 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
With the rapid development of nanotechnology, the potential releases of nanoparticles (NPs) have drawn considerable attention. Plants are essential fundamental components of all ecosystems, and the interaction between NPs and plants is an indispensable aspect of the risk assessment. Originally, this review focuses on NP phytotoxicity, which is an important precondition to promote the application of nanotechnology and to avoid the potential ecological risks. Both enhancive and inhibitive effects of various NPs on different plants’ growth have been documented. In this paper, the influence factors of nanotoxicity and the mechanisms of these toxic effects are also summarized. Subsequently, the defense mechanisms are presented as well. Eventually, this review puts forward the prospects of research direction of the environmental behavior and the biological toxicity of NPs, hoping to bring new ideas to the further research on NP phytotoxicity.
Abbreviations: 1O2: singlet oxygen; AA: ascorbate; ABA: abscisic acid; APX: ascorbate peroxidase; B-Ag NPs: biosynthesized silver nanoparticles; Bt: Bacillus thuringiensis; CAT: catalase; CDs: carbon dots; Cu/Zn-SOD: copper/Zinc SOD; DHA: dehydroascorbate; DHAR: dehydroascorbate reductase; GA: gibberellic acid: GA-Ag NPs: coated silver nanoparticles; GPOX: guaiacol peroxidase; GPX: glutathione peroxidase; GR: glutathione reductase; GSH: glutathione; GSSG: oxidized glutathione; GST: glutathione S-transferases; •OH: hydroxyl radical; HA: high amylose; IAA: indole-3-acetic acid; LA: low amylose; MA: medium amylose; MDA: malondialdehyde; MDHAR: monodehydroascorbate reductase; NPs: nanoparticles; : superoxide radical; POD: promoting peroxidase; ROS: reactive oxygen species; SOD: superoxide dismutase; t-ZR: trans-zeatin-riboside
Introduction
Recent advances in nanotechnology have impacted industries including manufacturing, biomedical applications, electronics/telecommunications, agriculture and renewable energy, among others (Ma, White, et al. Citation2015). Nanoparticles (NPs) are broadly defined as particles having at least one dimension between 1 and 100 nm in diameter (Auffan et al. Citation2009). Because of their unique properties and novel features, NPs have been widely used in many aspects of daily life and energy production, including in catalysts, semiconductors, cosmetics, drug carriers and environmental energy (Nel et al. Citation2006). The large-scale and unrestricted use of NPs has led researchers to consider the problems, challenges and consequences of their environmental impact (Gottschalk et al. Citation2015; Tolaymat et al. Citation2015).
To date, the concentration of NPs in the environment is much lower than the toxic concentration (Batley et al. Citation2013). The potential health and environmental effects of NPs need to be thoroughly evaluated before they are widely commercialized. When NPs enter the soil through agricultural application, atmospheric deposition, rain erosion, surface runoff or other pathways, the NPs will accumulate in the soil as time goes because of their weak migration ability in soil. Exposure modeling also indicated that the concentrations of NPs in soil are higher than those in water or air, implying that soils might be the main source of NPs released into the environment (Gottschalk et al. Citation2009).
As primary producers, plants are key for any community to function as they are responsible for converting solar energy into organic matter that can be used by other trophic groups (McKee & Filser Citation2016). Plants serve as a potential pathway for the transportation of NPs (Rico et al. Citation2011). Through the food chain, NPs can be accumulated in high trophic-level consumers (Zhu et al. Citation2008). Organisms in the ecosystem could suffer from oxidative stress induced by NPs (Hong et al. Citation2014). In recent years, research in this area has been focused on the interaction between plants and NPs, and the effects of NPs on ecology, the food chain and human health; evaluating the pros and cons of NPs requires interdisciplinary knowledge (Tolaymat et al. Citation2015).
Both Stampoulis et al. (Citation2009) and Wang et al. (Citation2012) found that CuO NPs did not affect the germination of zucchini and maize, but suppressed root elongation. Another study showed that multi-walled carbon nanotubes (MWCNTs) had no negative effect on the seed germination and root elongation of zucchini species (Stampoulis et al. Citation2009). In the case of root growth, biomass was not affected by ZnO NPs at both 400 and 800 mg/kg (Zhao et al. Citation2013). However, Zhang, Zhang, et al. (Citation2015) reported that corn exposed to ZnO NPs showed no significant negative physiological effects. Most studies showed that NPs could produce toxic effects above a certain concentration (Rico et al. Citation2011). In contrast, some studies have reported that NPs might have a positive effect on plants. Mixed nano-TiO2 and nano-SiO2 was introduced into soybean (Glycine max), causing an increase in nitrate reductase activity; this treatment hastened plant germination and increased growth by enhancing the absorption and utilization of water and fertilizer by the crop (Lu et al. Citation2001). Some studies showed that TiO2 NPs promoted nitrogen metabolism and photosynthesis, thereby improving spinach growth when provided at a suitable concentration (Hong et al. Citation2005; Zheng et al. Citation2005; Yang et al. Citation2006). Similarly, Li et al. (Citation2016) found that carbon dots (CDs) at a concentration of 0–1.0 mg/mL played a role in promoting mung bean growth. The results showed that CDs could enhance the ability of mung bean to absorb and utilize nutrients. Therefore, it was concluded that CDs induced a physiological response in the mung bean plant and had a positive effect on the growth of mung bean (Li et al. Citation2016). The unique optical properties of the CDs allowed their transport by means of the apoplastic paths from the roots to the stems and leaves by the vascular system (Li et al. Citation2016). These studies have increased our understanding of NPs in phytotoxicology. However, toxicity mechanisms have not yet been completely elucidated for most NPs, and little is known about the potential effects of plants and NPs on their subsequent fate in the food chain. This paper mainly summarizes the research on the ecological toxicity of NPs to plants in recent years. In addition, the effects of NPs on biomass were briefly generalized. We also analyzed the toxicity mechanisms of NPs in terms of cells, molecules and enzyme activity, and compared the effects of different factors on their phytotoxicity. Finally, some suggestions are put forward on the existing problems and potential future research directions. This study will contribute to the promotion of scientific research related to the ecological risks of NPs and promote the sustained and healthy development of nanotechnology.
Effects of NPs on plants
Effect of NPs on plant physiological indices
The main plant physiological indices of the toxic effects of NPs are the germination percentage, root elongation, biomass and leaf number (Lee et al. Citation2010). NPs can have substantial negative effects, such as reduction in seed germination and suppression of plant elongation, and can even cause plant death. Several previous plant nanotoxicity studies have determined the inhibition of plant species such as soybean, maize, wheat (Triticum aestivum), ryegrass and barley by exposure to NPs (MWCNTs, single-wall carbon nanotubes, ZnO NPs, Ag NPs and Fe NPs); several aspects of plant growth were affected including seed germination, shoot length, biomass and gene expression (Dimkpa et al. Citation2012; El-Temsah & Joner Citation2012; Riahi-Madvar et al. Citation2012; Yan et al. Citation2013; Ghosh et al. Citation2015). Growth inhibition was observed when Bacillus thuringiensis (Bt)-transgenic cotton was exposed to SiO2 NPs (Le Van et al. Citation2014). When wheat plants were grown in a sand matrix, CuO NPs inhibited their growth and changed the structure of the roots (Dimkpa et al. Citation2012; Tang et al. Citation2016). Shaw and Hossain (Citation2013) showed that CuO NPs significantly reduced the fresh weights and root length of Arabidopsis seedlings, and the germination rate and biomass of rice seeds.
Ma et al. (Citation2010) and López-Moreno, de la Rosa, Hernández-Viezcas, Peralta-Videa, et al. (Citation2010) found that rare earth oxide NPs (CeO2, La2O3, Gd2O3 and Yb2O3) had a detrimental effect on plant growth in radish, tomato, rape, lettuce, wheat, cabbage, cucumber and corn when added to roots at high concentrations. TiO2 NPs increased the content of total chlorophyll and catalase (CAT) and decreased ascorbate peroxidase (APX) content in leaves (Servin et al. Citation2013). A detailed discussion on the effects of NPs on plant enzyme activities will be provided in the next section.
