ABSTRACT
In this study, triacontanol (TRIA) and nitric oxide (NO) interaction on arsenic (As)-induced oxidative stress tolerance in coriander (Coriandrum sativum L.) plants was investigated. The results showed that As had a significant adverse effect on the plant’s biomass. The seedlings pretreated with TRIA and NO significantly increased growth reduction induced by the metalloid. The obtained results indicated that the application of TRIA and sodium nitroprusside (SNP) generally reduced oxidative markers such as of electrolyte leakage percentage, malondialdehyde and H2O2 contents under As toxicity, while application of As treatment without TRIA + SNP increased these oxidative parameters compared to the control. The non-enzymatic antioxidant contents such as total phenol, anthocyanin, carotenoid, ascorbic acid and reduced glutathione (GSH) were extracted and assayed from both control and treated plants. It was found that TRIA + SNP treatments have a profound effect on the antioxidant metabolism and caused an enhancement in non-enzymatic antioxidant potentials under As toxicity in coriander. Moreover, the results revealed a mutually amplifying reaction between TRIA and NO in reducing As-induced damages.
Introduction
Heavy metal (HM) toxicity as one of the major abiotic stresses induces hazardous effects in crop plants. A common consequence of HM toxicity is the excessive accumulation of reactive oxygen species (ROS) and methylglyoxal (MG), both of which can cause peroxidation of lipids, oxidation of protein, inactivation of enzymes, DNA damage and/or interact with other vital constituents of plant cells. Higher plants have evolved antioxidant defense systems to scavenge ROS and MG. In addition, HMs may be sequestered by organic acids, glutathione (GSH) or by specific metal-binding ligands in cells. GSH as a central molecule of antioxidant defense systems is involved in either direct or indirect control of ROS and MG and their reaction products in plant cells, thus protecting the plant from HM-induced oxidative damage (Hossain et al. Citation2012). Cells have evolved an elaborate system of enzymatic and non-enzymatic antioxidants which help to scavenge these indigenously generated ROS. Various enzymes involved in ROS-scavenging have been manipulated, over-expressed or down-regulated to add to the present knowledge and for understanding the role of the antioxidant systems (Ahmad et al. Citation2010; Gupta et al. Citation2013; Zu et al. Citation2016). When the first mechanisms of chloroplast redox regulation were being discovered, ROS were regarded as by-products of potentially beneficial reactions. Indeed, it remains the case that reduction of O2 to superoxide by the thylakoid electron transport chain can prevent over-reduction (redox poisoning) and contribute to chloroplast ATP pools via pseudo-cyclic phosphorylation. Nevertheless, as they became implicated in diverse stress responses, ROS subsequently gained a reputation as damaging molecules (Foyer & Noctor Citation2012).
As as HM affects the growth and development of plants and causes toxicity resulting in various biochemical and physiological disorders (Liu et al. Citation2005). As toxicity depends on many factors such as concentration of this pollutant, its exposure duration and physiological state of plants. As is released into the environment in both inorganic and organic forms. Arsenate (As(V)) and arsenite (As(III)) are the inorganic and phytoavailable forms of As in soil solution. Arsenate competes with phosphate and is considered as an analogue of phosphate. In addition, arsenate acts as an uncoupler of oxidative phosphorylation because it replaces phosphate in ATP to form unstable ADP–As and leads to the disruption of energy flow in cells (Stoeva et al. Citation2005). Even though As is not a redox metal, there is significant evidence that exposure of plants to inorganic As results in the generation of ROS, for example, O2−, OH− and hydrogen peroxide (H2O2), causes oxidative damage to biomolecules such as lipids and proteins, and eventually cause cell death (Gunes et al. Citation2009).
Triacontanol (TRIA) is a potent plant growth regulator, which has been reported to enhance growth and yield of several crop species when applied exogenously (Gatica et al. Citation2008; Naeem et al. Citation2011). TRIA has been reported to enhance photosynthesis and water and mineral nutrients’ uptake, regulate activities of various enzymes and increase the level of various organic compounds in leaf tissues (Ries & Houtz Citation1983; Ries Citation1991; Naeem et al. Citation2009, Citation2010). TRIA is known as an antioxidizing agent because it can effectively inhibit both enzymatic and non-enzymatic peroxidative breakdown of lipids (Ramanarayan et al. Citation2000). It is reported that TRIA after initial application elicits a metabolite or a secondary messenger, 9-β-L(+) adenosine, which rapidly induces a variety of physiological responses, and influences the activities of enzymes involved in carbohydrate metabolism (dry weight increase) (Khan et al. Citation2007; Naeem et al. Citation2009; Li et al. Citation2016). Nitric oxide (NO) is a free radical involved in numerous and diverse physiological processes in plants (Lamattina et al. Citation2003). Evidence has been obtained for the involvement of NO in plant growth and development, as well as in defense responses (Lamattina et al. Citation2003; Yu et al. Citation2005). Recently, an increasing number of articles have reported the effects of exogenous NO on alleviating HM toxicity in various plants (Jin et al. Citation2010; Xiong et al. Citation2010; Panda et al. Citation2011). Evidence has been obtained for the relation of NO and plant growth regulators (Palavan-Unsal & Arisan Citation2009; Yadav et al. Citation2011; Shen et al. Citation2013). However, there are not any studies that have identified the effects of TRIA and NO on stress-induced changes in the antioxidative defense system in plants under HM stresses. Thus, the objectives of the present study are to study the effect of plant growth regulator, TRIA, on change of non-enzymatic antioxidant contents in C. sativum plants under As stress and evaluation of amplifying reactions between TRIA and NO on reducing As-induced damages in this plant.
