high-risk NMIBC have also reported encouraging results [Citation66]. These results were promising, but it is however acknowledged that the number of phase II studies is limited and randomized phase III studies are required [Citation67].
Two phase III trials (SPI-611 and 612) were completed and the results were presented at the American Urological Association annual meeting in 2016 [Citation68,Citation69]. Both studies had similar designs and a single dose of apaziquone (4 mg/40 ml) was administered intravesically to patients with low-risk NMIBC (TaT1 and G1/G2 lesions) within 6 h of TURBT and retained for 1 h. The control arm consisted of the administration of the placebo in both studies. The primary endpoint was the 2-year recurrence rate and the secondary endpoint was time to recurrence. Taking both studies together, 1614 patients were enrolled across 152 centers in the USA, Poland, and Canada (study 612 only). The studies were analyzed individually and both did not meet their primary endpoint of a statistically significant difference in the 2-year recurrence rate between treatment and placebo arms. When the results of the two trials were pooled, however, statistically significant differences were obtained in both the 2-year recurrence rate (apaziquone 38.8% vs. placebo 45.5%; p = 0.0218) and time to recurrence (apaziquone 18.2 months vs. placebo 16.8 months; P = 0.0096; Hazard ratio 0.79) [Citation68]. Furthermore, post hoc analysis of data from both studies demonstrated that a significant difference in time to recurrence (Hazard ratio 0.48; p = 0.0096) was observed in the 117 patients who received apaziquone >30 min post-TURBT compared to placebo (100 patients). Patients in the apaziquone 31–90 min subgroup also showed a significant absolute decrease in the 2-year recurrence rate (apaziquone 28.2% vs. placebo 50%; p = 0.001) resulting in a 43.6% relative reduction in 2-year recurrence rates [Citation69], but no improvements were found in the other 2 subgroups (<30 min, later than 90 min). The result in the <30 min could be explained by the fact that blood released during surgery could inactivate apaziquone [Citation70]. Loadman et al. [Citation70] indeed demonstrated that EO9 is rapidly degraded by blood in vitro. However, the FDA’s Oncologic Drugs Advisory Committee recently determined that apaziquone did not show sufficient statistical evidence of treatment effect of apaziquone over placebo based on the primary endpoint results of individual studies and that the post hoc analysis conducted by Spectrum Pharmaceuticals was hypothesis generating. The FDA have granted an SPA agreement for a new phase III study to address the issues arising from SPI-611 and 612, particularly with regards to the timing of apaziquone administration post-TURBT. Spectrum plans to initiate enrolment in this study.
Preclinical toxicology studies in mice were reported in 1993 and the most obvious finding was a lack of myelosuppression, a result that was considered unique for what was essentially an alkylating agent [Citation2]. No myelosuppression was also reported in the phase I and II studies when EO9 was administered intravenously [Citation59,Citation71]. Dose-limiting toxicity in these studies was reversible proteinuria that was attributed to high levels of NQO1 found within the kidney. In the phase I/II study following intravesical administration to patients with NMIBC, 6 patients received escalating doses of apaziquone (0.5–16 mg/40 ml) weekly for 6 weeks. A further 6 patients received weekly apaziquone for 6 weeks at the highest nontoxic dose (4 mg/40 ml). Grade 2 and 3 dysuria and hematuria were observed at doses above 8 mg/40 ml, but 4 mg/40 ml was well tolerated [Citation61]. Apaziquone was also well tolerated in phase II studies when administered either as 6 weekly infusions at 4 mg/40 ml [Citation62] and as a single dose administered within 6 h of TURBT [Citation60]. In phase III studies (single dose of 4 mg/40 ml administered within 6 h of TURBT), apaziquone was well tolerated with a safety profile that was indistinguishable from placebo, with a similar incidence of adverse events (80.0% vs. 78.5%, respectively) with dysuria as the most common treatment-related adverse event in both the apaziquone and placebo group 4.6 versus 4.1%, respectively) [Citation68]. Hence, the safety profile of apaziquone is very good and at 4 mg/40 ml, apaziquone was well tolerated following both single and multiple instillations. This compares favorably with other agents such as the structurally related (but mechanistically very different) anticancer drug mitomycin C used to treat NMIBC where rare but severe side effects have been reported [Citation19–Citation22]. Of course, the occurrence of rare adverse events caused by apaziquone cannot be ruled out until larger numbers of patients have been treated, but the early signs are that apaziquone has a favorable toxicity profile. In the context of managing elderly NMIBC patients with comorbidities, the excellent safety profile of apaziquone is very encouraging.
