Abstract
Peptic-tryptic (PT) digested prolamins from spelt wheat Triticum aestivum ssp. spelta and landraces ERSA 6 and ERSA 8 of farro wheat T. turgidum ssp. dicoccum were found to agglutinate K562(S) cells, and exert strong toxic effects on Caco-2/TC7 cells. Cytotoxicity of spelt prolamins against Caco-2/TC7 cells was greatly reduced by 10-mer peptide QQPQDAVQPF. By contrast, the PT digests from monoccum wheat (Triticum monococcum) and farro landraces Prometeo, L5563, L5540 and L5558 did not exhibit any negative effects on K562(S) and Caco-2/TC7 cells. Toxic genotypes ERSA 6 and ERSA 8 were found to share the same gliadin pattern, which was absent in inactive landraces. Monococcum, farro and spelt wheats differed from each other in their responses to antibodies specific for 13-mer cytotoxic sequence FPGQQQPFPPQQP and 10-mer peptide QQPQDAVQPF. This latter sequence was found to occur in high amounts in common wheat line FG, Phaseoulus vulgaris, Ph. coccineus and Lens culinaria.
Introduction
Celiac disease (CD), the intestinal inflammatory disorder induced by prolamins in susceptible individuals, occurs as a result of the interplay between genetic and environmental factors. In particular, the celiac patients possess human leukocyte antigen (HLA) alleles DQA1*05/DQB1*02 or DQA1*0301/DQB1*0302 encoding for the HLA class II molecules DQ2 and DQ8, respectively (Louka and Sollid Citation2003). However, celiac enteropathy develops in less than 4% of individuals possessing the DQ2 or DQ8 molecules (Sollid and Thorsby Citation1993), suggesting that additional genetic factors may increase the risk for CD. On the other hand, the occurrence of monozygotic twin pairs that are discordant for CD (Sollid and Thorsby Citation1993) indicates that environment contributes to the development of the disease as well.
The environmental factor that triggers CD is the ingestion of prolamins, the alcohol-soluble proteins present in the endosperm of cereal grain (Shewry and Tatham Citation1990). Prolamins of common wheat (Triticum aestivum) are the main components of gluten, which is responsible for the unique viscoelastic properties of wheat dough, leading to a myriad of food products and industries (Miflin et al. Citation1983). In particular, wheat prolamins consist of two groups of polypeptides, gliadins and glutenins. On the whole, a single cultivar of common wheat contains 65–80 different prolamin molecules encoded by genes clustered into 20 loci on six chromosomes (Payne Citation1987; Pogna Citation2002). Furthermore, the prolamin patterns of wheat plants show an extremely high level of polymorphism, which includes variation in the presence/absence, amino acid sequence, amount and chemical properties of each prolamin component (Payne Citation1987; Metakovsky Citation1991; Jackson et al. Citation1996).
Feeding trials and in vitro or in vivo screens based on celiac intestine mucosa found α/β-, γ- and possibly ω-gliadins to be noxious (Ciclitira et al. Citation1984; Howdle et al. Citation1984). Moreover, α/β-gliadins, γ-gliadins and glutenin subunits were shown to agglutinate human myelogenous leukemia K562(S) cells, an exclusive property of grain proteins harmful for celiac patients (De Vincenzi et al. Citation1995, Citation1996a, Citationb), and stimulate T cell clones derived from jejunal mucosa or peripheral blood of celiac individuals (van de Wal et al. Citation1999; Shan et al. Citation2002; Vader et al. Citation2002; Gianfrani et al. Citation2003). In addition, a few peptides were shown to elicit a strong and rapid T cells response in nearly all celiac patients, these immunodominant sequences occurring in α- or γ-gliadins (Sjostrom et al. Citation1998; van de Wal et al. Citation1998; Shan et al. Citation2002). On the other hand, α-gliadin-derived 13-mer peptide FPGQQQPFPPQQP (residues 31 to 43) was found to be weakly immunogenic but very active in triggering mucosal lesions and enterocyte apoptosis (Maiuri et al. Citation1996). On the whole, these findings suggest two distinct effects of prolamins in CD: one is a rapid cytotoxic effect on the intestinal epithelium; the other is an immune response involving T cells that recognize specific prolamin epitopes.
There is experimental evidence for a natural variation in noxiousness of prolamins from the different cereal species. Rye and barley show some degree of ‘toxicity’ in CD, whereas rice and maize have generally been considered safe for celiac patients (Dicke et al. Citation1953; Cornell and Townley Citation1974; Baker and Read Citation1976; Anand et al. Citation1978). Furthermore, recent works have provided impressive results showing that oats is tolerated by CD patients, with the only exception of certain individuals possessing peculiar DQ2-restricted T cells (Picarelli et al. Citation2001; Janatuinen et al. Citation2002; Kilmartin et al. Citation2003; Arentz-Hansen et al. Citation2004). More interestingly, large differences in the harmfulness profile of prolamins have been found in the genus Triticum, which contains diploid, tetraploid and hexaploid species.
Common (or bread) wheat and its primitive sub-group spelt wheat (T. aestivum ssp. spelta) contain the A, B and D genomes, which accumulated in a single plant as a consequence of natural hybridization between the diploid D-genome wheat T. tauschii and the tetraploid AB-genome wheat T. turgidum. This latter species originated through spontaneous hybridization between the diploid A-genome T. urartu and B-genome species T. speltoides or longissima or searsii ().
Table 1 The main wheat (Triticum and Aegilops) species and their genome compositions.
About 10,000 years ago, T. monococcum, an A-genome wheat species closely related genetically to T. urartu and characterized by hulled kernels, was domesticated in the Fertile Crescent and cultivated for more than 6000 years in the Mediterranean basin and Central Europe to prepare bread and beer. During the Bronze Age, the tetraploid AB-genome species T. turgidum ssp. dicoccum was the most cultivated wheat in Egypt. This hulled species was the main cereal crop during the Roman period under the Latin name of ‘farrum’, and still survives as a crop in marginal areas of Italy, Turkey and Balkan countries where is used for feeding livestock and human consumption. Hulled farro wheat underwent a natural mutation that originated the free–threshing wheat species T. turgidum ssp. durum (also known as durum wheat) currently grown in Mediterranean countries for pasta.