Materials and methods
Plant material, growth condition and treatment procedures
The seeds of C. sativum were washed with sterile distilled water and transferred to plastic pots containing perlite. Fresh nutrient solution (pH 5.7 ± 0.1) for using was prepared every time from the stock solutions (Hoagland & Arnon Citation1950). The plants were kept in a greenhouse with photoperiod 16/8 h light/dark, 28/18°C day/night and 50–60% relative humidity. TRIA was purchased from SIGMA-ALDRICH Italy. At the three-leaved stage, seedlings were pretreated with treatment solutions contained TRIA (0(control) and 10 μM) and sodium nitroprusside (SNP) with or without as a NO donor (0 and 100 μM) that added to the nutrient solution. After 72 h of initial pretreatment, plants were irrigated by half-strength Hoagland’s solution containing sodium arsenate salt (Na2HASO4, 200 µM) for 6 days. At the end of the experiment, the leaves of the plants were harvested and immediately were frozen in liquid nitrogen and stored at −80°C for future analysis.
Determination of growth parameters
At the end of the experiments, plants were uprooted and shoot weight was recorded. The samples were dried in an oven at 70°C and DW was obtained.
Electrolyte leakage, MDA and H2O2 assay
The electrolyte leakage (EL) was determined as described by Ben Hamed et al. (Citation2007) in leaves’ tissue of the coriander plants. The level of lipid peroxidation in coriander leaves was determined as the amount of 2-thiobarbituric acid-reactive substances (TBARS) by means of malondialdehyde (MDA) and other aldehydes content formed as described by Heath and Packer (Citation1968) and Meirs et al. (Citation1992), respectively. The concentration of MDA and other aldehydes was calculated using an extinction coefficient of 15.5 mM−1 mm−1 and 0.00457 mM−1 mm−1 expressed as Μmol g−1 FW. The H2O2 content of both control and treated plants was determined after reaction with potassium iodide (KI) according to Velikova et al. (Citation2000).
Determination of phenolic compounds and anthocyanin
Carotenoids was extracted from fresh leaves with 80% acetone and quantified by measuring the absorbance of subsolution at 646.8, 663.2 and 470 nmol L−1 and using an equation as described by Lichtenthaler (Citation1987). The total amount of phenolic compounds in the coriander leaves was determined using Folin–Ciocalteu’s reagent according to the method of Singleton and Rossi (Citation1965). Fifty microliters of the methanolic extract was mixed with 450 ll of distilled water and 250 ll of 2 N Folin–Ciocalteu’s reagent. The mixture added to 1.25 ml of 20% Na2CO3 was incubated at 25°C for 20 min and then centrifuged at 2000 g for 10 min. The absorbance of the supernatant was measured at 735 nm and the standard curve was prepared using gallic acid. Anthocyanin was extracted by grinding 0.1 g leaf samples with 10 ml of methanol containing 1% (v/v) HCl and determined according to Wagner (Citation1979).
Measurement of ascorbate, dehydroascorbate (DHA) and GSH content
The plants were homogenized with 5% metaphosphoric acid at 4°C. The homogenate was centrifuged at 20,000 g for 15 min at 4°C and the supernatant was collected for analysis of ascorbate (ASA) and GSH. ASA and DHA were determined according to the method of Kampfenkel et al. (Citation1995). Briefly, total ASA was determined after reduction of DHA to ASC with DTT, and the concentration of DHA was estimated from the difference between total ascorbate pool (ASC plus DHA) and ASC. A standard curve was developed based on ASA in the range of 0–50 lg/ml. The GSH content was determined by the spectrophotometric method of Ellman (Citation1959) where GSH was oxidized in 2.6 cm3 of a sodium phosphate buffer (pH 7 .0) containing 0.2 cm3 of a sample extract and 0.2 cm3 of 6 mM 5,5'-dithiobis-(2 -nitrobenzoic) acid (DTNB). The absorbance was monitored at 412 nm. The GSH content was calculated from a standard curve constructed using GSH over the range 0–100 μM.