Effect of NPs on plant hormones
Plant hormones are active organic materials that are produced by plant metabolism, which can regulate physiological responses during plant growth and mediate responses to challenges (Santner et al. Citation2009). The content and activity of plant hormones are considered an important index of toxicity in plants. Le Van et al. (Citation2015) observed that CeO2 NPs had no significant effect on indole-3-acetic acid (IAA), abscisic acid (ABA) and gibberellic acid (GA) in the leaves of Bt-transgenic and conventional cotton compared with the control group. When conventional cotton was exposed to 500 mg/L CeO2 NPs, the content of trans-zeatin-riboside (t-ZR) in the leaves decreased by 25% when compared with the control group alone. Gui, Deng, et al. (Citation2015) reported that the IAA and ABA content in the roots of transgenic and non-transgenic rice was increased in response to γFe2O3. NPs had a significant influence on the production of plant hormones. Hao et al. (Citation2016) demonstrated that decreases in phytohormone concentrations were evident in rice seedlings upon exposure to carbon nanotubes (). According to Bleecker and Kende (Citation2000), because Ag ions inhibited the production of ethylene, the interaction between IAA and ethylene would be substantially weakened.
Table 1. Summary of the plant hormones upon NPs exposure.
Effect of NPs on crop quality
Previous studies of hydroponic plants have shown that the accumulation of NPs in the environment could profoundly change the soil-based food crop quality and yield (Priester et al. Citation2012). Rani et al. (Citation2016) demonstrated that protein was insensitive to stimulation by Ag NPs when compared with carbohydrates, with protein content increased only at high Ag NP concentrations (100 mg/L). Rico et al. (Citation2014) showed that ZnO increased the starch and protein content and decreased the micronutrient (Cu and Mo) concentrations in cucumber. Rice treated with nCeO2 had less Fe, S, prolamin, glutelin, lauric and valeric acids, and starch than the control, and the antioxidant properties of the treated rice were weakened (Rico, Morales, et al. Citation2013). Rico et al. (Citation2014) also showed CeO2 NPs changed the content of amino acids, fatty acids, nonreducing sugars and phenolics in plants. Le Van et al. (Citation2016) also documented the effects of NPs on plant nutrient content.
Factors affecting NP phytotoxicity
In combination with recent studies and our own knowledge, we have concluded that the main factors influencing the effects of NPs on plants are the characteristics of the nanomaterials themselves (concentration/size/category/stability), the plant seed (size/species), the plant growth medium, the plant growth stage and the NP coating material. We will discuss the influence of various factors below.
Effects of different nanomaterials on NP phytotoxicity
Song et al. (Citation2013) demonstrated that treatment of tomato with Ag NPs resulted in a reduction in biomass and root length. TiO2 NPs could significantly improve the germination rate of seeds; however, bulk TiO2 can have inhibitory effects on seed germination. Similar results were observed in Feizi et al. (Citation2013) and Hawthorne et al. (Citation2012). Because of the high reactivity of small materials, NPs usually show greater toxicity than the same material of larger size (Oberdürster Citation2000). However, other studies have shown that phytotoxicity increases with particle size. For example, Yasur and Rani (Citation2013) affirmed that all Ag NP treatment groups had no effect on the growth of castor, but in the treatments where the Ag was used in its bulk form, inhibition was observed. This was confirmed by the work of Lee et al. (Citation2010). On the fifth day of NP exposure, synthesized silver NP treatments had higher Ag accumulation than biosynthesized silver NP (B-Ag NP) treatments (10 and 100 mg/L) in water hyacinth; at the high concentration (100 mg/L), B-Ag NPs improved plant growth (Rani et al. Citation2016).
Effects of different seeds on NP phytotoxicity
To some extent, growth and antioxidant defense responses differ from species to species. Some studies have speculated that the differences in the toxicity of NPs might be related to the differences in seed size, the single leaf and the xylem structure of double-leaf plants (Lee et al. Citation2008). We have mentioned the response of different plant seeds being exposed to the same nanomaterial in the previous section. The responses of different species to the same NPs must also be discussed. Three rice varieties (high (HA), medium (MA) and low (LA) amylose) were cultivated in soil amended with CeO2 NPs; a comparison between the treatments showed that relative to the control, the Ce content in HA was not significantly different. Treatment with CeO2 NPs enhanced the Ce content in MA and LA treatments (Rico, Morales, et al. Citation2013). Transgenic seeds are likely to have different responses when compared with conventional seeds (Gui, Deng, et al. Citation2015). When exposed to ZnO NPs, leaf surface area was statistically reduced for cultivar Zhu Liang You 06 (Oryza sativa) when compared with cultivar Qian You No. 1 (Oryza sativa) under control conditions for both cultivars (Salah et al. Citation2015).
Effects of different growth media on NP phytotoxicity
NPs must be studied in the specific environments in which they occur, as their characteristics differ widely depending on the environmental conditions (Song et al. Citation2013). When the biomass of radishes was compared in silty loam (∼2.21% ± 0.04% soil organic matter) and loamy sand (∼11.87% ± 0.56% soil organic matter), the former had significantly higher root biomass and the presence of 1000 mg/kg CeO2 NPs made no substantial difference. The Ce accumulation in fine roots and in the storage root showed that growth in loamy sand was higher than that in silty loam (Zhang, Musante, et al. Citation2015). Kidney bean plants exposed to CeO2 NPs for 52 days in a low organic matter soil usually possessed higher Ce concentration than the same tissues collected from an organic matter-enriched soil (Majumdar et al. Citation2016). Likewise, different levels of phytotoxicity caused by CeO2 NPs were found in lettuce seedlings incubated with three kinds of media, including agar (Cui et al. Citation2014), potting mix soil (Gui, Zhang, et al. Citation2015) and sand (Zhang et al. Citation2017). Schlich and Hund-Rinke (Citation2015) showed that Ag NP toxicity decreased with increasing clay content and pH, but seemed to be unaffected by the organic carbon content of the soil.
Effect of different growth stages on NP phytotoxicity
Photosynthetic parameters changed throughout the whole growth period in cucumber; seedling leaf size decreased when compared with the control when treated with 200 mg/L of CeO2 NPs and CuO NPs, but when mature leaves were measured, there were no significant differences among the treatments (Hong et al. Citation2016). At 15 days, the CAT and APX activities in the roots showed a sharp decrease when compared with the treatments at day 7, except for the control treatment. Total soluble protein content in roots showed the opposite results, with the exception of the 500 mg/L CeO2 NP treatment (Majumdar et al. Citation2014).
Effect of different coating material on NP phytotoxicity
Coating not only alters the function of NPs, but also changes its effects on soil–plant systems. The types of coating also influence the toxicity and solubility of NPs. The suspension of Ag NPs led to the increased germination rate of 5 of 11 species, whereas exposure to coated silver NPs (GA-Ag NPs) only significantly contributed to the germination rate of one species. This was because of the presence of a co-ligand in the soil, which caused a preferential reduction in the bioavailability and toxicity of Ag+. The toxicity of GA-Ag NPs was longer lasting than that of AgNO3 (Yin et al. Citation2012). No significant differences in the biomass production rate (fresh weight) of rice were found among plants treated with Fe-Co-filled carbon nanotubes (FeCo-CNTs) compared with the non-stressed plants; MWCNTs and Fe-CNTs significantly decreased the biomass production rate at 30 and 50 mg/L (Hao et al. Citation2016).
Toxicity and detoxicity mechanisms in plants induced by NPs
With regard to the effects of NPs on plants, recent reports have revealed ambiguous results. Many reports found NPs positive effects, whereas, some reports that show negative effects. Plant species, NP properties (size, shape, types, structure and defects, surface coating, etc.) and culture media may all potentially cause the divergent results. In this section, the phytotoxicity and detoxicity mechanisms will be reviewed.
Phytotoxicity mechanism in plants induced by NPs
In general, reactive oxygen species (ROS) contain both free radicals such as hydroxyl radical (•OH) and superoxide radical (), and non-radical molecules such as singlet oxygen (1O2) and hydrogen peroxide (H2O2) (Gill & Tuteja Citation2010). ROS is the product of aerobic metabolism in an ordinary plant, which acts as signaling molecules (Thannickal & Fanburg Citation2000), whereas excess ROS would cause kinds of adverse effects called oxidative stress, which emerges when the ROS level exceeds the defense mechanisms (It will be described in detail in the next section), and is able to pose threat to cells by inducing DNA damage, protein oxidation, electrolyte leakage, lipid peroxidation and membrane damage, finally causing cell death (Meriga et al. Citation2004; Sharma et al. Citation2012). Published studies on metal and metal-based NP phytotoxicity suggest that NPs can induce oxidative stress in many plant species (Dimkpa et al. Citation2012; Shaw & Hossain Citation2013; Cui et al. Citation2014; Zhang, Ma, et al. Citation2015, Citation2017; Ma, Zhang, Zhang, He, Li, et al. Citation2015). To clarify ZnO NP phytotoxicity to ryegrass, Lin and Xing (Citation2008) proposed that the particle-dependent ROS formation and lipid peroxidation occurred on the surface of cellular membranes. Likewise, due to a high correlation between the decrease in viable cells and ROS generation at concentrations of Ag NPs up to 10 mg/mL over a week of treatment period, the phytotoxicity of Ag NPs was caused by ROS generation (Oukarroum et al. Citation2013).