Prolamins from monococcum wheat were found unable to agglutinate the K562(S) cells and trigger lesions in cultured intestinal mucosa from celiac patients (Auricchio et al. Citation1982; De Vincenzi et al. Citation1996c). In addition, T. monococcum accessions poor in T-cell stimulatory sequences have been recently described by Molberg et al. (Citation2005). On the other hand, gliadin-derived peptides from durum wheat were found to exert much less adverse effects on intestinal mucosa from celiac patients as compared with those from common wheat (Auricchio et al. Citation1982), whereas a few durum wheat cultivars were not recognized by T-cell clones specific for some immunodominant α-gliadin epitopes (Molberg et al. Citation2005). Reduced or no damages were also caused by bread wheat genotypes after removal of some α-, γ- or ω-gliadins (Frisoni et al. Citation1995; De Vincenzi et al. Citation1996a). More recently, low amounts of T-cell-stimulatory sequences have been observed in two T. aestivum accessions by Spaenij-Dekking et al. (Citation2005). Finally, gliadin sequence QQPQDAVQPF with a molecular mass of 1157.5 Da isolated from the durum wheat cv. Adamello was found to prevent agglutination of K562(S) cells induced by prolamins from bread wheat (De Vincenzi et al. Citation1997).
Wheat genotypes poor in noxious prolamins are of considerable interest in breeding programs aimed at developing wheat cultivars useful for CD prevention and tolerated by most celiac patients. Therefore, in the present work, prolamins from three hulled wheat species, i.e. monococcum (T. monococcum), farro (T. turgidum ssp. dicoccum) and spelt wheat (T. aestivum ssp. spelta), which represent different periods of wheat cultivation, were analyzed for their compositions in cytotoxic sequences, and for their effects on K562(S) cells and human colon adenocarcinoma Caco-2/TC7 cells grown in vitro.
Materials and methods
Plant and chemical materials
Seeds of monococcum wheat (T. monococcum, genome AA, 2n = 14), farro wheat (T. turgidum ssp. dicoccum, genome AABB, 2n = 28), spelt wheat (T. aestivum ssp. spelta, genome AABBDD, 2n = 42) and common wheat (T. aestivum, genome AABBDD, 2n = 42) from the collection maintained by the Istituto Sperimentale per la Cerealicoltura, Rome, Italy, were used in the present study.
The 10-mer QQPQDAVQPF peptide (De Vincenzi et al. Citation1997) and the polyclonal antibodies against this latter peptide and 13-mer peptide FPGQQQPFPPQQP were synthesized by Primm Company (Milan, Italy).
Extraction and digestion of prolamins
After removing the hulls, seeds (100 g) were milled with an experimental mill and added to 400 ml (w/v) of 0.5 M NaCl. The suspension was stirred at 4°C for 1 h and then centrifuged at 2000 g for 15 min at 4°C. Proteins in the supernatant (albumin and globulin) were eliminated. This operation was repeated twice. The pellet was resuspended in 300 ml of 70% (w/v) ethanol, extracted for 1 h at room temperature under stirring, and centrifuged at 2000 g for 20 min at 20°C. The supernatant containing prolamins was frozen and freeze-dried. Prolamins were submitted to peptic-tryptic (PT) sequential digestion as described by De Ritis et al. (Citation1979). At the end of the procedure, the PT digest was heated for 30 min at 100°C, lyophilised and stored at −20°C. The protein content of the PT digest was determined according to Lowry et al. (Citation1951).
Extraction and fractionation of gliadin by A-PAGE
Extraction of gliadins was performed as described previously (Pogna et al. Citation1990). Gliadin polypeptides were extracted from single crushed seeds (25 mg) with 75 µl of 70% (v/v) ethanol for 1 h at room temperature under costant agitation. After centrifugation at 15,000 g for 10 min, the protein suspension (25 µl) was mixed with 25 µl of an aqueous solution containing 50% glycerol and 0.1% (w/v) pyronine Y. An aliquot (25 µl) of the suspension was fractionated by acid polyacrylamide gel electrophoresis (A-PAGE) at pH 3.1 in a Hoeffer SE 600 apparatus (Amersham) using a 7.5% acrylamide gel (T = 7.5% and C = 0.37%) at 450 constant voltage until 1 hour after the dye reached the bottom of the gel. The separating gel (16×18 cm), 1.5 mm thick, was prepared by adding to acrylamide 80 mg of ascorbic acid, 8 ml of sodium lactate buffer (3.4 g/l of 97% NaOH adjusted to pH 3.1 with lactic acid) and 120 µl of 1% ferrous sulphate, brought to 80 ml with distilled water. After cooling at 4°C, the acrylamide solution was mixed with 24 µl of 2.5% hydrogen peroxide and immediately poured into the gel cassette. The electrophoretic buffer was 0.17 g/l of 97% NaOH adjusted to pH 3.1 with lactic acid.
Extraction and fractionation of total proteins by SDS-PAGE
Total proteins from individual crushed seeds (25 mg) were extracted with 0.5 ml of a solution containing 0.25 M Tris-HCl buffer (pH 6.8), 0.12% (w/v) SDS, 10% (v/v) glycerol, 0.2% (w/v) pyronin Y and 5% 2-mercaptoethanol and shaken for 1 h at room temperature. After incubation at 80°C for 20 min and centrifugation at 15,000 g for 10 min, an aliquot (20 µl) of the protein suspension was fractionated on SDS-PAGE running gels prepared with 15% (w/v) acrylamide (T = 15% and C = 0.5%), 0.375 M Tris-HCl (pH 8.4) and 0.1% (w/v) SDS. Stacking gels contained 4.5% (w/v) acrylamide (T = 4.5% and C = 0.06%), 0.08M Tris-HCl (pH 6.8) and 0.1% (w/v) SDS. The electrophoresis buffer was 0.025M Tris-glycine (pH 8.3) and 0.1% (w/v) SDS. The gels (160×180×1.5 mm) were run at 18 mA/gel. Electrophoresis was stopped 1 h after the tracking dye had reached the bottom of the gel. Comassie Brillant Blue R250 in 6% trichloracetic acid was used to stain both A-PAGE and SDS-PAGE gels.