PAL extraction and assay
Phenylalanine ammonia lyase enzyme (PAL) was extracted from a 0.3 g sample with 6.5 ml of 50 mM Tris–HCl buffer (pH 8.8) containing 15 mM B-mercaptoethanol in an ice-cooled mortar. The homogenate was centrifuged at 10,000 g for 15 min, and the supernatant was collected for enzyme assay. PAL activity was measured by a modified method of Tanaka et al. (Citation1974). Briefly, 1 ml of the extraction buffer, 0.5 ml of 10 mM L-phenylalanine, 0.4 ml of double-distilled water and 0.1 ml of enzyme extract were incubated at 37°C for 1 h. The reaction was terminated by the addition of 0.2 ml of 25% TCA and the cinnamic acid (CA) concentration was quantified with the absorbance measured at 290 nm. One unit of PAL activity is equal to 1 mol of CA produced per min.
Statistical analysis
The data were analyzed by one-way ANOVA, using SPSS software, Version 18 for Windows. Duncan’s multiple range test (DMR) was used to separate means for significant treatment (p ≤ .05). The reported values are means of three replicates ± SE.
Results
Effect of exogenous TRIA, SNP and As on growth parameters
As shown in , it is clear that As exposure had maximum reducing effect on the shoot fresh/dry weight in As-treated plants. TRIA and SNP alone did not significantly affect the growth of coriander plants compared to control plants, but TRIA and SNP treatments alleviated height reduction and increased shoots’ fresh and dry weight under As stress. The beneficial effects of TRIA and NO were seen by all growth parameters and were shown to be statistically significant especially when these compounds were supplied together.
Table 1. The effects of exogenous TRIA and SNP on growth, lipid peroxidation, protein contents and H2O2 in coriander seedlings under As stress.
Lipid peroxidation and H2O2 content
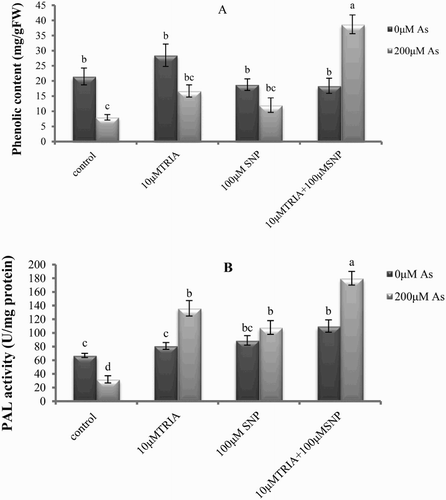
compares EL percentage, MDA and other aldehyde contents in coriander plants subjected to As with or without TRIA and SNP pretreatment. MDA and other aldehyde contents increased upon As exposure in leaves of the plants without TRIA or SNP treatment, but decreased in seedlings previously exposed to TRIA, SNP and TRIA + SNP (). As stress caused significant accumulations of H2O2 in coriander leaves compared to the control. Application of TRIA, SNP and TRIA + SNP considerably reduced the accumulation of H2O2 in leaves by about 39.2, 34.8 and 55.03%, respectively ().
Effect of exogenous TRIA and SNP on PAL activity under As stress
In order to investigate the mechanism of the difference of phenolic content by treatments in this study, we determined PAL activity in the leaves of coriander under experimental conditions. As shown in (B), PAL activity in plants treated with As decreased compared to the control. Pretreatment with TRIA, SNP and TRIA + SNP before As supply significantly increased PAL activity in the leaves compared to the control, while maximum increasing effect on the PAL activity was when TRIA and SNP were supplied together. ((B)).
Effect of As treatments on total soluble phenols and anthocyanin contents
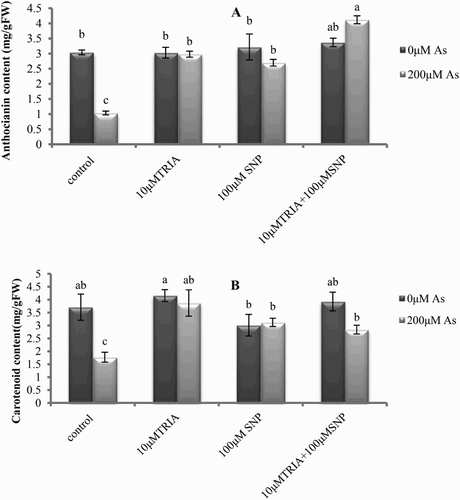
In this study, As exposure decreased total soluble phenols and anthocyanin content in the leaves of treated plants compared to the control ((A) and (A)) However, it was observed that exogenously applied TRIA + SNP in C. sativum not only compensated the reduction of these compounds but also enhanced significantly phenols pool in As + TRIA + SNP-treated plants in comparison to the other treatments ((A)).