Recently, ROS-sensitive dye DAB (3,3′-diaminobenzidine) was used to find the accumulation of H2O2 in the plant roots treated with CeO2 and La2O3 NPs (Tarasenko et al. Citation2012). Results clearly showed that the insoluble deep brown colored product was produced under the treatment of NPs, which was visualized easily by human eyes (Zhang, Ma, et al. Citation2015; Ma, Zhang, Zhang, He, Li, et al. Citation2015). Similarly, the treatment with 800 mg/kg CeO2 NPs caused the accumulation of H2O2 concentration at 35 μM, which was almost 10 times higher than that of control, whereas experimental results followed even there was oxidative stress happened, no lipid peroxidation occurred, did not induce ion leakage in either roots or shoots, which means that membrane integrity was intact (Zhao et al. Citation2012). As ROS H2O2 could be converted into more toxic •OH, which cannot be detoxified by any known enzymatic system, and no relevant reports on •OH determination in plants induced by metal-based NPs exposure exist (Ma, White, et al. Citation2015), the cellular damage was inevitable. Among all of ROS, the most reactive is •OH, which has a single unpaired electron. Therefore, it could interact with all biological molecules and causes subsequent cellular damages such as lipid peroxidation, protein damage, membrane destruction (Foyer et al. Citation1997; Freinbichler et al. Citation2011) and ultimately cell premature death.
If the ROS level exceeds the threshold, not only was the normal cellular functioning affected directly, but also oxidative stress was aggravated through the production of lipid-derived radicals (Montillet et al. Citation2005), which could react with and damage proteins and DNA (Sharma et al. Citation2012). Lipid peroxidation causes the most damaging process in every living organism, and membrane damage usually acts as an indicator of lipid damage under all sorts of stresses. Malondialdehyde (MDA), one of the final products of peroxidation of unsaturated fatty acids in phospholipids, causes the cell membrane damage (Tanou et al. Citation2009; Halliwell & Gutteridge Citation2015). It has been demonstrated that in plants exposed to various abiotic stresses, such as metal-based NPs, enhanced lipid peroxidation occurs due to the ROS generation.
Generally, oxidative stress byproducts and/or ROS can induce covalent modification of a protein, called protein oxidation. Excess ROS generation may induce the modification of site-specific amino acids, fragmentation of peptide chains, aggregation of cross-linked reaction products, altered electric charge and increased susceptibility of proteins to proteolysis (Sharma et al. Citation2012). Oxidative stress damaged tissues usually contain an enhanced concentration of carbonylated proteins, which are widely used as a marker of protein oxidation (Møller & Kristensen Citation2004). Sulfur and thiol groups containing amino acids are highly vulnerable sites to be attacked by ROS. An H atom from cysteine residues can be abstracted by activated oxygen to form a thiyl radical, which will be cross-linked to another thiyl radical to form a disulfide bridge (Hancock et al. Citation2006). In a recent study, the phytotoxicity and genotoxicity of Ag NPs on germinating wheat seedlings were investigated. It was found that 10 mg/L Ag NPs caused the alteration of all sorts of proteins which are vastly related to cell metabolism (Vannini et al. Citation2014). Likewise, according to Mirzajani et al. (Citation2014), exposure of Oryza sativa L. to Ag NPs led to the identification of 28 responsive proteins, for example, decrement/increment in abundance, which were involved in various important physiological and biochemical processes, for example, Ca2+ regulation and signaling, oxidative stress tolerance, cell wall and RNA/DNA/protein direct damage, transcription and protein degradation, ultimately leading to cell division and apoptosis. Hossain et al. (Citation2016) evaluated the phytotoxicity of Al2O3, ZnO and Ag NPs in soybean seedlings at the proteome level; 16 common proteins in soybean leaves were significantly changed, which were predominantly associated with protein degradation and photosystem.
ROS can lead to oxidative damages in nuclear, chloroplastic and mitochondrial DNA (Imlay & Linn Citation1988). DNA molecule is a cell’s genetic material and any damage to the DNA would cause changes in the encoded proteins, which may result in malfunctions and/or complete inactivation of the encoded proteins. Enhanced DNA degradation has been observed in plants exposed to kinds of environmental stresses such as metal-based NPs and carbon nanomaterials (López-Moreno, de la Rosa, Hernández-Viezcas, Castillo-Michel, et al. Citation2010; Shen et al. Citation2010; Kumari et al. Citation2011; Khodakovskaya et al. Citation2012, Citation2013; Shaymurat et al. Citation2012; Dimkpa et al. Citation2013). For instance, López-Moreno, de la Rosa, Hernández-Viezcas, Castillo-Michel, et al. (Citation2010) discovered that high concentrations of CeO2 NPs had negative effects on DNA of G. max. Similarly, Allium cepa roots suffered various negative effects such as total chromosomal aberrations and increased mitotic index when exposed to bismuth oxide NPs (Liman Citation2013). Several studies have also investigated the potential genotoxic effects of TiO2 NPs on various species of plants (Ghosh et al. Citation2010; Klančnik et al. Citation2011; DEMİR et al. Citation2014; Pakrashi et al. Citation2014). A study on Nicotiana tabacum and A. cepa has found that genotoxic effects occur with exposure to different concentrations of TiO2 NPs (100 nm). Furthermore, damages to the chromosome and micronuclei of A. cepa were observed, and DNA shearing and fragmentation were also found, herein causing the reduction in root growth (Ghosh et al. Citation2010). However, another study on castor bean (Ricinus communis) seeds under Ag NPs exposure reported an increased ROS production and associated antioxidant defense mechanisms: promoting peroxidase (POD) and superoxide dismutase enzyme (SOD) activity, and this caused an increase in phenolic acids. Phenols are synthesized in plants for defense against pathogens; therefore, ROS generation in this case may not be viewed as entirely negative as ROS also enhances root elongation up to a certain concentration (Yasur & Rani Citation2013).
Generation of ROS, which is induced by NPs directly or indirectly, plays a critical role in phytotoxicity mechanism. The production of ROS is based on the physicochemical properties of NPs as well as the test species. Various determinants, such as size and shape, solubility and particle dissolution, metal ions released from metal and metal oxide NPs, biotransformation of NPs, light and so forth, may cause the ROS generation and phytotoxicity (Dimkpa et al. Citation2012; Rui et al. Citation2015; Zhang, Ma, et al. Citation2015). Zhang, Ma, et al. (Citation2015) compared the toxicity of three types of CeO2 NPs to different kinds of Lactuca genus plants. Compared to the control, 7 nm CeO2 caused a significant increase in the MDA level, which means the membrane damage happened in the root cells. However, there was no obvious discrepancy in the MDA level among control, 25 nm CeO2 and its bulk counterpart treatment. Other reports have studied the effects of size and shape of NPs on various plant species as well (Hawthorne et al. Citation2012; Syu et al. Citation2014). In addition, Ma, Wang, et al. (Citation2015) demonstrated that plant responses to CeO2 exposure varied with particle sizes and the growth stages of plants.
Compared to high-solubility NPs such as ZnO NPs and Ag NPs (Xia et al. Citation2008; Oukarroum et al. Citation2013), CeO2 NPs are generally regarded as stable regardless of the biological or environmental systems involved, which were always used as a model material to study the phytotoxicity mechanisms of NPs. Moreover, recent studies indicated that CeO2 NPs can be biotransformed to CePO4 and Ce(CH3COO)3 in several plant species (Zhang et al. Citation2012; Cui et al. Citation2014; Rui et al. Citation2015; Gui, Zhang, et al. Citation2015; Ma, Zhang, Zhang, He, Zhang, et al. Citation2015). The release of Ce3+ ions from NPs was confirmed to play a critical role in the phytotoxicity of CeO2 NPs to lactuca plants in the agar media (Cui et al. Citation2014), aqueous suspensions (Zhang, Ma, et al. Citation2015) and potting soil (Gui, Zhang, et al. Citation2015). A subsequent finding of the present research group was that CeO2 NPs transformation occurred on the root surface of cucumber, rather than in plant tissues, and stated that root exudates at the nano–bio interface play a fundamental role in the biotransformation of CeO2 NPs. Furthermore, phosphates play a vital role in the transformation process of CeO2 NPs in plants (Rui et al. Citation2015). It is well known that phosphates widely exist in the environment and are used as a basic component of many culture media for toxicity testing. A recent study has also confirmed that the phytotoxicity to romaine lettuce of CeO2 NPs was determined by phosphates in a sand culture (Zhang et al. Citation2017).