K562(S) cell culture and agglutination test
The K562(S) subclone of human myelogenous leukemia origin was cultured as described previously (Auricchio et al. Citation1984). Cells were harvested by centrifugation, washed twice with calcium-free and magnesium-free phosphate-buffered saline solution (PBSS), and resuspended in this buffer at a concentration of 108 cells/ml. The cell suspension (25 µl) was added to each well of a 96-well microtitre plate containing different PT-digest concentrations obtained by serial diluitions (1:1) with PBSS. The final total volume was 100 µl. After 30 min incubation at room temperature, a drop of cell suspension was analysed with a microscope to count clumped and single cells. The agglutination test was repeated twice, using control wells as appropriate.
Caco-2/TC7 cell culture
The human colon adenocarcinoma Caco-2/TC7 cells were grown in a controlled atmosphere of 5% CO2 at 37°C in the Dulbecco's modified essential medium (DMEM) containing 4.5 g/l glucose, 2 mM L-glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin, 1% nonessential amino acids, 1% HEPES and 10% heat-inactivated fetal calf serum (FCS), as described previously (De Angelis et al. Citation1998). Experiments were performed using cells between passages 85 and 100.
Caco-2/TC7 cell viability
Caco-2/TC7 cells were seeded at a density of 5×103 cell/well in a 96-multiwell plate. Twenty-four hours after seeding, cells were exposed to 1 mg/ml of the PT digest for 48, 72 and 96 h. Cell viability was measured by the Neutral Red uptake assay (Borenfreund and Puerner Citation1985). The absorbance was read on a Novapath microplate reader (Biorad, Hercules, CA, USA) at a wavelength of 540 nm.
Apoptosis of Caco-2/ TC7 cells
Caco-2/TC7 cells were seeded in 100 mm plates at a density of 3.2×103 cell/cm2. On the fifth day after plating, cells were washed with serum-free medium and treated with 1 mg/ml of PT digest for 24 h in complete culture medium with 5% FCS. Staurosporine (1 µM) was used as a positive control. Apoptosis was evaluated using the In Situ FLICA Pan-Caspase Detection Kit (Chemicon International, Temecula, CA, USA), a fluorescent-based assay for detection of active caspases. The green fluorescent signal produced by FLICA was measured by a 96-well plate reader using an excitation wavelength of 490 nm and an emission wavelength of 520 nm. The fluorescent signal was a direct measure of the amount of active caspases present in the Caco-2/ TC7 cells.
Nitric oxide release by Caco-2/TC7 cells
Caco-2/TC7 cells were plated at a density of 2.5×104 cells/well and grown for 7 days. Following 24 h of incubation at 37°C in the presence of 1mg/ml of PT digest, 500 µl of culture medium were taken from the plates and mixed with an equal volume of Griess reagent (1% sulfanilic acid in 0.5N HCl and 0.1% N′-1-naphtyl-ethylendiamine-hydrochloride). Absorbance at 540 nm was measured after 30 min of incubation at room temperature in the dark. The combined concentrations of NO2- and NO3-, the degradation products of the nitric oxide in the culture medium, were determined by the Griess reaction (Green et al. Citation1982). The nitrite concentration was determined by reference to a standard curve of sodium nitrite and values were reported as µM nitrite /mg protein/ml.
Transepithelial electrical resistance of Caco-2/TC7 cells
Caco-2/TC7 cells were plated on polycarbonate (Falcon) filters with 0.45 mm pore diameter and 0.9 cm2 area, and left to grow for 20 days in order to achieve a high differentiation level displaying tight junctions and polarization. The transepithelial electrical resistance (TER) of differentiated Caco-2/TC7 monolayers was measured using a Millicell electrode (Millipore Co., Bedford, MA, USA), and expressed in Ohms×cm2. Two hours before the treatment with the PT digests, cells were equilibrated in phosphate-buffered saline (PBS) solution containing glucose and 5% FCS. TER values were measured before (TER0) and after (TER1) 30 min exposure to 1 mg/ml of PT-digested prolamins, and the TER1/ TER0 ratio was calculated.
Immnunofluorescence
Caco-2/TC7 cells were grown on slides and let differentiate for 21 days. Cells were exposed to the different digests for 30 min and 4 h and then washed gently three times with PBS and fixed/permeabilized in 100% ethanol at −20°C for 20 min. Non specific background was blocked with 5% powdered milk in PBS and 0.1% Tween (1 h at room temperature). Monolayers were incubated overnight with primary antibody to zonula occludens protein-1 (ZO-1) at 1/400 dilution (Zymed Laboratories Inc., San Francisco, USA). After washing with PBS the slides were probed with Alexa-Fluor- 488/555 (1/100) (Molecular Probe) secondary antibody for 1 hour and rinsed again with PBS.
Western blotting
Western blotting was performed in a Bio-Rad semi-dry transfer cell using nitrocellulose membranes (Immobilon-NCMillipore). SDS-PAGE gels were equilibrated for 30 min in a transfer buffer, pH 9.2, containing 80 mM Tris, 13 mM glycine and 20% (v/v) methanol in distilled water, whereas A-PAGE gels were equilibrated for 1 hour in the same transfer buffer added with 1% 2-mercaptoethanol. Gels were then transferred between two double layers of 3 MM chromatography paper (Whatman) and electro-blotted at 14 V for 40 min. After the transfer, membranes were maintained for 1 h in PBS buffer containing 5% (w/v) powdered milk as a blocking agent, and incubated for 16 h in the same buffer containing 0.2% (w/v) blocking agent and the specific antiserum diluted 1:500. After incubation with a 1:2500 dilution of a goat anti-rabbit horseradish peroxidase conjugate (Promega. USA), blots were stained with 4-chloro-1-naphthol and hydrogen peroxide.