Interaction effect between TRIA, SNP and As on total protein, ASC, DHA and GSH content
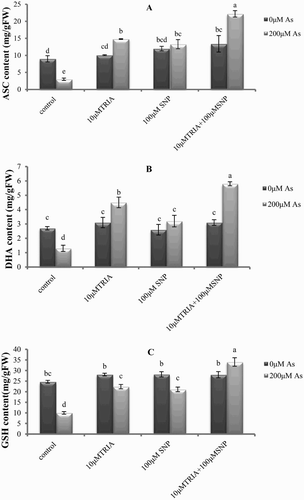
The ASC and GSH contents of C. sativum leaves exposed to As with or without the pretreatments are presented in . As caused a significant decrease in ASC and GSH content in the absence of any pretreatment. A similar trend was observed for leaf protein content (). However, these reductive effects were not observed in plants pretreated with TRIA and SNP separately. This effect was significantly greater in the plants pretreated with both TRIA and SNP compared to the plants exposed to these elicitors separately.
Discussion
In this study, the MDA and other aldehydes as lipid peroxidation markers were higher than the control in As-treated plants confirmed while As elevated membrane damage (). Membrane damage leads to unbalanced nutrient uptake and water content in plant cells (Vazquez et al. Citation2008). Metal toxicity can affect the permeability of the plasma membrane and As has been reported to interact with the water balance (Garg & Singla Citation2011). In this research, H2O2 content and electrolytes’ leakage percentage (as an important membrane damage index) increased in plants under As stress compared to the control plants (). It has been suggested that the extents of lipid peroxidation and membrane permeability were closely related to higher levels of ROS during either senescence or under stress conditions (Weckx & Clijsters Citation1997). Our findings show that TRIA and SNP interaction inhibited MDA and H2O2 generation compared to stress condition in coriander (). In this study, As had a significant adverse effect on biomass shoots of coriander seedlings. This result indicates that the coriander seedling is sensitive to As and the growth of the shoot is inhibited by As exposure. One of the most significant effects of HM toxic influence on plants is largely a strong and fast inhibition of the growth process of the above- and underground parts (Garg & Singla Citation2011). As has no known beneficial function, and its presence in the plant interferes with the metabolism and alters the uptake of other essential elements (Shri et al. Citation2009). Arsenate is known to interfere in phosphate (P) uptake by plant cells since P transporters had high affinity to As, therefore competing with P anions (Finnegan & Chen Citation2012). Competition between As and P physiologically results in blocking the electron transport chain at the level of cell membranes, and therefore ATP synthesis is significantly inhibited (Pigna et al. Citation2009). This leads to the disruption of energy flows in cells and finally represses the growth and development of plants. The growth-promoting effects of TRIA on various attributes especially those on fresh and dry weights, leaf-area and nodulation have been explored by Sharma and Dietz (Citation2006) and Khan et al. (Citation2007) in various medicinal crops. A significant enhancement in TRIA values from treated plants could presumably be ascribed to the well-known effect of exogenously applied TRIA on elongation of internodes through cell division and cell extensibility (Li et al. Citation2016). In this experiment, the reduction in biomass and induction of lipid peroxidation under toxicity condition were partially alleviated by applying TRIA or SNP (). Singh et al. (Citation2009) demonstrated that exogenously supplied NO significantly provides resistance to rice against As-induced toxicity and has an ameliorating effect against As-induced oxidative stress. As an antioxidant, NO could directly quench the ROS and modulates various cellular physiological processes to limit oxidative injury (Singh et al. Citation2009). NO supplied exogenously provides resistance against stress induced by HMs like Cd in rice (Panda et al. Citation2011) and As in fescue (Jin et al. Citation2010). There is some evidence that the exogenous application of TRIA inhibited lipid peroxidation in spinach and peanut plants’ (Ramanarayan et al. Citation2000; Verma et al. Citation2011) leaves and improved membrane integrity by differentially modulating membrane lipid composition (Swamy et al. Citation2009). Perveen et al. (Citation2011) found that TRIA-induced improvement in growth might have been due to its effect on the performance of antioxidant enzymes like POD under salt stress (Perveen et al. Citation2011). It was in agreement with the results obtained in the present study that TRIA or SNP decreased EL and MDA accumulation (). In this experiment, the reduction in biomass and induction of lipid peroxidation under toxicity condition were partially alleviated by applying TRIA or SNP. This is evidenced by the decreased level of MDA and EL in the leaves of TRIA-treated coriander. Such a process was facilitated by the active oxygen scavenging system, including several antioxidant enzymes and enhancing membrane stability. Our data suggested that TRIA might have played a key role in protecting the structure and function of cell membranes under As toxicity.