Lin and Xing (Citation2008) investigated the phytotoxicity of ZnO NPs on Lolium perenne (ryegrass), and found that concentrated ZnO NPs in the rhizosphere solution and absorbed on the root surface caused potential effects on the plant’s growth. Results also revealed that the concentration of released Zn2+ ions was far below the toxic threshold to the plant and, herein, the phytotoxicity effect cannot be attributed to ions dissolution. Toxicity by dissolution of meta-based NPs was also excluded from the main mechanisms in some other investigations (Kirchner et al. Citation2005; Nel et al. Citation2006; Griffitt et al. Citation2007; Zhang, Zhang, et al. Citation2015). Recently a study was conducted on the toxicogenomic effects of CuO NPs on Arabidopsis thaliana by using microarray analysis. The results also agreed with previous opinions that the released Cu2+ ions from CuO NPs contributed to partial toxicity during CuO NPs exposure (Tang et al. Citation2016). Compared with Ag+ ions, Ag NPs showed higher toxicity on the root elongation of A. thaliana, even though seeds germination was not affected. Results also found that seedlings adsorbed very few amounts of Ag+, which could not affect chloroplast structure compared to Ag NPs. Ag NPs could change the balance of oxidation and antioxidant systems and further affect the homeostasis of water and other small molecules within the plant body. A potential mechanism of phytotoxicity is that Ag NPs adsorbed on the root surface, which disrupted the structure of the thylakoid membrane and decreased chlorophyll content, herein causing an inhibitory effect on plant growth (Qian et al. Citation2013). As for other NPs, the phytotoxicity of ZnO NPs may also be ascribed to its photocatalytic activity, which promotes the level of ROS production under irradiation with energy at/or above its band gap energy and induces phytotoxicity (Ma, Williams, et al. Citation2013).
However, when the composition, concentration, size, morphology and surface coating of NPs changed, the results of NPs would also be changed. Therefore, the phytotoxicity mechanism of metal-oxide NPs, for example, ZnO NPs and Ag NPs, may be derived from the NPs’ physicochemical properties to impact seed germination rates, root elongation and so forth, which need further research. In order to enhance our understanding, advanced molecular approaches such as proteomics and genomics must be also extensively implemented.
Detoxification mechanism in plants induced by NPs
As mentioned above, certain environmental conditions, such as heavy metal, salt stress, nutrient deficiency and diverse metal-based NPs exposure, clearly affect the level of cellular ROS, for example, H2O2 and , 1O2 and •OH. ROS is a signaling or damaging molecule, which plays a vital role in the subtle equilibrium between the levels of ROS generation and scavenging. ROS have been involved in intercellular signaling cascades as second messengers, which regulate various plant responses in plant cells such as gravitropism (Joo et al. Citation2001), programmed cell death (Mittler Citation2002), stomatal closure (Kwak et al. Citation2003) and so forth. They also regulate the activities of numerous components by signaling, including transcription factors, protein phosphatases and protein kinases (Cheng & Song Citation2006). However, excess ROS could cause oxidative stress in plants under NPs exposure. To protect themselves against these toxic oxygen intermediates, herein, plant cells and its organelles such as mitochondria, peroxisomes and chloroplast make use of antioxidant defense systems to scavenge excess ROS production. Antioxidant defense systems of plants contain both nonenzymatic antioxidants including thiols, glutathione (GSH), phenolics, ascorbate (AA) (Singh et al. Citation2015) and so forth, and enzymatic components such as CAT, SOD, guaiacol peroxidase (GPOX), APX, glutathione reductase (GR), monodehydroascorbate reductase, dehydroascorbate reductase (DHAR), glutathione S-transferases (GST) and glutathione peroxidase (GPX).
Nonenzymatic agents of the antioxidant defense system
Thiols and AA are the most significant low molecular weight antioxidants. AA is a crucial antioxidant that can resist oxidative stress induced by the promoted level of ROS generation. Owing to the intrinsic ability to donate electrons of AA in diverse nonenzymatic and enzymatic reactions, it can scavenge and •OH and regenerate α-tocopherol from the tocopheroxyl radical to protect membranes directly (Noctor & Foyer Citation1998). It also acts as the first barrier of defense against the potential negative external oxidants. Shaw and Hossain et al. investigated the impact of CuO NPs on rice seedlings. They found consistent increases in AA activity to ensure a higher scavenging rate of H2O2 in seedling leaves when exposed to less than 1.0 and 1.5 mM CuO NPs (Shaw & Hossain Citation2013). Similarly, Rico, Morales, et al. (Citation2013) observed that the 500 mg/L CeO2 NPs treatment altered levels of AA and free thiols, causing enhanced membrane oxidative damage and photosynthetic stress in the shoots.
GSH, one of the fundamental metabolites (a nonprotein thiol with low molecular weight) in plants, plays a key role in intercellular antioxidant defense against ROS-induced oxidative stress. In plant tissues, GSH is commonly found in all cell compartments, including chloroplasts, mitochondria, vacuole, cytosol, as well as in peroxisomes and endoplasmic reticulum (Foyer & Noctor Citation2003). To preserve the normal state of cells, GSH plays an essential role in coping with the oxidative damage induced by ROS. As an antioxidant, GSH acts as a proton donor in the organic free radicals and/or in the presence of ROS, scavenging ROS and reducing to a disulfide form, oxidized glutathione (GSSG). Additionally, via the AA–GSH cycle, GSH takes part in the regeneration of another potential water-soluble antioxidant product such as AA (Foyer & Halliwell Citation1976). Although some researchers have begun to evaluate the levels of GSH in plants under metal and metal-oxide NPs exposure, the reason for similar phytotoxicity caused by NPs is unclear. Recently, it was first reported that CeO2 and In2O3 NPs caused sulfur assimilation gene regulation and GSH biosynthesis in Arabidopsis (Ma, Chhikara, et al. Citation2013). GSH and/or GSSG quantification could be regarded as an alternative method to evaluate whether or not the biosynthesis of GSH plays an important role in the detoxification process under NPs exposure. Likewise, enhancing the GSSG level in plants was induced by other NPs exposure (Dimkpa et al. Citation2012, Citation2013), whereas there is no direct connection between the enhanced level of GSSG production and the decrease in GSH, which converts H2O2 into H2O (Faisal et al. Citation2013; Shaw & Hossain Citation2013).
Due to possessing antioxidant properties, phenolic compounds can scavenge ROS species, chelate transition metal ions and inhibit lipid peroxidation by means of trapping the lipid alkoxyl (–OCH3) polyphenols. Carotenoids, in particular, are the member of lipophilic antioxidants and can detoxify many forms of ROS (Young Citation1991). Tocopherols, containing α-, β-, γ- and δ-tocopherol, represent a class of lipophilic antioxidants that participate in the scavenging of lipid peroxidation radicals, oxygen-free radicals and 1O2 (Diplock et al. Citation1989). By chemically reacting with O2 and physically quenching in chloroplasts, tocopherols can protect lipids and other membrane components, herein protecting the membrane structure and PS II (Ivanov & Khorobrykh Citation2003). However, the investigation of phenolic compounds, carotenoids and tocopherols in response to metal-based NPs exposure is very scarce and the role of the above compounds against NPs-induced stress is also unclear. Understanding the response of the nonenzymatic defense system in plants under the exposure of NPs is very vital in accurately assessing the potential risks of NPs to plants in the near future.