Statistical analysis
Each experiment was run in duplicate and repeated three times. Data expressed as means were analysed using the two sided Student's t-test for unpaired variables.
Results
Different gliadin patterns in farro wheats
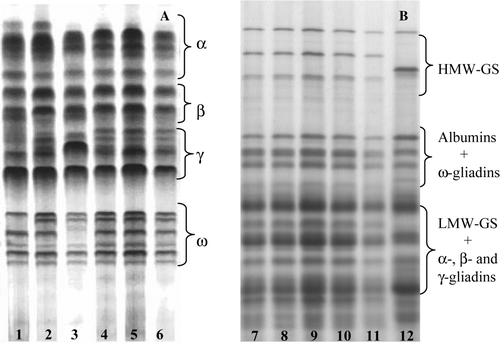
Upon A-PAGE, gliadins from the dicoccum landraces appeared as 18 to 25 bands, which are classified as α, β-, γ- and ω-gliadins according to their mobilities in . Fractionation of 10 single seeds from each dicoccum landrace revealed two to three different gliadin compositions (biotypes) in Ersa 8 (biotypes a and b), Prometeo (biotypes c, d and e) and L5563 (biotypes f and g), whereas Ersa 6 (biotype b), L5540 (biotype h) and L5558 (biotype k) exhibited a single gliadin pattern (A). Fractionation of total proteins by SDS-PAGE revealed a wide genetic variability for high-molecular weight glutenin subunits (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS) amongst the dicoccum wheats (B). The banding patterns of gliadins, HMW-GS and LMW-GS of the endosperm of dicoccum wheats were quite distinctive with respect to those of monococcum, durum, spelt and common wheats (data not shown).
Agglutination of K562(S) cells
PT-digested prolamins from bread wheat, rye and barley were found to possess the exclusive property of driving agglutination of the K562(S) cells (De Vincenzi et al. Citation1995, Citation1996a, Citationb). Here, the minimal agglutinating concentration (MAC) of PT-digested prolamins from both spelt and common wheat required to agglutinate 100% of K562(S) cells was 73 mg/l (). The same MAC value was observed for farro wheat landraces Ersa 6 and Ersa 8, whereas prolamins from landraces Prometeo, L5563, L5540 and L5558, and from monococcum wheat Cv. Monlis did not show any agglutination activity even when tested at a concentration as high as 5000 mg/l. In the presence of 1 mg/ml of the QQPQDAVQPF sequence, the 10-mer protective peptide (PP) present in the PT-digested prolamins of durum wheat cv. Adamello (De Vincenzi et al. Citation1997), cell agglutination by spelt prolamins was completely inhibited, and the K562(S) cells maintained their normal appearance.
Table 2 Agglutination of K562(S) cells by PT-digested prolamins from different wheat species.
Effects of PT-digested prolamins on Caco-2/TC7 cells
Viability of Caco-2/TC7 cells as measured by the neutral red (NR) uptake was significantly inhibited by 1 mg/ml of digested prolamins from common wheat cv. S. Pastore, spelt wheat and dicoccum wheat landraces Ersa 6 and Ersa 8, as compared with untreated control cells (). By contrast, NR uptake by Caco-2/TC7 cells treated with prolamins from monococcum or dicoccum wheat landraces Prometeo, L5540, L5558 and L5563, was not significantly different from that of control cells during the entire time of exposure to prolamins. Moreover, the 10-mer protective peptide (PP) added to the cell culture at a concentration of 1 mg/ml reduced remarkably the viability impairment caused by spelt prolamins.
Table 3 Neutral red (NR) uptake, active caspases (AC) production, nitric oxide (NO) release and transepithelial electric resistance (TER) in Caco-2/TC7 cells treated with PT-digested prolamins from different wheat species.
The amounts of active caspases (AC) present in the Caco-2/TC7 cells treated with 1 mg/ml of PT-digested prolamins from dicoccum landraces Ersa 6 and Ersa 8 and spelt wheat were similar to that observed in common wheat cv. S. Pastore, and significantly higher that those measured in untreated cells (). On the contrary, prolamins from monococcum or dicoccum wheats Prometeo, L5540, L5558 and L5563 caused a small, not significant increase of caspase activity with respect to control cells. The strong pro-apoptotic activity induced by prolamins from spelt wheat was reduced by addition of 1 mg/ml of the protective peptide. Finally, the Caco-2/TC7 cells exposed to prolamins from spelt wheat and farro landraces Ersa 6 and Ersa 8 showed high values of NO concentration and low TER1/TER0 ratios as compared to control cells (). Untreated cells and those treated for 24 h with digested prolamins from monococcum and farro landraces Prometeo, L5540, L5558 and L5563 showed small, not significant differences in NO concentration and transepithelial electric resistance (). Exposition of the Caco-2/TC7 cells to spelt prolamins in the presence of 1mg/ml of protective peptide caused a moderate release of nitric oxide in the culture medium, and a small impairment of their transepithelial electrical resistance.
ZO-1 level in Caco-2/TC7 cells
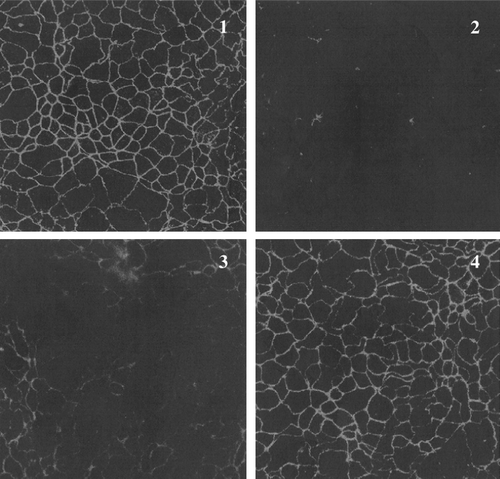
Zonula occludens protein-1 (ZO-1), the human membrane-associated protein that localizes at both the tight and adherens junctions found at sites of cell-cell contact, showed a different response to prolamins from the wheat genotypes analyzed, as revealed by confocal immunoflorescence microscopy (). In particular, prolamins from spelt wheat and farro landrace Ersa 6 induced ZO-1 disruption in a very short time, whereas dicoccum landraces Prometeo, L5540, L5558 and L5563 did not cause any alteration of this protein. ZO-1 in cells treated with spelt or Ersa 6 prolamins returned to its basal state three hours after prolamin exposure.