There are several reports which show that TRIA reduces the level of membrane damages due to its action as an antioxidant compound to inhibit peroxidation of membrane lipids (Ramanarayan et al. Citation2000; Khan et al. Citation2009). TRIA diminished lipid peroxidation probably through the stimulation of non-enzymatic (ASA and GSH) antioxidant machinery responsible for regulation of ROS balance during HM stress. Cellular antioxidants such as phenolic compounds play an important role in inducing resistance of plants to metals by protecting macromolecules and membranes against attacks induced via free radicals (Sanchez-viveros Citation2010). Antioxidant action of phenolic compounds is due to their high tendency to chelate metals. The negative effects of As on the growth of the coriander might be related to impaired synthesis of soluble phenols and other antioxidant compounds such as GSH and ASC by which the coriander was not able to alleviate the oxidative stress induced by the accumulation of this metalloid in tissues. There have been many reports of induced accumulation of phenolic compounds, PAL and peroxidase activity in plants treated with a high concentration of metals (Michalak Citation2006). The induction of phenolic compound biosynthesis was observed in pepper in response to Cu (Diaz et al. Citation2001) and in maize in response to Al (Winkel-Shirley Citation2002). In this study, exogenously applied TRIA and SNP increased the content of phenolic compounds in coriander leaves ((A)). Induction of phenolic content after TRIA application has been reported in green gram (Kumaravelu et al. Citation2000). According to Kovacik et al. (Citation2009) exogenous application of SNP causes accumulation of soluble phenol by stimulating PAL activity in N-deficient Matrica chamomilla roots. In a study with tobacco bright-yellow 2 cells, only the combined application of NO and ROS generators caused a remarkable increase in PAL activity. Both ROS and NO may stimulate expression of the pal gene (De Pinto et al. Citation2002). In addition, Durner et al. (Citation1998) reported that NO induced activation of PAL gene (pal) expression and PAL enzyme activity in tobacco. Also, TRIA may be responsible for the activation of PAL in green gram (Kumaravelu et al. Citation2000). However, we found no data focused on the changes in PAL activity or phenolic metabolism-related enzymes in TRIA-treated plants.
The GSH level has been shown to correlate with the plant adaptation to extreme HM stress and reduced GSH pool shows marked alterations in response to metal stress (Singh et al. Citation2006). ASA is also known to operate as an antioxidant either in direct chemical interaction with ROS or during the reaction catalyzed by ascorbate peroxidase (APX) (Singh et al. Citation2006). During the present study, reduced levels of ASA and GSH were observed in As-treated plants ((A) and (C)). This result is similar to those of Hasanuzzaman and Fujita (Citation2013), who reported a decrease in ASC and GSH contents and the GSH/GSSG ratio in As-treated wheat (Triticum aestivum). This might be attributed to the toxicity of the As (Sanchez-viveros Citation2010). Therefore, the measured decline in the contents of ASC and GSH in C. sativum could be partially due to its consumption while acting as antioxidant to limit lipid peroxidation. Moreover, GSH as a sulfur-containing tripeptide thiol is involved in plant protection against HMs as a precursor in the synthesis of phytochelatins (PCs) and in the scavenging of ROS by the ascorbate–glutathione cycle (Sharma & Dietz Citation2006; Xiong et al. Citation2010). GSH levels in plant tissues are known to increase under metal stress (Koricheva et al. Citation1997). In this study, decreasing GSH content in coriander may be caused by increased conversion of GSH to PC in response to As toxicity. Recently, Gupta et al. (Citation2009) demonstrated that PC synthesis is stimulated by As supply in Brassica juncea due to the over-expression of the PC gene. The PC–metal complex is often sequestered in the vacuoles (Sharma & Dietz Citation2006). The chemical structure of PCs contains repetitions of the dipeptide Glu–Cys. NO is able to react with Cys residues (S-nitrosylation), especially when these are surrounded by acidic amino acids like Glutamate, and NO reacts rapidly with GSH, the precursor of PCs, to form S-nitrosoglutathione (Stamler et al. Citation1997). However, Michele et al. (Citation2009) indicated that PCs might similarly be nitrosylated. They believed that a mechanism through which NO might modulate the PC capability to chelate HMs is by direct nitrosylation.