Enzymatic antioxidants of the antioxidant defense system
SOD, the most effective intracellular metalloenzyme, plays a fundamental role in the antioxidant defense system against oxidative stress by catalyzing highly toxic ROS () to less toxic H2O2 and O2. It contains three isozymes including iron SOD (Fe-SOD), manganese SOD (Mn-SOD) and copper/zinc SOD (Cu/Zn-SOD) (Fridovich Citation1989), which are ubiquitous not only in aerobic organisms but also in subcellular compartments to prevent against the toxic effects of enhanced levels of ROS production (Scandalios Citation1993). Several reports have recorded that the promotion of SOD is related to the increase in tolerance of plants against environmental stress, such as NP toxicity (Ma et al. Citation2016; Zhang et al. Citation2017), which means that SOD could act as an indirect selection criterion for researching oxidative stress. While working on tomato, Faisal et al. (Citation2013), showed that a significantly promoted levels of SOD under 0∼1000 mg/L NiO NPs exposure, whereas the decline of SOD activity revealed that the antioxidant defense system was compromised. Ma et al. (Citation2016) have recorded that the SOD activity in rice was elevated under 250 mg/L CeO2 NPs exposure, whereas at a higher concentration treatment there was no obvious change in enzyme activity. Rajeshwari et al. (Citation2015) also found similar results using 1 and 100 mg/L Al2O3 NPs exposed on onion root. Once H2O2 is overproduced either from alternative abiotic stress or from SOD defense system induction, to avoid oxidative stress such as lipid peroxidation, CAT, APX and oxidized GSH contain the main antioxidant enzyme pathway that would be activated to detoxify H2O2.
CAT, among all of the antioxidant enzymes, is the enzyme that was first discovered and characterized and plays an indispensable role in ROS detoxification under stress (Garg & Manchanda Citation2009). It is widely found in tetrameric heme-containing enzymes and converts H2O2 into O2 and H2O directly. APX, regarded as the most significant ROS scavenger to protect higher plants, avoids oxidative stress and plays a central role in regulating the AA–GSH cycle. Compared to CAT and POD, APX has a higher affinity for H2O2. APX can convert the low toxic H2O2 to nontoxic H2O by AA oxidation into dehydroascorbate and MDA. GR takes part in both enzymic and nonenzymic oxidation–reduction cycles as an antioxidant. It is an NADPH-dependent enzyme that catalyzes the oxidation of GSH to GSSG, which can maintain a high ratio of GSH/GSSG in the cells (Rao et al. Citation2006). There are two steps involved in the catalytic mechanism. Originally, in order to achieve a cysteine and a thiolate anion, on interaction with NADPH flavin is oxidized and a disulfide bridge is reduced. Then, the reduction of GSSG is involved in thiol–disulfide interchange reactions (Ghisla & Massey Citation1989). GPX, a catalyzer, uses GSH to reduce lipid hydroperoxides and organic hydroperoxides, thus exhibiting a positive effect in plants against environmental stress. GR, APX and DHAR are involved in the AA–GSH that can control the excess levels of ROS or oxidative state in plants. Rico et al. (Citation2015) found that APX can reduce the level of H2O2, which was formed by SOD converting it to H2O. Owing to DHAR existence, regenerated AA was used by APX for the diminution of H2O2 again. Likewise, various antioxidant enzymes can be activated by numerous NPs, for example, Fe3O4 NPs, Co3O4 NPs and CeO2 NPs induced CAT; Au NPs, MnO2 NPs, Fe3O4 NPs, CuO NPs, Co3O4 NPs and CeO2 NPs induced GPX and fullerene; and Pt NPs and CeO2 NPs induced SOD (Wei & Wang Citation2013). Majumdar et al. (Citation2014), while working on Phaseolus vulgaris, showed that phytotoxicity in plants caused by CeO2 NPs exposure disturbed the antioxidant defense system. Several studies have demonstrated the activities of CAT and APX where plant species are under NPs exposure. In CeO2 NPs-induced ROS generation, the CAT activity was reduced significantly in roots, suggesting that a detoxification pathway may be involved in the AA–GSH or GPX cycle (Rico, Morales, et al. Citation2013). Compared to the control, Servin et al. (Citation2013) have recorded the effects of TiO2 NPs in cucumber and found that NPs can induce a significant CAT activity ranging from 250 to 750 mg/L, whereas no obvious difference in APX activity was found, except a marked decrease at 500 mg/L. A dose-dependent response of SOD and CAT activity was employed in tomatoes under NiO NPs treatment (Faisal et al. Citation2013). Ma et al. (Citation2016) investigated the defense mechanisms of A. thaliana and found that the activities of CAT, SOD, POD and APX were significant ameliorated under CeO2 NPs exposure, while the elevations were only found for POD and SOD under In2O3 NPs exposure. Similarly, an article on the effects of Nd2O3 NPs and its bulk counterparts in pumpkin found that not only pumpkin growth was inhibited, but also the K, Ca, Mg and S levels in pumpkin tissues were decreased under 100 mg/L Nd2O3 NPs and bulk exposure. They also reported that the enzymatic activities of SOD and POD elevated, whereas that of CAT and APX reduced, which could be due to other pathways scavenging H2O2 or their biosynthesis was inhibited under overproduced H2O2 (Chen et al. Citation2016). These evidence indicated that the antioxidant enzyme response mechanism might be based on the NP concentration, plant species, media and NP types (). As shown in , which is presented in the published literature on plants and NMs (nanomaterials), NMs can cause a great number of adverse effects on plants at both the cellular (cell membrane damage, chromosomal aberration and chlorophyll synthesis disruption) and physiological (biomass decrease, root length inhibition and so on) levels. Recent researchers are devoting their efforts to identify the mechanisms of plant defense against NMs-induced oxidative stress.
Figure 1. Schematic diagram of potential cell, DNA damages and detoxification pathways under NMs exposure.
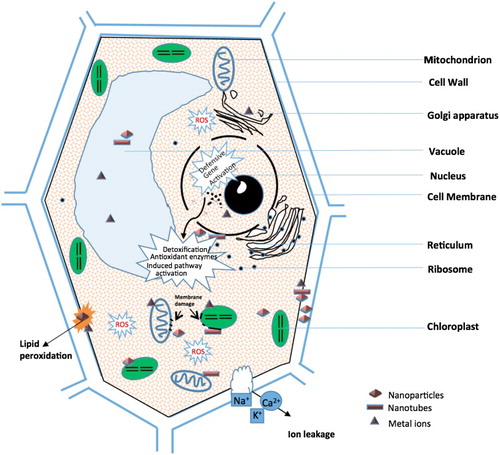
Table 2. Summary of the plant antioxidant enzymes upon NPs exposure.
Rapid progress has been made in recent years though; however, there are many gaps and uncertainties in our knowledge of ROS-dependent damage and its effects on plants. It is critical to understanding NP phytotoxicity and the antioxidant defense system as plant response mechanisms.
Future research needs
Scientists have made some consensus on the environmental behavior and ecological effects of nanomaterials; but there are still a lot of controversies and problems that need to be further studied.
Further understanding of the environmental fate and mechanism of NPs
It is not enough to rely on the current detection methods for phytotoxicity only; how to correctly use the positive effect and eliminate the negative effect of NPs is the research motivation and goal of researchers. On the one hand, the dose- and time-dependent negative effects of NPs on plant growth observed in experimental conditions should lead to a more careful use in crop management. On the other hand, the phytotoxic mechanism of NPs must be thoroughly understood before being applied in the field environment under the toxic concentration; an effective method to reduce the toxicity also needs to be developed.
Change the exposure time, concentration and mode in the experiment
Unfortunately, very few reports covered the life cycle of plants treated with NPs to make a conclusive assessment of their long-term risks and benefits. Lin et al. (Citation2009) further found that soluble organic matter treated C70 and NWCNTs can reduce the seed setting rate, and even affect the next generation. So it is necessary to strengthen the research on the toxicity and absorption of NPs to plants in a long-term dose exposure test. Similarly, increasing the diversity of material exposure is also important, such as changing short-term exposure with high concentration to long-term exposure with low concentration.
Make the experiment condition more close to the real field environment
These limited data, as show above, are only based on laboratory or greenhouse studies. Due to the results of the single element in the laboratory and the properties of NMs are greatly influenced by environmental media, the results of field trails and laboratory studies are often contradictory. Therefore, it is needed to strengthen the toxicity and absorption of NPs in soil or simulated soil (e.g. quartz sand). In addition, the study on the effects of NPs on population and ecological structure should be strengthened in the future.
Analyze problems using multidisciplinary knowledge objectively
The study of López-Moreno, de la Rosa, Hernández-Viezcas, Castillo-Michel, et al. (Citation2010) found that the plant germination rate decreased, but the amount of root elongation had been promoted. In this case, how do we evaluate the effects of NPs on plants? It is done by assuming that under moderate concentrations the root elongation could promote plant growth and increase yield, so as to make up for the loss caused by the germination rate and even exceed, resulting in an increase in the total production, which would use the knowledge of economics. Furthermore, for the analysis of the crops quality, food safety or food nutrition knowledge is needed as support.