Western blotting of prolamins in wheat species
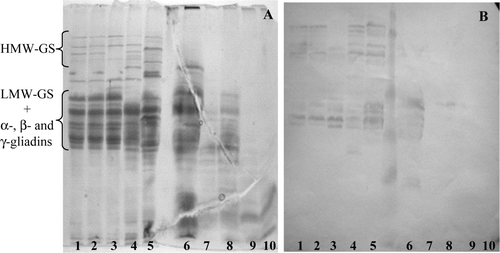
The antiserum developed against the P31–43 peptide (sequence FPGQQQPFPPQQP), an α-gliadin-derived fragment claimed to be very active in driving enterocyte damage, reacted with several HMW glutenin subunits in the SDS-PAGE patterns of dicoccum wheat landraces (B, lanes 1–5). Immunoreactive bands were also found in the gel region containing LMW-GS and α-, β- and γ-gliadin bands. However, no reaction was observed in the SDS-PAGE patterns of PT-digested prolamins (B, lanes 6–10).
Figure 3. (A) SDS-PAGE patterns of (1–5) native and (6–10) PT-digested endosperm proteins, and (B) their reactions with anti-FPGQQQPFPPQQP (P31–43) antiserum. The dicoccum wheat landraces analysed are (1, 6) Leonessa 1; (2, 7) Leonessa 4; (3, 8) Leonessa 5; (4, 9) Ersa 6 and (5, 10) Ersa 8.
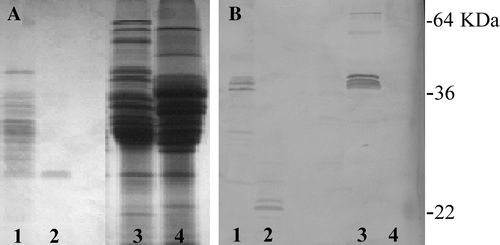
The gliadin QQPQDAVQPF sequence is a 10-mer molecule isolated from the PT-digested prolamins of durum wheat cv. Adamello and found to prevent agglutination of K562(S) cells induced by ethanol-soluble proteins from bread wheat (De Vincenzi et al. Citation1997). Western immunoblotting of PT-digested prolamins from cv. Adamello fractionated by SDS-PAGE and exposed to the polyclonal antiserum developed against this protective peptide (PP) revealed a pair of bands whose MWs were in the range of 22 KDa (B, lane 2). Interestingly, no immunoreactive band was found in the SDS-PAGE pattern of native (undigested) prolamins extracted from cv. Adamello (B, lane 4). Amongst dozens of durum or common wheat cultivars immunoblotted with the anti-PP antiserum, only commom wheat line FG showed four reactive bands, about 45 KDa in size, in the SDS-PAGE pattern of native endosperm proteins (B, lane 3). These immunoreactive bands were partly resistant to the PT digestion, and were detected in the immunoblot of PT-digested proteins fractionated by SDS-PAGE (, lane 1).
Figure 4. (A) SDS-PAGE patterns of (1, 2) PT-digested and (3, 4) native endosperm proteins and (B) their reactions with anti-QQPQDAVQPF antiserum. (1, 3) common wheat cv. FG; (2, 4) durum wheat cv Adamello.
An immunoreactive band having an apparent MW of approximately 32 KDa occurred in the immunoblot of PT-digested prolamins from dicoccum landrace L5563 fractionated by SDS-PAGE and exposed to the anti-PP antiserum (, lane 1). A weak immunoreaction against a protein band of similar MW was also observed in PT-digested prolamins from landraces Prometeo and Ersa 8 (, lanes 6 and 7). However, spelt wheat, monococcum wheat or dicoccum wheat landraces showed no reactive protein in the immunoblots of native prolamins exposed to the anti-PP antiserum ().
Figure 5. (A) Immunoblotting of anti-PP antiserum against (B) PT-digested prolamins fractionated by SDS-PAGE. The dicoccum wheat landraces are (1) L5563, (2) L5558, (3) L5540, (4) Filosini, (5) Prometeo, (6) Ersa 8, (7) Ersa 6, (8) Leonessa 5 and (9) Leonessa 4. Arrow indicates a band protein showing a weak immunoreaction.
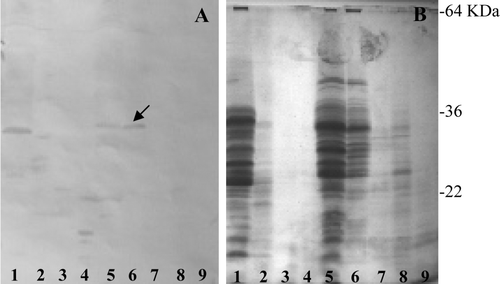
Figure 6. Immunoblotting of anti-PP antiserum against kernel proteins fractionated by SDS-PAGE. (1) T. monococcum; (2) T. aestivum line FG; (3) T. aestivum ssp. spelta and (4) Phaseolus vulgaris.