4. Conclusion
There is a clear need for new therapeutic approaches to treating NMIBC [Citation18]. From both a pharmacoeconomic and patient perspective, the development of new therapies that can delay the recurrence of NMIBC and are well tolerated would have a significant financial impact on health-care providers. Apaziquone demonstrated excellent activity against NMIBC in marker lesion studies, but the clinical improvement in phase III studies required for drug approval is yet to be demonstrated. It is important to state that the design of the phase III studies was significantly different from the phase I/II studies. With hindsight, the presence of blood in the bladder shortly after TURBT should have been considered as inactivating metabolism of apaziquone by blood [Citation70] and this could have had a significant bearing on the outcome of these trials. A further phase III study has been designed and the results of this trial will be definitive.
5. Expert opinion
Apaziquone was initially developed as a bioreductive drug designed to target NQO1 rich and/or hypoxic tumors. Initial clinical studies following intravenous administration failed to show ant-tumor activity at doses that caused toxicity and it was not developed further. These early clinical trials did not include potential biomarkers (e.g. hypoxia, NQO1) or test the drug in combination with other modalities and it is feasible that the lack of target expression in the patient’s tumors could account for its poor efficacy in clinical trials [Citation72]. Moreover, the drug showed a very unfavorable pharmacokinetic profile leading to poor drug delivery to tumors and development was halted, until it was recognized that such a profile would be ideal for local administration. Any leakage to the systemic circulation would immediately be taken care of by a rapid degradation and elimination. Early clinical studies by bladder instillation showed very promising data with limited local and no systemic toxicity. Significant activity against marker lesions in phase I and II studies clearly demonstrates that apaziquone has anticancer activity, but this failed to translate into positive results in phase III trials. Whilst the two, randomized phase III studies failed to reach statistical significance, there was some evidence from post hoc analysis of pooled results of significant activity. The FDAs Oncologic Drugs Advisory Committee were not convinced of the validity of the statistical analysis and a further phase III trial is required to test the hypotheses generated from the completed studies. Despite these disappointing results, it is our opinion that the development of apaziquone should continue and future studies should take several aspects into account such as the instability at acidic conditions and protein binding, as well as timing of the instillation (between 31 and 90 min) after surgery. The stability issue of apaziquone is taken care off by using a buffered formulation (pH 9.2) for the drug, which prolonged stability of apaziquone during instillation, even when mixed with acid urine [Citation31]. Other future applications of apaziquone would be regional treatment for other malignancies, even in the prevention setting. Moreover, apaziquone is an excellent radiosensitizer, which offers possibilities for combinations with radiotherapy, including bladder cancer, but also other types of localized tumors would be eligible. From a wider perspective, the developmental history of this drug shows that compounds with poor pharmacological profile are not necessarily precluded from further development as loco-regional therapies.
Box 1. Drug summary box: apaziquone
Declaration of interest
GJ Peters has received research funding from Spectrum pharmaceuticals, but not for apaziquone, HR Hendriks is the owner of Hendriks consulting. G Reddy is an employee and shareholder of Spectrum pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Acknowledgement
This paper was written in the framework of the EORTC Pharmacology and Molecular Mechanism (PAMM) Group.
Additional information
Funding
References
- Oostveen EA, Speckamp WN. Mitomycin analogs I. Indolequinones as (potential) bisalkylating agents. Tetrahedron. 1987;43:255–262.