Develop a series of safety evaluation and toxicological risk assessment standards
At present, the standard research methods and evaluation indexes of the toxicity of nanomaterials are relatively simple. The analysis of morphological, anatomical, physiological and biochemical indexes cannot fully reflect the plant toxicity to NPs. NPs have unique physical and chemical properties, so the obtained scientific knowledge is still needed further inspection. A series of safety evaluation and toxicological risk assessment standards must be formulated, including exposure route, safe exposure dose, etc. The purpose of this work is to provide a scientific basis for the application of NPs.
Others
As to the accumulation of NPs in plants, the problem should also be analyzed objectively. For example, if NPs accumulate in the tomato leaves, not the edible part, then the NPs will not enter the human body through the consumption of the fruit, and it will not affect our health. At this point, we should focus on the study of NPs in the environment and how to recycle them and reduce their release. The research on cucumber, pumpkin, radish, lettuce and other edible plants should be based on the same theory.
To our knowledge, there are few studies reported on the effect and fate of NPs under extreme conditions. When the study of NPs in agricultural soils has matured to a certain extent, the study realms should be expanded, such as saline land, tropics droughts, floods and some other extreme conditions, in order to observe whether the special properties of NPs in extreme environments will produce special reactions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. 2009. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 4:634–641. doi: 10.1038/nnano.2009.242
- Batley GE, Kirby JK, McLaughlin MJ. 2013. Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res. 46:854–862. doi: 10.1021/ar2003368
- Bleecker AB, Kende H. 2000. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 16:1–18. doi: 10.1146/annurev.cellbio.16.1.1
- Chen G, Ma C, Mukherjee A, Musante C, Zhang J, White JC, Dhankher OP, Xing B. 2016. Tannic acid alleviates bulk and nanoparticle Nd2O3 toxicity in pumpkin: a physiological and molecular response. Nanotoxicology. 10:1243–1253. doi: 10.1080/17435390.2016.1202349
- Cheng Y, Song C. 2006. Hydrogen peroxide homeostasis and signaling in plant cells. Sci China. Ser C Life Sci/Chinese Acad Sci. 49:1–11.
- Cui D, Zhang P, Ma Y, He X, Li Y, Zhang J, Zhao Y, Zhang Z. 2014. Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environ Sci Nano. 1:459–465. doi: 10.1039/C4EN00025K
- Demir E, Kaya N, Kaya B. 2014. Genotoxic effects of zinc oxide and titanium dioxide nanoparticles on root meristem cells of Allium cepa by comet assay. Turk J Biol. 38:31–39. doi: 10.3906/biy-1306-11
- Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ. 2012. Cuo and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res. 14:1–15. doi: 10.1007/s11051-012-1125-9
- Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ. 2013. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol. 47:1082–1090. doi: 10.1021/es302973y
- Diplock AT, Machlin L, Packer L, Pryor W. 1989. Vitamin E: biochemistry and health implications. New York: The New York Academy of Sciences.
- El-Temsah YS, Joner EJ. 2012. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol. 27:42–49. doi: 10.1002/tox.20610
- Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Hegazy AK, Musarrat J. 2013. Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater. 250–251:318–332. doi: 10.1016/j.jhazmat.2013.01.063
- Feizi H, Kamali M, Jafari L, Moghaddam PR. 2013. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere. 91:506–511. doi: 10.1016/j.chemosphere.2012.12.012
- Foyer CH, Halliwell B. 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 133:21–25. doi: 10.1007/BF00386001
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. 1997. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 100:241–254. doi: 10.1111/j.1399-3054.1997.tb04780.x
- Foyer CH, Noctor G. 2003. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant. 119:355–364. doi: 10.1034/j.1399-3054.2003.00223.x
- Freinbichler W, Colivicchi MA, Stefanini C, Bianchi L, Ballini C, Misini B, Weinberger P, Linert W, Varešlija D, Tipton KF, Corte LD. 2011. Highly reactive oxygen species: detection, formation, and possible functions. Cell Mol Life Sci. 68:2067–2079. doi: 10.1007/s00018-011-0682-x
- Fridovich I. 1989. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 264:7761–7764.
- Garg N, Manchanda G. 2009. ROS generation in plants: boon or bane? Plant Biosyst. 143:81–96. doi: 10.1080/11263500802633626
- Ghisla S, Massey V. 1989. Mechanisms of flavoprotein-catalyzed reactions. Eur J Biochem. 181:1–17. doi: 10.1111/j.1432-1033.1989.tb14688.x
- Ghosh M, Bandyopadhyay M, Mukherjee A. 2010. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 81:1253–1262. doi: 10.1016/j.chemosphere.2010.09.022
- Ghosh M, Bhadra S, Adegoke A, Bandyopadhyay M, Mukherjee A. 2015. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat Res/Fundam Mol Mech Mutagen. 774:49–58. doi: 10.1016/j.mrfmmm.2015.03.004
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 48:909–930. doi: 10.1016/j.plaphy.2010.08.016
- Gottschalk F, Lassen C, Kjoelholt J, Christensen F, Nowack B. 2015. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int J Environ Res Public Health. 12:5581–5602. doi: 10.3390/ijerph120505581
- Gottschalk F, Sonderer T, Scholz RW, Nowack B. 2009. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol. 43:9216–9222. doi: 10.1021/es9015553
- Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS. 2007. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol. 41:8178–8186. doi: 10.1021/es071235e
- Gui X, Deng Y, Rui Y, Gao B, Luo W, Chen S, Van Nhan L, Li X, Liu S, Han Y, et al. 2015. Response difference of transgenic and conventional rice (Oryza sativa) to nanoparticles (γFe2O3). Environ Sci Pollut Res. 22:17716–17723. doi: 10.1007/s11356-015-4976-7
- Gui X, Zhang Z, Liu S, Ma Y, Zhang P, He X, Li Y, Zhang J, Li H, Rui Y, et al. 2015. Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PloS one. 10:e0134261. doi: 10.1371/journal.pone.0134261
- Halliwell B, Gutteridge JM. 2015. Free radicals in biology and medicine. USA: Oxford University Press.
- Hancock J, Desikan R, Harrison J, Bright J, Hooley R, Neill S. 2006. Doing the unexpected: proteins involved in hydrogen peroxide perception. J Exp Bot. 57:1711–1718. doi: 10.1093/jxb/erj180
- Hao Y, Yu F, Lv R, Ma C, Zhang Z, Rui Y, Liu L, Cao W, Xing B, Choi J. 2016. Carbon nanotubes filled with different ferromagnetic alloys affect the growth and development of rice seedlings by changing the C:N ratio and plant hormones concentrations. PloS One. 11:e0157264. doi: 10.1371/journal.pone.0157264
- Hawthorne J, Musante C, Sinha SK, White JC. 2012. Accumulation and phytotoxicity of engineered nanoparticles to Cucurbita pepo. Int J Phytoremediation. 14:429–442. doi: 10.1080/15226514.2011.620903
- Hong J, Peralta-Videa JR, Rico C, Sahi S, Viveros MN, Bartonjo J, Zhao L, Gardea-Torresdey JL. 2014. Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ Sci Technol. 48:4376–4385. doi: 10.1021/es404931g
- Hong J, Wang L, Sun Y, Zhao L, Niu G, Tan W, Rico CM, Peralta-Videa JR, Gardea-Torresdey JL. 2016. Foliar applied nanoscale and microscale CeO2 and CuO alter cucumber (Cucumis sativus) fruit quality. Sci Total Environ. 563–564:904–911. doi: 10.1016/j.scitotenv.2015.08.029
- Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P. 2005. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res. 105:269–280. doi: 10.1385/BTER:105:1-3:269
- Hossain Z, Mustafa G, Sakata K, Komatsu S. 2016. Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J Hazard Mater. 304:291–305. doi: 10.1016/j.jhazmat.2015.10.071
- Imlay JA, Linn S. 1988. DNA damage and oxygen radical toxicity. Sci(Washington). 240:1302–1309. doi: 10.1126/science.3287616
- Ivanov B, Khorobrykh S. 2003. Participation of photosynthetic electron transport in production and scavenging of reactive oxygen species. Antioxid Redox Signal. 5:43–53. doi: 10.1089/152308603321223531
- Joo JH, Bae YS, Lee JS. 2001. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 126:1055–1060. doi: 10.1104/pp.126.3.1055
- Khodakovskaya MV, Kim BS, Kim JN, Alimohammadi M, Dervishi E, Mustafa T, Cernigla CE. 2013. Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small. 9:115–123. doi: 10.1002/smll.201201225
- Khodakovskaya MV, de Silva K, Biris AS, Dervishi E, Villagarcia H. 2012. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano. 6:2128–2135. doi: 10.1021/nn204643g
- Kirchner C, Liedl T, Kudera S, Pellegrino T, Muñoz Javier A, Gaub HE, Stölzle S, Fertig N, Parak WJ. 2005. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 5:331–338. doi: 10.1021/nl047996m
- Klančnik K, Drobne D, Valant J, Koce JD. 2011. Use of a modified allium test with nanoTiO2. Ecotoxicol Environ Saf. 74:85–92. doi: 10.1016/j.ecoenv.2010.09.001
- Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N. 2011. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater. 190:613–621. doi: 10.1016/j.jhazmat.2011.03.095
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22:2623–2633. doi: 10.1093/emboj/cdg277
- Le Van N, Ma C, Rui Y, Liu S, Li X, Xing B, Liu L. 2015. Phytotoxic mechanism of nanoparticles: destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci Rep. 5:116–118.