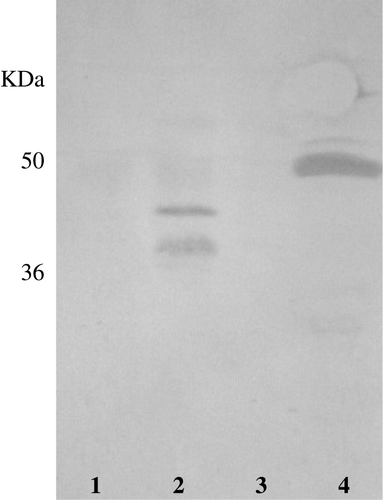
Interestingly, the anti-PP antiserum showed a strong reaction against a group of proteins, approximately 50 KDa in size, extracted with a 0.5 M NaCl solution from crushed kernels of Phaseolus vulgaris, Ph. coccineus and Lens culinaria (, lane 4).
Discussion
When fractionated by A-PAGE and SDS-PAGE, the dicoccum landraces analyzed here exhibited prolamin patterns quite distinctive with respect to those of monococcum, durum, spelt and common wheat. This observation is in agreement with earlier reports by Galterio et al. (Citation1994) that T. turgidum ssp. dicoccum is a source of prolamins with unusual primary structures. Moreover, dicoccum landraces Ersa 6 and Ersa 8 are related genetically to each other, as suggested by the presence of gliadin biotype b in both genotypes.
Earlier studies (De Vincenzi et al. Citation1996c; Molberg et al. Citation2005; Vincentini et al. Citation2007) showed diploid and tetraploid hulled wheat species T. monococcum and T. turgidum ssp. dicoccum to be poor in or devoid of noxious prolamins. This observation is in agreement with the absence of cytotoxic effects caused by monococcum and dicoccum landraces Prometeo, L5540, L5558 and L5563 on Caco-2/TC7 cells. However, farro landraces Ersa 6 and Ersa 8 were comparable to spelt and common wheat in triggering agglutination of K562(S) cells and damage of Caco-2/TC7 cells. In particular, PT-digested prolamins from landraces Ersa 6 and Ersa 8, together with those from spelt wheat, were found to be able to induce zonula occludens protein-1 disruption and apoptosis signalling, which contributes to epithelial barrier dysfunction and increased epithelial permeability. This finding is in agreement with recent works showing that gliadins are able to decrease transepithelial resistance in normal intestinal epithelial cells grown in vitro, and increase their permeability to small molecules. Moreover, gliadins were found to activate the zonulin signalling pathway and cause a cytoskeleton rearrangement with a redistribution of actin filaments in the intracellular subcortical compartment (Clemente et al. Citation2003). Finally, Caco-2 cells exposed to gliadin were found to release zonulin in the cell medium and return to baseline 60 minutes post-gliadin incubation (Drago et al. Citation2006).
The very toxic dicoccum genotypes Ersa 6 and Ersa 8 were found to share gliadin biotype b, which was absent in the inactive farro genotypes. On the other hand, cytotoxicity of Ersa 6 and Ersa 8 demonstrates that toxic prolamins are not confined to the D genome. In this context it is noteworthy that dicoccum accessions have been found to differ significantly from each other in the level of T-cell-stimulatory epitopes as well (Spaenij-Dekking et al. Citation2005).
The gliadin QQPQDAVQPF sequence isolated from the gliadin fraction of durum weat cv. Adamello was able to prevent agglutination of K562(S) cells and restrain cellular damages to Caco-2/TC7 cells caused by spelt prolamins. Taking into account the different mechanisms involved in the responses of K562(S) and Caco-2/TC7 cells to toxic prolamins, the protective effect of gliadin fragment QQPQDAVQPF is likely due to its interference in the recognition of prolamin peptides by those cells. It is noteworthy that prolamin-derived peptides able to prevent agglutination of K562(S) cells by toxic prolamins also occur in monococcum wheat (De Vincenzi et al. Citation1996c), suggesting that inactivity of prolamins from monococcum wheat against intestinal cells could be partly due to the presence of protective sequences in this species.
HMW glutenin subunits and a few prolamins of low molecular weights occurring in the total protein extracts of dicoccum landraces were found to react strongly with the polyclonal antiserum developed against the P31–43 peptide (sequence FPGQQQPFPPQQP), a chromosome-6A encoded gliadin able to drive mucosal lesions and enterocyte apoptosis (Maiuri et al. Citation1996). This finding suggests that toxic peptide P31–43 peptide is not confined to a single gliadin molecule but also occurs in prolamin polypeptides encoded by chromosomes 1A and 1B. After PT digestion, no immunoreactive band was detected in the SDS-PAGE prolamin pattern of all dicoccum landraces.
Common wheat line FG is quite unique in having the protective QQPQDAVQPF sequence in the primary structure of four ω-gliadin polypeptides, these molecules being partly resistant to PT digestion. However, reduced amounts of immunoreactive peptides were also found in the PT-digested prolamins of durum wheat cv. Adamello and dicoccum landraces L5563, Prometeo and Ersa 8. On the other hand, kernels of Phaseolus vulgaris, Ph. coccineus and Lens culinaria were found to contain high amounts of storage proteins possessing the protective QQPQDAVQPF sequence.
The present findings suggest that wheat is an heterogeneous group of genotypes with a wide variability in their prolamin composition and, as a consequence, in their effects on the intestinal epithelium. More interestingly, prolamins from four dicoccum landraces were found to lack intestinal cell toxicity and cause very small changes, if any, in ZO-1 localization and intestinal permeability. Furthermore, monococcum wheat did not show any negative effects on K562(S) or Caco-2/TC7 cells, supporting early observations that this wheat species is devoid or low in prolamin sequences able to trigger lesions in cultured intestinal mucosa from celiac patients (Auricchio et al. Citation1982; De Vincenzi et al. Citation1996c). A number of accessions of T. monococcum has also been found to possess low amount of T-cell stimulatory sequences from α- or γ-gliadins (Molberg et al. Citation2005; Spaenij-Dekking et al. Citation2005). Therefore, dicoccum and monococcum wheat could offer novel dietary opportunity for prevention of celiac disease in individuals at risk.
Acknowledgements
This work has been partly financed by the ‘Regione Lombardia’ through the ‘MONICA’ research project.