- Hendriks HR, Pizao PE, Berger DP, et al. EO9: a novel bioreductive alkylating indoloquinone with preferential solid tumour activity and lack of bone marrow toxicity in preclinical models. Eur J Cancer. 1993;29A:897–906.
- Phillips RM. Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs. Cancer Chemother Pharmacol. 2016;77:441–457.
- Hunter FW, Wouters BG, Wilson WR. Hypoxia-activated prodrugs: paths forward in the era of personalised medicine. Br J Cancer. 2016;114:1071–1077.
- Phillips RM, Loadman PM, Cronin BP. Evaluation of a novel in vitro assay for assessing drug penetration into avascular regions of tumours. Br J Cancer. 1998;77:2112–2119.
- Phillips RM, Hendriks HR, Peters GJ, et al. EO9 (apaziquone): from the clinic to the laboratory and back again. Br J Pharmacol. 2013;168:11–18.
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403.
- Cheng L, Montironi R, Davidson DD, et al. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol. 2009;22(Suppl 2):S70–95.
- Witjes JA, Hendricksen K. Intravesical pharmacotherapy for non-muscle-invasive bladder cancer: a critical analysis of currently available drugs, treatment schedules, and long-term results. Eur Urol. 2008;53:45–52.
- Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447–461.
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029.
- Abern MR, Owusu RA, Anderson MR, et al. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. J Natl Compr Canc Netw. 2013;11:477–484.
- Perlis N, Zlotta AR, Beyene J, et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol. 2013;64:421–430.
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90, quiz 435.
- Jarow J, Maher VE, Tang S, et al. Development of systemic and topical drugs to treat non-muscle invasive bladder cancer. Bladder Cancer. 2015;1:133–136.
- Barocas DA, Globe DR, Colayco DC, et al. Surveillance and treatment of non-muscle-invasive bladder cancer in the USA. Adv Urol. 2012;2012:421709.
- Kamat AM, Lamm DL. Intravesical therapy for bladder cancer. Urology. 2000;55:161–168.
- Panach-Navarrete J, Ferrandis-Cortes C, Sales-Maicas MA, et al. Mitomycin extravasation after postoperative instillation. Arch Esp Urol. 2015;68:633–636.
- Lim D, Izawa JI, Middlebrook P, et al. Bladder perforation after immediate postoperative intravesical instillation of mitomycin C. Can Urol Assoc J. 2010;4:E1–3.
- De Jager R, Guinan P, Lamm D, et al. Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology. 1991;38:507–513.
- Lamm DL. BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology (Williston Park). 1995;9:947-52, 55, discussion 55-65.
- Svatek RS, Hollenbeck BK, Holmang S, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66:253–262.
- Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27:295–300.
- Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939–949.
- Soria F, Moschini M, Korn S, et al. How to optimally manage elderly bladder cancer patients? Transl Androl Urol. 2016;5:683–691.
- EO9 to be licenced by Kyowa Hakko Kogyo. Ann Oncol. 1994;5:2.
- van der Schoot SC, Nuijen B, Flesch FM, et al. Development of a bladder instillation of the indoloquinone anticancer agent EO-9 using tert-butyl alcohol as lyophilization vehicle. AAPS Pharmscitech. 2007;8:E78–E87.
- van der Schoot SC, Vainchtein LD, Beijnen JH, et al. Bladder instillations: formulation selection based on stability characteristics and in vitro simulation studies. Int J Pharm. 2007;329:135–141.
- Vainchtein LD, Rosing H, Mirejovsky D, et al. Stability experiments in human urine with EO9 (apaziquone): a novel anticancer agent for the intravesical treatment of bladder cancer. J Pharm Biomed Anal. 2007;43:285–292.
- Comer E, Murphy WS. The bromoquinone annulation reaction: a formal total synthesis of EO9. Archive Org Chem. 2003;2003:286–296.
- Cotterill AS, Moody CJ, Roffey JRA. An improved synthesis of the indolequinone anticancer agent EO9. Tetrahedron. 1995;51:7223–7230.