- Le Van N, Rui Y, Cao W, Shang J, Liu S, Nguyen Quang T, Liu L. 2016. Toxicity and bio-effects of CuO nanoparticles on transgenic Ipt-cotton. J Plant Interact. 11:108–116. doi: 10.1080/17429145.2016.1217434
- Le Van N, Rui Y, Gui X, Li X, Liu S, Han Y. 2014. Uptake, transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J Nanobiotechnol. 12:50. doi: 10.1186/s12951-014-0050-8
- Lee WM, An YJ, Yoon H, Kweon HS. 2008. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem. 27:1915–1921. doi: 10.1897/07-481.1
- Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJ. 2010. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem. 29:669–675. doi: 10.1002/etc.58
- Li W, Zheng Y, Zhang H, Liu Z, Su W, Chen S, Liu Y, Zhuang J, Lei B. 2016. Phytotoxicity, uptake, and translocation of fluorescent carbon dots in mung bean plants. ACS Appl Mater Interfaces. 8:19939–19945. doi: 10.1021/acsami.6b07268
- Liman R. 2013. Genotoxic effects of bismuth (III) oxide nanoparticles by allium and comet assay. Chemosphere. 93:269–273. doi: 10.1016/j.chemosphere.2013.04.076
- Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC. 2009. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small. 5:1128–1132. doi: 10.1002/smll.200800677
- Lin D, Xing B. 2008. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Technol. 42:5580–5585. doi: 10.1021/es800422x
- López-Moreno ML, de la Rosa G, Hernández-Viezcas JÁ, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL. 2010. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol. 44:7315–7320. doi: 10.1021/es903891g
- López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Peralta-Videa JR, Gardea-Torresde JL. 2010. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem. 58:3689–3693. doi: 10.1021/jf904472e
- Lu C, Zhang C, Wen J, Wu G, Tao M. 2001. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 21:168–171.
- Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP. 2013. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng. 1:768–778. doi: 10.1021/sc400098h
- Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z. 2010. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere. 78:273–279. doi: 10.1016/j.chemosphere.2009.10.050
- Ma C, Liu H, Guo H, Musante C, Coskun SH, Nelson BC, White JC, Xing B, Dhankher OP. 2016. Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ Sci Nano. 3:1369–1379. doi: 10.1039/C6EN00189K
- Ma X, Wang Q, Rossi L, Zhang W. 2015. Cerium oxide nanoparticles and bulk cerium oxide leading to different physiological and biochemical responses in Brassica rapa. Environ Sci Technol. 50:6793–6802. doi: 10.1021/acs.est.5b04111
- Ma C, White JC, Dhankher OP, Xing B. 2015. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol. 49:7109–7122. doi: 10.1021/acs.est.5b00685
- Ma H, Williams PL, Diamond SA. 2013. Ecotoxicity of manufactured ZnO nanoparticles – a review. Environ Pollut. 172:76–85. doi: 10.1016/j.envpol.2012.08.011
- Ma Y, Zhang P, Zhang Z, He X, Li Y, Zhang J, Zheng L, Chu S, Yang K, Zhao Y, Chai Z. 2015. Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicology. 9:262–270. doi: 10.3109/17435390.2014.921344
- Ma Y, Zhang P, Zhang Z, He X, Zhang J, Ding Y, Zhang J, Zheng L, Guo Z, Zhang L, et al. 2015. Where does the transformation of precipitated ceria nanoparticles in hydroponic plants take place? Environ Sci Technol. 49:10667–10674. doi: 10.1021/acs.est.5b02761
- Majumdar S, Peralta-Videa JR, Bandyopadhyay S, Castillo-Michel H, Hernandez-Viezcas JA, Sahi S, Gardea-Torresdey JL. 2014. Exposure of cerium oxide nanoparticles to kidney bean shows disturbance in the plant defense mechanisms. J Hazard Mater. 278:279–287. doi: 10.1016/j.jhazmat.2014.06.009
- Majumdar S, Peralta-Videa JR, Trujillo-Reyes J, Sun Y, Barrios AC, Niu G, Flores-Margez JP, Gardea-Torresdey JL. 2016. Soil organic matter influences cerium translocation and physiological processes in kidney bean plants exposed to cerium oxide nanoparticles. Sci Total Environ. 569–570:201–211. doi: 10.1016/j.scitotenv.2016.06.087
- McKee MS, Filser J. 2016. Impacts of metal-based engineered nanomaterials on soil communities. Environ Sci Nano. 3:506–533. doi: 10.1039/C6EN00007J
- Meriga B, Reddy BK, Rao KR, Reddy LA, Kishor PBK. 2004. Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). J Plant Physiol. 161:63–68. doi: 10.1078/0176-1617-01156
- Mirzajani F, Askari H, Hamzelou S, Schober Y, Römpp A, Ghassempour A, Spengler B. 2014. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol Environ Saf. 108:335–339. doi: 10.1016/j.ecoenv.2014.07.013
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410. doi: 10.1016/S1360-1385(02)02312-9
- Møller IM, Kristensen BK. 2004. Protein oxidation in plant mitochondria as a stress indicator. Photochem Photobiol Sci. 3:730–735. doi: 10.1039/B315561G
- Montillet JL, Chamnongpol S, Rustérucci C, Dat J, Van De Cotte B, Agnel JP, Battesti C, Inzé D, Van Breusegem F, Triantaphylides C. 2005. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138:1516–1526. doi: 10.1104/pp.105.059907
- Nel A, Xia T, Mädler L, Li N. 2006. Toxic potential of materials at the nanolevel. Science. 311:622–627. doi: 10.1126/science.1114397
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol. 49:249–279. doi: 10.1146/annurev.arplant.49.1.249
- Oberdürster G. 2000. Toxicology of ultrafine particles: in vivo studies. Philos Trans Roy Soc London A Math Phys Eng Sci. 358:2719–2740. doi: 10.1098/rsta.2000.0680
- Oukarroum A, Barhoumi L, Pirastru L, Dewez D. 2013. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ Toxicol Chem. 32:902–907. doi: 10.1002/etc.2131
- Pakrashi S, Jain N, Dalai S, Jayakumar J, Chandrasekaran PT, Raichur AM, Chandrasekaran N, Mukherjee A, Bansal V. 2014. In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS One. 9:e87789. doi: 10.1371/journal.pone.0087789
- Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An YJ, et al. 2012. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proceed Nat Acad Sci. 109:E2451–E2456. doi: 10.1073/pnas.1205431109
- Qian H, Peng X, Han X, Ren J, Sun L, Fu Z. 2013. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci. 25:1947–1956. doi: 10.1016/S1001-0742(12)60301-5
- Rajeshwari A, Kavitha S, Alex SA, Kumar D, Mukherjee A, Chandrasekaran N, Mukherjee A. 2015. Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip – effects of oxidative stress generation and biouptake. Environ Sci Pollut Res. 22:11057–11066. doi: 10.1007/s11356-015-4355-4
- Rani PU, Yasur J, Loke KS, Dutta D. 2016. Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol Plant. 38:1–9. doi: 10.1007/s11738-016-2074-1
- Rao K, Raghavendra A, Reddy K. 2006. Physiology and molecular biology of stress tolerance in plants. Netherlands: Springer.