Although multiple factors can affect the GSH pool in plants, some conditions are known to modify the GSH biosynthesis pathway at the transcriptional level. However, this regulation was observed in response to HMs, SNP treatment (Innocenti et al. Citation2007). Our data show that GSH content is increased in plants by NO and TRIA application ((C)). This result is similar to those of Li et al. (Citation2016), who reported an increase in GSH and ASA contents in TRIA-treated rice plants. Innocenti et al. (Citation2007) indicated that the application of SNP increased GSH in Medicago. They believed that NO triggered an increase of the endogenous GSH amount in M. truncatula roots through the stimulation of GSH synthesis gene transcript accumulation. In addition to a direct ROS-scavenging activity and to the modulation of lipid peroxidation, NO may also protect cells against oxidative processes by stimulating GSH synthesis (Innocenti et al. Citation2007). Since ASC and GSH are able to detoxify ROS by a direct scavenging or by acting as substrate in the enzymatic reactions (APX and GR), an elevation or protection in their contents with TRIA and SNP pretreatments enhanced the tolerance against As-induced oxidative stress in the coriander plants (). Also, our results showed that TRIA and SNP may act synergistically to increase coriander resistance against As HM toxicity by enhancing non-enzymatic antioxidant pool or PAL activity.
Conclusion
The reduction of growth and induction of lipid peroxidation indicated that excess As induces oxidative stress in coriander. Application of TRIA and SNP before As exposure supported considerably higher non-enzymatic antioxidant contents, especially when seedlings were subjected to both compounds (TRIA and SNP). In summary, our results suggest that there was a mutually amplifying reaction between TRIA and NO in reducing As-induced damages. However, whether TRIA and NO participate synergically in regulating the transcript level and the activity of key enzymes remains unknown and further research is required to understand the signaling relationships between TRIA and NO in plant defenses against HM stress.
Acknowledgements
The authors acknowledge Shahid Bahonar University for providing research funds.
Disclosure statement
No potential conflict of interest was reported by the authors
ORCID
Elham Asadi Karam http://orcid.org/0000-0001-9352-7095
References
- Ahmad P, Abdul Jaleel C, Salem MA, Nabi G, Sharma S. 2010. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotech. 30:161–175. doi: 10.3109/07388550903524243
- Ben Hamed K, Castagna A, Salem E, Ranieri A, Abdelly C. 2007. Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul. 53:185–194. doi: 10.1007/s10725-007-9217-8
- De Pinto MC, Tommasi F, De Gara L. 2002. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco bright-yellow 2 cells. Plant Physiol. 130:698–708. doi: 10.1104/pp.005629
- Diaz J, Bernal A, Pomar F, Merino F. 2001. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 161:179–188. doi: 10.1016/S0168-9452(01)00410-1
- Durner J, Wendehenne D, Klessig DF. 1998. Defense gene induction in tobacco by nitric oxide, cyclic CMP and cyclic ADP-ribose. Proc Natl Acad Sci USA. 95:10328–10333. doi: 10.1073/pnas.95.17.10328
- Ellman Gl. 1959. Tissue sulfydryl groups. Arch Biochem Biophys. 82:70–77. doi: 10.1016/0003-9861(59)90090-6
- Finnegan PM, Chen W. 2012. Arsenic toxicity: the effects on plant metabolism. Front Physiol. 3:1–18. doi: 10.3389/fphys.2012.00182
- Foyer CH, Noctor G. 2012. Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ. 35:199–201. doi: 10.1111/j.1365-3040.2011.02453.x
- Garg N, Singla P. 2011. Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett. 9:303–321. doi: 10.1007/s10311-011-0313-7
- Gatica AM, Arrieta G, Espinosa AM. 2008. Direct somatic embryogenesis in Coffea arabica L cvs catura and catuai: effect of triacontanol, light condition, and medium consistence. Agron Costarric. 32:139–147.
- Gunes A, Pilbeam DJ, Inal A. 2009. Effect of arsenic-phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil. 314:211–220. doi: 10.1007/s11104-008-9719-9
- Gupta DK, Inouheb M, Rodrı´guez-Serrano M, Romero-Puertas MC, Sandalio LM. 2013. Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere. 90:1987–1996. doi: 10.1016/j.chemosphere.2012.10.066
- Gupta M, Sharma P, Sarin NB, Sinha AK. 2009. Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosensory. 74:1201–1208.
- Hasanuzzaman M, Fujita M. 2013. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology. 22:584–596. doi: 10.1007/s10646-013-1050-4
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplast: I. Kinetic and stochiometry of fatty acid peroxidation. Biochem Biophys. 125:189–190. doi: 10.1016/0003-9861(68)90654-1
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ. 347:1–3.
- Hossain MA, Piyatida P, da Silva JAT, Fujita M. 2012. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 55:872–8757.