- Kinugawa M, Arai H, Nishikawa H, et al. Facile synthesis of the key intermediate of EO9 via the formation of the indole skeleton using the Nenitzescu reaction. J Chem Soc Perkin Trans. 1995;1:2677–2678.
- Kinugawa M, Masuda Y, Arai H, et al. Large scale synthesis of the high quality indoloquinone antitumor agent EO 9 via [Bis(trifluoroacetoxy)iodo]benzene oxidation of 4-aminoindole. Synthesis. 1996;1996:633–636.
- Kinugawa M, Rai H, Ogasa T, et al. Development of large-scale synthetic process for antitumor agent EO9. J Synth Org Chem Jpn. 1999;57:401–406.
- Butler J, Spanswick VJ, Cummings J. The autoxidation of the reduced forms of EO9. Free Radic Res. 1996;25:141–148.
- Bailey SM, Lewis AD, Patterson LH, et al. Involvement of NADPH: cytochrome P450 reductase in the activation of indoloquinone EO9 to free radical and DNA damaging species. Biochem Pharmacol. 2001;62:461–468.
- Walton MI, Smith PJ, Workman P. The role of NAD(P)H: quinone reductase (EC 1.6.99.2, DT-diaphorase) in the reductive bioactivation of the novel indoloquinone antitumor agent EO9. Cancer Commun. 1991;3:199–206.
- Bailey SM, Wyatt MD, Friedlos F, et al. Involvement of DT-diaphorase (EC 1.6.99.2) in the DNA cross-linking and sequence selectivity of the bioreductive anti-tumour agent EO9. Br J Cancer. 1997;76:1596–1603.
- Maliepaard M, Wolfs A, Groot SE, et al. Indoloquinone EO9: DNA interstrand cross-linking upon reduction by DT-diaphorase or xanthine oxidase. Br J Cancer. 1995;71:836–839.
- Phillips RM. Bioreductive activation of a series of analogues of 5-aziridinyl-3-hydroxymethyl-1-methyl-2-[1H-indole-4, 7-dione] prop-beta-en-alpha-ol (EO9) by human DT-diaphorase. Biochem Pharmacol. 1996;52:1711–1718.
- Phillips RM, Ward TH. Influence of extracellular pH on the cytotoxicity and DNA damage of a series of indolequinone compounds. Anticancer Res. 2001;21:1795–1801.
- Fitzsimmons SA, Workman P, Grever M, et al. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996;88:259–269.
- Plumb JA, Gerritsen M, Milroy R, et al. Relative importance of DT-diaphorase and hypoxia in the bioactivation of EO9 by human lung tumor cell lines. Int J Radiat Oncol Biol Phys. 1994;29:295–299.
- Robertson N, Haigh A, Adams GE, et al. Factors affecting sensitivity to EO9 in rodent and human tumour cells in vitro: DT-diaphorase activity and hypoxia. Eur J Cancer. 1994;30A:1013–1019.
- Smitskamp-Wilms E, Peters GJ, Pinedo HM, et al. Chemosensitivity to the indoloquinone EO9 is correlated with DT-diaphorase activity and its gene expression. Biochem Pharmacol. 1994;47:1325–1332.
- Plumb JA, Workman P. Unusually marked hypoxic sensitization to indoloquinone EO9 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int J Cancer. 1994;56:134–139.
- Traver RD, Siegel D, Beall HD, et al. Characterization of a polymorphism in NAD(P)H: quinone oxidoreductase (DT-diaphorase). Br J Cancer. 1997;75:69–75.
- Workman P. Enzyme-directed bioreductive drug development revisited: a commentary on recent progress and future prospects with emphasis on quinone anticancer agents and quinone metabolizing enzymes, particularly DT-diaphorase. Oncol Res. 1994;6:461–475.
- Collard J, Matthew AM, Double JA, et al. EO9: relationship between DT-diaphorase levels and response in vitro and in vivo. Br J Cancer. 1995;71:1199–1203.
- Kal HB, Karim ABMF, Hendriks HR. The efficacy of EO9 with radiation in experimental rat tumours. Ann Oncol. 1994;5(Suppl 5):88.