References
- Anand , BS , Piris , J and Truelove , SC . 1978 . The role of various cereals in coeliac disease . Q J Med , 185 : 101 – 110 .
- Arentz-Hansen , EH , Fleckenstein , B , Molberg , O , Scott , H , Koning , F , Jung , G , Roepstoff , P , Lundin , KEA and Sollid , LM . 2004 . The molecular basis for oat intolerance in celiac patients . Plos Med , 1 ( 1 ) : 84 – 92 .
- Auricchio , S , De Ritis , G , De Vincenzi , M , Minetti , M , Sapora , O and Silano , V . 1984 . Agglutination activity of gliadin-derived peptides from bread wheat: Implications for coeliac disease pathogenesis . Biochem Biophys Res Communic , 21 : 428 – 433 .
- Auricchio , S , De Ritis , G , De Vincenzi , M , Occorsio , P and Silano , V . 1982 . Effects of gliadin-derived peptides from bread and durum wheats on small intestine cultures from rat fetus and coeliac children . Pediatr Res , 16 : 1004 – 1010 .
- Baker , PG and Read , AE . 1976 . Oats and barley toxicity in coeliac patients . Postgrad Med J , 52 : 264 – 268 .
- Borenfreund , E and Puerner , JA . 1985 . Toxicity determined in vitro by morphological alterations and neutral red uptake absorption . Toxicol Lett , 24 : 119 – 124 .
- Ciclitira , PJ , Evans , DJ and Fagg , NLK . 1984 . Clinical testing of gliadin fractions in celiac patients . Clin Sci , 66 : 357 – 361 .
- Clemente , MG , De Virgiliis , S , Kang , JS , Macatagney , R , Musu , MP , Di Pierro , MR , Drago , S , Congia , M and Fasano , A . 2003 . Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function . Gut , 52 ( 2 ) : 218 – 223 .
- Cornell , HJ and Townley , RW . 1974 . The toxicity of certain cereal proteins in coeliac disease . Gut , 15 : 862 – 869 .
- De Angelis , I , Vincentini , O , Brambilla , G , Stammati , A and Zucco , F . 1998 . Characterization of furazolidone apical relative effect to human polarized intestinal cell line . Toxicol Appl Pharmacol , 152 : 119 – 127 .
- De Ritis , G , Occorsio , P , Auricchio , S , Gramenzi , F , Morisi , G and Silano , V . 1979 . Toxicity of wheat flour and protein-derived pepdides for in vitro developing intestine from rat fetus . Pediatric Res , 13 : 1255 – 1261 .
- De Vincenzi , M , Dessì , MR , Giovannini , C , Maialetti , F and Mancini , E . 1995 . Agglutinating activity of wheat gliadin peptide fractions in coeliac disease . Toxicology , 96 ( 1 ) : 29 – 35 .
- De Vincenzi , M , Dessì , M , Luchetti , R , Pogna , NE , Redaelli , R and Galterio , G . 1996a . Toxicity of bread wheat lines lacking prolamins encoded by the Gli-B1/Gli-B5/Glu-B3 and Gli-D1/Glu-D3 loci in coeliac disease as determined by their agglutinating activity . ATLA , 24 : 39 – 48 .
- De Vincenzi , M , Luchetti , R , Peruffo , ADB , Curioni , A , Pogna , NE and Gasbarrini , G . 1996b . In vitro assessment of acetic-acid-soluble proteins (glutenin) toxicity in celiac disease . J Biochem Toxicol , 11 : 205 – 210 .
- De Vincenzi , M , Luchetti , R , Giovannini , C , Pogna , NE , Saponaro , C , Galterio , G and Gasbarrini , G . 1996c . In vitro toxicity testing of alchool-soluble proteins from diploid wheat Triticum monococcum in celiac disease . J Biochem Toxicol , 11 : 313 – 318 .
- De Vincenzi , M , Stammati , A , Luchetti , R , Silano , M , Gasbarrini , G and Silano , V . 1997 . Structural specificities and significance for coeliac disease of wheat gliadin peptides able to agglutinate or to prevent agglutination of K562(S) cells . Toxicology , 127 : 97 – 106 .
- Dicke , WM , Weijers , HA and Van de Kamer , JH . 1953 . The presence in wheat of a factor having a deleterious effect in cases of coeliac disease . Acta Paediatr , 42 : 34 – 42 .
- Drago , S , El Asmar , R , Di Pierro , M , Clemente , M , Tripathi , A , Sapone , A , Thakar , M , Iacono , G , Carroccio , A , D'Agate , C , Not , T , Zampini , L , Catassi , C and Fasano , A . 2006 . Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines . Scand J Gastroenterol , 41 : 408 – 419 .
- Frisoni , M , Corazza , GR , Lafiandra , D , De Ambrogio , E , Filippini , C , Bonvicini , F , Borasio , E , Porceddu , E and Gasbarrini , G . 1995 . Wheat deficient in gliadins: Promising tool for treatment of coeliac disease . Gut , 36 : 375 – 378 .
- Galterio , G , Cappelloni , M , Desiderio , E and Pogna , NE . 1994 . Genetic, technological and nutritional characteristics of three Italian populations of ‘farrum’ (Triticum turgidum ssp. dicoccum) . J Genet Breed , 48 : 391 – 398 .
- Gianfrani , C , Troncone , R and Mugione , P . 2003 . Celiac disease association with celiac disease + T cell responses: identification of a novel gliadin-derived HLA-DQ2-restricted epitope . J Immunol , 170 : 2719 – 2726 .
- Green , LC , Wagner , DA , Glogowski , J , Skipper , PL , Wishnok , JS and Tannenbaum , SR . 1982 . Analysis of nitrate, nitrite and [15N] nitrate in biological fluids . Anal Biochem , 126 : 131 – 138 .
- Howdle , PD , Ciclitira , PJ and Simpson , FO . 1984 . Are all gliadins toxic in coeliac disease? An in vitro study of α-, β-, γ-, ω-gliadins . Scand J Gastroenterol , 19 : 41 – 47 .