- Innocenti G, Pucciariello Ch, Gleuher ML, Hopkins J, Stefano MD, Delledonne M, Puppo A, Baudouin E, Frendo P. 2007. Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta. 225:1597–1602. doi: 10.1007/s00425-006-0461-3
- Jin JW, Xu YF, Huang YF. 2010. Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr J Biotechnol. 9:1619–1627. doi: 10.5897/AJB10.704
- Kampfenkel K, Van Montagu M, Inzb D. 1995. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 225:165–167. doi: 10.1006/abio.1995.1127
- Khan MMA, Bhardwaj G, Naeem M, Moinuddin F, Mohammad F, Singh, M, Nasir S, Idrees M. 2009. Response of tomato (Solanum lycopersicum L.) to application of potassium and triacontanol. Acta Hort. (ISHS). 823:199–208. doi: 10.17660/ActaHortic.2009.823.29
- Khan R, Khan MMA, Singh M, Nasir S, Naeem M, Siddiqui MH, Mohammad F. 2007. Gibberellic acid and triacontanol can ameliorate the opium yield and morphine production in opium poppy (Papaver somniferum L.). Acta Agri Scand Section B – Soil Plant Sci. 57:307–312.
- Koricheva J, Roy S, Vranjic JA, Haukioja E, Hughes PR, Han-ninen O. 1997. Antioxidant responses to simulated acid rain and heavy metal deposition in birch seedlings. Environ Pollut. 95:249–258. doi: 10.1016/S0269-7491(96)00071-1
- Kovacik J, Klejdus B, Backor M. 2009. Nitric oxide signals ROS scavenger-mediated enhancement of PAL activity in nitrogen deficient Matricaria chamomilla roots: side effects of scavengers. Free Radic Biol Med. 46:1686–1693. doi: 10.1016/j.freeradbiomed.2009.03.020
- Kumaravelu G, Livingstone VD, Ramanujam MP. 2000. Triacontanol-induced changes in the growth, photosynthetic pigments, cell metabolites, flowering and yield of green gram. Biol Plant. 43:287–290. doi: 10.1023/A:1002724831619
- Lamattina L, Garca-Mata C, Graziano M, Pagnussat G. 2003. Nitric oxide: the versatility of an extensive signal molecule. Ann Rev Plant Biol. 54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752
- Li X, Zhong Q, Li Y, Li G, Ding Y, Wang S, Liu Z, Tang S, Ding C, Chen L. 2016. Triacontanol reduces transplanting shock in machine-transplanted rice by improving the growth and antioxidant systems. Front Plant Sci. doi:10.3389/fpls.2016.00872
- Lichtenthaler HK. 1987. Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 148:350–382. doi: 10.1016/0076-6879(87)48036-1
- Liu Y, Zhu YG, Chen BD, Christie P, Li XL. 2005. Influence of the arbuscular mycorrhizal fungus Glomus mosseae on uptake of arsenate by the as hyperaccumulator fern Pteris vittata L. Mycorrhiza. 15:187–192. doi: 10.1007/s00572-004-0320-7
- Meirs S, Philosophhadas S, Aharoni N. 1992. Ethylene increased accumulation of fluorescent lipid peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci. 117:128–132
- Michalak A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J Environ Stu. 15:523–530.
- Michele RD, Vurro E, Rigo Ch, Costa A, Elviri L, Valentin MD, Careri M, Zottini M, Toppi LS, Schiavo FL. 2009. Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol. 150:217–228. doi: 10.1104/pp.108.133397
- Naeem M, Khan MMA, Moinuddin MMA. 2011. Triacontanol: a potent plant growth regulator in agricultural crops. J Plant Interact. 7:129–142. doi: 10.1080/17429145.2011.619281
- Naeem M, Idrees M, Aftab T, Khan MMA, Moinuddin MMA. 2010. Changes in photosynthesis enzyme activities and production of anthraquinone and sennoside content of coffee senna (Senna occidentalis L.) by triacontanol Internat. Plant Dev Biol. 4:53–59.
- Naeem M, Khan MMA, Moinuddin MMA, Siddiqui MH. 2009. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureus L.). Sci Hort. 121:389–396. doi: 10.1016/j.scienta.2009.02.030
- Palavan-Unsal N, Arisan D. 2009. Nitric oxide signalling in plants. Bot. Rev. 75:203–229. doi: 10.1007/s12229-009-9031-2
- Panda P, Nath Sh, Chanu ThTh, Sharma GD, Panda SK. 2011. Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol. 33:1737–1747. doi: 10.1007/s11738-011-0710-3
- Perveen S, Shahbaz M, Ashraf A. 2011. Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak J Bot. 43:2463–2468.