- Phillips RM, Hulbert PB, Bibby MC, et al. In vitro activity of the novel indoloquinone EO-9 and the influence of pH on cytotoxicity. Br J Cancer. 1992;65:359–364.
- Choudry GA, Stewart PA, Double JA, et al. A novel strategy for NQO1 (NAD(P)H: quinoneoxidoreductase, EC 1.6.99.2) mediated therapy of bladder cancer based on the pharmacological properties of EO9. Br J Cancer. 2001;85:1137–1146.
- Siegel D, Beall H, Senekowitsch C, et al. Bioreductive activation of mitomycin C by DT-diaphorase. Biochemistry. 1992;31:7879–7885.
- Phillips RM, Naylor MA, Jaffar M, et al. Bioreductive activation of a series of indolequinones by human DT-diaphorase: structure-activity relationships. J Med Chem. 1999;42:4071–4080.
- Samuni AM, DeGraff W, Krishna MC, et al. Nitroxides as antioxidants: Tempol protects against EO9 cytotoxicity. Mol Cell Biochem. 2002;234-235:327–333.
- Schellens JH, Planting AS, Van Acker BA, et al. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. 1994;86:906–912.
- Hendricksen K, Gleason D, Young JM, et al. Safety and side effects of immediate instillation of apaziquone following transurethral resection in patients with nonmuscle invasive bladder cancer. J Urol. 2008;180:116–120.
- Puri R, Palit V, Loadman PM, et al. Phase I/II pilot study of intravesical apaziquone (EO9) for superficial bladder cancer. J Urol. 2006;176:1344–1348.
- van der Heijden AG, Moonen PM, Cornel EB, et al. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: toxicity and marker response. J Urol. 2006;176:1349-53; discussion 53.
- Gofrit ON, Zorn KC, Shikanov S, et al. Marker lesion experiments in bladder cancer–what have we learned? J Urol. 2010;183:1678–1684.
- Hendricksen K, van der Heijden AG, Cornel EB, et al. Two-year follow-up of the phase II marker lesion study of intravesical apaziquone for patients with non-muscle invasive bladder cancer. World J Urol. 2009;27:337–342.
- Jain A, Phillips RM, Scally AJ, et al. Response of multiple recurrent TaT1 bladder cancer to intravesical apaziquone (EO9): comparative analysis of tumor recurrence rates. Urology. 2009;73:1083–1086.
- Hendricksen K, Cornel EB, de Reijke TM, et al. Phase 2 study of adjuvant intravesical instillations of apaziquone for high risk nonmuscle invasive bladder cancer. J Urol. 2012;187:1195–1199.
- Yutkin V, Chin J. Apaziquone as an intravesical therapeutic agent for urothelial non-muscle-invasive bladder cancer. Expert Opin Investig Drugs. 2012;21:251–260.
- Karsh L, Shore N, Saltzstein D, et al. Integrated results of two multicenter, randomised, placebo controlled, double blind, phase 3 trials (SPI-611/612) of single dose intravesical apaziquone immediately following resection in patients with nonmuscle invasive bladder cancer. J Urol. 2016;95(Supplement):e290.
- Witjes JA, Karsh L, Soloway M, et al. Improved efficacy of adjuvant, single dose intravesical apaziquone by timing post-resection in two double blind, randomised, placebo-controlled phase 3 studies in non-muscle invasive bladder cancer. J Urol. 2016;195(Supplement):e136.
- Loadman PM, Bibby MC, Phillips RM. Pharmacological approach towards the development of indolequinone bioreductive drugs based on the clinically inactive agent EO9. Br J Pharmacol. 2002;137:701–709.
- Dirix LY, Tonnesen F, Cassidy J, et al. EO9 phase II study in advanced breast, gastric, pancreatic and colorectal carcinoma by the EORTC Early Clinical Studies Group. Eur J Cancer. 1996;32A:2019–2022.
- Connors TA. Bioreductive agents, hypoxic cells and therapy. Eur J Cancer. 1996;32A:1833–1834.