- Riahi-Madvar A, Rezaee F, Jalali V. 2012. Effects of alumina nanoparticles on morphological properties and antioxidant system of Triticum aestivum. Iran J Plant Physiol. 3:595–603.
- Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang JY, Peralta-Videa JR, Gardea-Torresdey JL. 2013. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol. 47:5635–5642. doi: 10.1021/es401032m
- Rico CM, Lee SC, Rubenecia R, Mukherjee A, Hong J, Peralta-Videa JR, Gardea-Torresdey JL. 2014. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J Agric Food Chem. 62:9669–9675. doi: 10.1021/jf503526r
- Rico CM, Majumdar S, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL. 2011. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem. 59:3485–3498. doi: 10.1021/jf104517j
- Rico CM, Morales MI, Barrios AC, McCreary R, Hong J, Lee WY, Nunez J, Peralta-Videa JR, Gardea-Torresdey JL. 2013. Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J Agric Food Chem. 61:11278–11285. doi: 10.1021/jf404046v
- Rico C, Peralta-Videa J, Gardea-Torresdey J. 2015. Nanotechnology and Plant Sciences. In: Manzer H. Siddiqui, Mohamed H. Al-Whaibi, Firoz Mohammad, editors. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants, Nanotechnology and Plant Sciences. Springer; p. 1–17.
- Rui M, Ma C, Hao Y, Guo J, Rui Y, Tang X, Zhao Q, Fan X, Zhang Z, Hou T. 2016. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci. 7:815. doi: 10.3389/fpls.2016.00815
- Rui Y, Zhang P, Zhang Y, Ma Y, He X, Gui X, Li Y, Zhang J, Zheng L, Chu S, et al. 2015. Transformation of ceria nanoparticles in cucumber plants is influenced by phosphate. Environ Pollut. 198:8–14. doi: 10.1016/j.envpol.2014.12.017
- Salah SM, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Weimin H, Mingyu N, Jin H. 2015. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep. 5:14278. doi: 10.1038/srep14278
- Santner A, Calderon-Villalobos LIA, Estelle M. 2009. Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol. 5:301–307. doi: 10.1038/nchembio.165
- Scandalios JG. 1993. Oxygen stress and superoxide dismutases. Plant Physiol. 101:7–12. doi: 10.1104/pp.101.1.7
- Schlich K, Hund-Rinke K. 2015. Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environ Pollut. 196:321–330. doi: 10.1016/j.envpol.2014.10.021
- Servin AD, Morales MI, Castillo-Michel H, Hernandez-Viezcas JA, Munoz B, Zhao L, Nunez JE, Peralta-Videa JR, Gardea-Torresdey JL. 2013. Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ Sci Technol. 47:11592–11598. doi: 10.1021/es403368j
- Sharma P, Jha AB, Dubey RS, Pessarakli M. 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012:1–26. doi: 10.1155/2012/217037
- Shaw AK, Hossain Z. 2013. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere. 93:906–915. doi: 10.1016/j.chemosphere.2013.05.044
- Shaymurat T, Gu J, Xu C, Yang Z, Zhao Q, Liu Y, Liu Y. 2012. Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): A morphological study. Nanotoxicology. 6:241–248. doi: 10.3109/17435390.2011.570462
- Shen CX, Zhang QF, Li J, Bi FC, Yao N. 2010. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am J Bot. 97:1602–1609. doi: 10.3732/ajb.1000073
- Singh VP, Singh S, Kumar J, Prasad SM. 2015. Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged luffa seedlings. Protoplasma. 252:1217–1229. doi: 10.1007/s00709-014-0753-6
- Song U, Jun H, Waldman B, Roh J, Kim Y, Yi J, Lee EJ. 2013. Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol Environ Saf. 93:60–67. doi: 10.1016/j.ecoenv.2013.03.033
- Stampoulis D, Sinha SK, White JC. 2009. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 43:9473–9479. doi: 10.1021/es901695c
- Syu Y, Hung JH, Chen JC, Chuang H. 2014. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem. 83:57–64. doi: 10.1016/j.plaphy.2014.07.010
- Tang Y, He R, Zhao J, Nie G, Xu L, Xing B. 2016. Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ Pollut. 212:605–614. doi: 10.1016/j.envpol.2016.03.019
- Tanou G, Molassiotis A, Diamantidis G. 2009. Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environ Exp Bot. 65:270–281. doi: 10.1016/j.envexpbot.2008.09.005
- Tarasenko V, Garnik EY, Shmakov V, Konstantinov YM. 2012. Modified alternative oxidase expression results in different reactive oxygen species contents in Arabidopsis cell culture but not in whole plants. Biol Plant. 56:635–640. doi: 10.1007/s10535-012-0115-1
- Thannickal VJ, Fanburg BL. 2000. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 279:L1005–L1028.
- Tolaymat T, El Badawy A, Sequeira R, Genaidy A. 2015. A system-of-systems approach as a broad and integrated paradigm for sustainable engineered nanomaterials. Sci Total Environ. 511:595–607. doi: 10.1016/j.scitotenv.2014.09.029
- Vannini C, Domingo G, Onelli E, De Mattia F, Bruni I, Marsoni M, Bracale M. 2014. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol. 171:1142–1148. doi: 10.1016/j.jplph.2014.05.002
- Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B. 2012. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol. 46:4434–4441. doi: 10.1021/es204212z
- Wei H, Wang E. 2013. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 42:6060–6093. doi: 10.1039/c3cs35486e
- Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. 2008. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2:2121–2134. doi: 10.1021/nn800511k
- Yan S, Zhao L, Li H, Zhang Q, Tan J, Huang M, He S, Li L. 2013. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J Hazard Mater. 246–247:110–118. doi: 10.1016/j.jhazmat.2012.12.013
- Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P. 2006. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res. 110:179–190. doi: 10.1385/BTER:110:2:179
- Yasur J, Rani PU. 2013. Environmental effects of nanosilver: impact on castor seed germination, seedling growth, and plant physiology. Environ Sci Pollut Res. 20:8636–8648. doi: 10.1007/s11356-013-1798-3
- Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES. 2012. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS One. 7:e47674. doi: 10.1371/journal.pone.0047674
- Young AJ. 1991. The photoprotective role of carotenoids in higher plants. Physiol Plant. 83:702–708. doi: 10.1111/j.1399-3054.1991.tb02490.x
- Zhang P, Ma Y, Liu S, Wang G, Zhang J, He X, Zhang J, Rui Y, Zhang Z. 2017. Phytotoxicity, uptake and transformation of nano-CeO2 in sand cultured romaine lettuce. Environ Pollut. 220:1400–1408. doi: 10.1016/j.envpol.2016.10.094
- Zhang P, Ma Y, Zhang Z, He X, Li Y, Zhang J, Zheng L, Zhao Y. 2015. Species-specific toxicity of ceria nanoparticles to Lactuca plants. Nanotoxicology. 9:1–8. doi: 10.3109/17435390.2013.855829
- Zhang P, Ma Y, Zhang Z, He X, Zhang J, Guo Z, Tai R, Zhao Y, Chai Z. 2012. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano. 6:9943–9950. doi: 10.1021/nn303543n
- Zhang W, Musante C, White JC, Schwab P, Wang Q, Ebbs SD, Ma X. 2015. Bioavailability of cerium oxide nanoparticles to Raphanus sativus L. in two soils. Plant Physiol Biochem. 110:185–193. doi: 10.1016/j.plaphy.2015.12.013
- Zhang R, Zhang H, Tu C, Hu X, Li L, Luo Y, Christie P. 2015. Phytotoxicity of ZnO nanoparticles and the released Zn (II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ Sci Pollut Res. 22:11109–11117. doi: 10.1007/s11356-015-4325-x
- Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR, Tang X, Niu G, Jin L, Varela-Ramirez A, et al. 2012. Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 6:9615–9622. doi: 10.1021/nn302975u
- Zhao L, Sun Y, Hernandez-Viezcas JA, Servin AD, Hong J, Niu G, Peralta-Videa JR, Duarte-Gardea M, Gardea-Torresdey JL. 2013. Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J Agric Food Chem. 61:11945–11951. doi: 10.1021/jf404328e
- Zheng L, Hong F, Lu S, Liu C. 2005. Effect of nano-TiO2. Biol Trace Elem Res. 104:83–91. doi: 10.1385/BTER:104:1:083
- Zhu H, Han J, Xiao JQ, Jin Y. 2008. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monitor. 10:713–717. doi: 10.1039/b805998e