- Pigna M, Cozzolino V, Violante A, Meharg AA. 2009. Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Pollut. 197:371–380. doi: 10.1007/s11270-008-9818-5
- Ramanarayan K, Bhut A, Shripathi V, Swamy GS, Rao KS. 2000. Triacontanol inhibits both enzymatic and nonenzymatic lipid peroxidation. Phytochemistry. 55:59–66. doi: 10.1016/S0031-9422(00)00201-6
- Ries SK. 1991. Triacontanol and its second messenger 9 -b-L(C)-adenosine as plant growth substances. Plant Physiol. 95:986–989. doi: 10.1104/pp.95.4.986
- Ries SK, Houtz R. 1983. Triacontanol as a plant growth regulator. Hort Science. 18:654–662.
- Sanchez-Viveros G. 2010. Short-term effects of arsenate-induced toxicity on growth, chlorophyll and carotenoid contents, and total content of phenolic compounds of Azolla filiculoides. Water Air Soil Pollut. 217:455–462. doi: 10.1007/s11270-010-0600-0
- Sharma SS, Dietz KJ. 2006. The significance of amino acid and amino acid-derived molecules in plant responses and adaption to heavy metal stress. J Exp Bot. 57:711–726. doi: 10.1093/jxb/erj073
- Shen Q, Wang YT, Tian H, Guo FQ. 2013. Nitric oxide mediates cytokinin functions in cell proliferation and meristem maintenance in arabidopsis. Mol Plant. 6:1214–1225. doi: 10.1093/mp/sss148
- Shri M, Kumar S, Chakrabarty D, Kumar-Trivedi P, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R. 2009. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf. 72:1102–1110. doi: 10.1016/j.ecoenv.2008.09.022
- Singh HP, Kaur Sh, Batish DR, Sharma VP, Sharma N, Kohli RK. 2009. Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide. 20:289–297. doi: 10.1016/j.niox.2009.02.004
- Singh N, Ma LQ, Srivastava M, Rathinasabapathi B. 2006. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 170:274–282. doi: 10.1016/j.plantsci.2005.08.013
- Singleton VL, Rossi JA. 1965. Colorimetry of total phenolics with phospho-molybdic-phosphotungstic acid reagents. Am J Enol. Vitic16:144–158.
- Stamler JS, Toone EJ, Stuart AL, Sucher NJ. 1997. NO signals: translocation, regulation, and a consensus motif. Neuron. 18:691–696. doi: 10.1016/S0896-6273(00)80310-4
- Stoeva N, Berova M, Vassilev A, Zlatev Z. 2005. Effect of arsenic on some physiological parameters in bean plants. Biol. Planta. 49:293–296. doi: 10.1007/s10535-005-3296-z
- Swamy GS, Ramanarayan K, Inamdar LS, Inamdar SR. 2009. Triacontanol and jasmonic acid differentially modulate the lipid organization as evidenced by the fluorescent probe behavior and 31P nuclear magnetic resonance shifts in model membranes. J Membrane Biol. 228:165–177. doi: 10.1007/s00232-009-9169-1
- Tanaka Y, Kojima M, Uritani I. 1974. Properties, development and cellular-localization of cinnamic acid 4-hydroxylase in cut injured sweet potato. Plant Cell Physiol. 15:843–854.
- Vazquez S, Esteban E, Carpena RO. 2008. Evolution of arsenate toxicity in nodulated white lupine in a long-term culture. J Agric Food Chem. 56:8580–8587. doi: 10.1021/jf801673c
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of endogenous polyamines. Plant Sci. 151:59–66. doi: 10.1016/S0168-9452(99)00197-1
- Verma A, Malik CP, Gupta VK, Bajaj BK. 2011. Effcts of in vitro triacontanol on growth, antioxidant enzymes, and photosynthetic characteristics in Arachis hypogaea L. Braz J Plant Physiol. 23:271–277.
- Wagner GJ. 1979. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 64:88–93. doi: 10.1104/pp.64.1.88
- Weckx JEJ, Clijsters HMM. 1997. Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol Biochem. 35:405–410.
- Winkel-Shirley B. 2002. Biosynthesis of flavonoids and effects of stress. Plant Biol. 5:218–223.
- Xiong J, Fu G, Tao L, Zhu Ch. 2010. Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys. 497:13–20. doi: 10.1016/j.abb.2010.02.014
- Yadav S, David A, Bhatla, SC. 2011. Nitric oxide accumulation and actin distribution during auxin-induced adventitious root development in sunflower. Sci Hort. 129, 159–166. doi: 10.1016/j.scienta.2011.03.030
- Yu C, Hung KT, Kao C. 2005. Nitric oxide reduces Cu toxicity and Cu-induced NH4 accumulation in rice leaves. Plant Physiol. 162:1319–1330. doi: 10.1016/j.jplph.2005.02.003
- Zu YQ, Sun JJ, He YM, Wu J, Feng GQ, Li Y. 2016. Effects of arsenic on growth, photosynthesis and some antioxidant parameters of Panax notoginseng growing in shaded conditions. Inter JAd Agri Res. 4:78–88.