- Kilmartin , C , Lynch , S , Abuzakouk , M , Wieser , H and Feighery , C . 2003 . Avenin fails to induce a Th1 response in coeliac tissue following in vitro culture . Gut , 52 : 47 – 52 .
- Jackson , EA , Morel , MH , Sontag-Strohm , T , Branlard , G , Metakowsky , EV and Redaelli , R . 1996 . Proposal for combining the classification systems of alleles of Gli-1 and Glu-3 loci in bread wheat (Triticum aestivum L) . J Genet Breed , 50 : 321 – 336 .
- Janatuinen , EK , Kemppainen , TA , Julkunen , RJ , Kosma , VM , Maki , M , Heikkinen , M and Uusitupa , MI . 2002 . No harm from five-year ingestion of oats in coeliac disease . Gut , 50 : 332 – 335 .
- Louka , AS and Sollid , LM . 2003 . HLA in coeliac disease: Unravelling the complex genetics of a complex disorder . Tissue Antigens , 61 : 105 – 117 .
- Lowry , OH , Rosebrough , NJ , Farr , AL and Randall , RJ . 1951 . Protein measurement with Folin phenol reagent . J Biological Chem , 93 : 265 – 275 .
- Maiuri , L , Troncone , R and Mayer , M . 1996 . In vitro activities of A-gliadin-related synthetic peptides: Damaging effect on the atrophic coeliac mucosa and activation of mucosal immune response in the treated celiac mucosa . Scand J Gastroenterol , 31 : 247 – 253 .
- Metakovsky , EV . 1991 . Gliadin allele identification in common wheat II. Catalogue of gliadin alleles in common wheat . J Genet Breed , 45 : 325 – 344 .
- Miflin , BJ , Field , JM and Shewry , PR . 1983 . “ Cereal storage proteins and their effects on technological properties ” . In Seed proteins , Edited by: Daussant , J , Mosse , J and Vaughan , J . 255 – 319 . London : Academic Press .
- Molberg , O , Kjersti Uhlen , A , Jensen , T , Solheim Flaete , N , Fleckenstein , B , Arentz-Hansen , H , Raki , M , Lundin , KEA and Sollid , LM . 2005 . Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease . Gastroenterology , 128 : 393 – 401 .
- Payne , PI . 1987 . Genetics of wheat storage proteins and the effect of allelic variation on breadmaking quality . Ann Rev Plant Physiol , 38 : 141 – 153 .
- Picarelli , A , Di Tola , M , Sabbatella , L , Gabrielli , F , Di Cello , TE , Anania , MC , Mastracchio , A , Silano , M and De Vincenzi , M . 2001 . Immunologic evidence of no harmful effect of oats in coeliac disease . Am J Clin Nutrit , 74 : 137 – 140 .
- Pogna , NE . 2002 . Genetic improvement of plant for coeliac disease . Digestive Liver Dis , 34 : 154 – 159 .
- Pogna , NE , Autran , JC , Mellini , F , Lafiandra , D and Feillet , P . 1990 . Chromosome 1B-encoded gliadins and glutenin subunits in durum wheat: Genetics and relationship to gluten strenght . J Cereal Sci , 11 : 15 – 34 .
- Sjostrom , H , Lundin , KE , Molberg , O , Korner , R , McAdam , SN , Anthonsen , D , Quarsten , H , Noren , O , Roepstorff , P , Thorsby , E and Sollid , LM . 1998 . Identification of a gliadin T-cell epitope in celiac disease: General importance of gliadin deamidation for intestinal T-cell recognition . Scand J Immunol , 48 : 111 – 115 .
- Shan , L , Molberg , O , Parrot , I , Hausch , F , Filiz , F , Gray , GM , Sollid , LM and Khosia , C . 2002 . Structural basis for gluten intolerance in celiac sprue . Science , 297 : 2275 – 2279 .
- Shewry , PR and Tatham , AS . 1990 . The prolamins storage proteins of cereal seeds: Structure and evolution . Biochem J , 267 : 1 – 12 .
- Sollid , LN and Thorsby , E . 1993 . HLA susceptibility genes in celiac disease: Genetic mapping and role in pathogenesis . Gastroenterology , 105 : 910 – 922 .
- Spaenij-Dekking , L , Kooy-Winkelaar , Y , Van Veelen , P , Drijfhout , JW , Jonker , H , Van Soest , L , Smulders , MJM , Bosch , D , Gilissen , LJWJ and Koning , F . 2005 . Natural variation in toxicity of wheat: Potential for selection of non-toxic varieties for celiac disease patients . Gastroenterology , 129 : 797 – 806 .
- Vader , W , Kooy , Y , van Veelen , P , de Ru , A , Harris , D , Benckhuijsen , W , Pena , S , Mearin , L , Drijfhout , JW and Koning , F . 2002 . The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides . Gastroenterology , 122 : 1729 – 1737 .
- Van de Wal , Y , Kooy , YMC , van Veelen , P , Vader , W , August , SA , Drijfhout , JW , Pena , SA and Koning , F . 1999 . Glutenin is involved in the gluten-driven mucosal T cell response . Eur J Immunol , 29 : 3133 – 3139 .
- Van de Wal , Y , Kooy , YM , van Veelen , PA , Pena , SA , Mearin , LM , Molberg , O , Lundin , KE , Sollid , LM , Mutis , T , Benckhuijsen , WE , Drijfhout , JW and Koning , F . 1998 . Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin . Proc Natl Acad Sci USA , 95 : 10050 – 10054 .
- Vincentini , O , Maialetti , F , Gazza , L , Silano , M , Dessì , M , De Vincenzi , M and Pogna , NE. 2007 . Environmental factors of celiac disease: Cytotoxicity of hulled wheat species Triticum monococcum, T. turgidum ssp. dicoccum and T. aestivum ssp. spelta . J Gastroenterol Hepatol , 22 : 1816 – 1822 .