ABSTRACT
Introduction
More than 30% of peripherally inserted central catheters (PICCs) and midline catheters experience complications. Most complications are related to thrombotic cellular adherence to catheter materials.
Areas covered
This manuscript outlines PICC and midline catheter complications, the need to reduce complications and how hydrogel catheters may provide a solution to address these unmet needs based on available evidence.
Expert opinion
Patients commonly require PICC or midline catheters for treatment to establish a reliable form of intravenous access. Catheters, while reliable in most cases, are not without complications, including occlusion, thrombosis and infection, each related to cellular adherence to the catheter material. Hydrophilic catheter coatings and composites have been developed to mitigate these thrombotic complications, reduce adherence of blood and bacterial cells to catheters and provide greater patient safety with these devices. Hydrogel materials are highly biocompatible and have been effective in reducing cellular adherence and the formation of biofilms on surfaces. Smooth hydrophilic catheter surfaces are potentially more comfortable for the patient, with reduced friction during insertion and removal. A catheter constructed of hydrophilic biomaterial, a hydrogel composite material, may minimize thrombotic complications in PICC and midline catheters, improving catheter performance and outcomes for patients.
1. Overview of the market
An estimated 80% of patients admitted into acute care for treatment require some form of intravenous (IV) access. Intravenous catheters are a crucial component in the medical field as they are used for administering medication, fluids, and nutrients directly into the bloodstream, but they are not without risk. The primary goal of IV management is to maintain catheter function while preventing catheter-related complications. When an IV device fails, becomes occluded, or develops thrombotic or infectious complications, medical treatment is hampered, and the patient requires more invasive procedures, such as re-insertion of the device.
Peripherally inserted central catheters (PICC) and midline catheters are often used instead of peripheral catheters to provide longer-term, more reliable, functional IV access. According to iData Vascular Access Research Report 2023, more than 2.5 million (M) PICCs and 1.1 M midline catheters are purchased in the United States (U.S.A.) each year [Citation1]. These PICC and midline catheters are inserted into a peripheral vein, usually in the upper arm, and are used for various medical procedures, including chemotherapy, antibiotics, blood transfusions, and hydration. Midline catheters terminate in the peripheral veins at or below the shoulder. PICC catheters are peripherally inserted but terminate in the larger vessels of the superior or inferior vena cava. Complications associated with PICC and midline catheters are venous thrombosis, catheter occlusion, and infection [Citation2–5]. Preventing complications is a priority and essential to the safety of the patient, the completion of intravenous therapy, and the efficient use of healthcare dollars. This manuscript outlines the impact of PICC and midline catheter complications and available evidence supporting the use of novel hydrogel composite catheter materials as a potential solution.
1.1. Basic materials and design
The material of the PICC or midline catheter plays a crucial role in determining performance, safety, and ease of use. There are several different types of materials used for intravenous catheters, each with its own unique properties and benefits (). Two primary types of catheter materials dominate the PICC and midline market, silicone and polyurethane. Polyurethane is most commonly used for PICC and midline catheters related to durability, flexibility and power injectability. Polyurethane is a synthetic polymer that is made from the reaction of polyisocyanate and polyalcohol [Citation6]. This reaction creates a material that is both strong and flexible, making it an ideal choice for catheter materials. Polyurethane catheters perform well and are known for their high kink resistance, which is a common problem with other types of catheter materials [Citation6]. This resistance to kinking allows for a stable and consistent flow of fluids and medication through the catheter [Citation6].
Table 1. Comparison of common catheter material properties and their performance.
Two of the main benefits of using polyurethane catheters are their biocompatibility and long-term durability. These catheters are safely left in place for extended periods of time, which is beneficial for patients who require long-term treatment. These characteristics make polyurethane an ideal material for catheter construction, as it can withstand the rigors of insertion into the bloodstream and prolonged dwell time.
Modifications and improvements in polyurethane composition for catheters have occurred over time with first, second and now third-generation materials that are more biocompatible [Citation7,Citation8]. Polyurethane catheter surfaces are inherently hydrophobic in nature and highly susceptible to protein adsorption however, modifications in the form of surface coatings and composite chemical material changes improve polyurethane catheter performance, providing anti-thrombotic and anti-microbial benefits [Citation6,Citation8,Citation9].
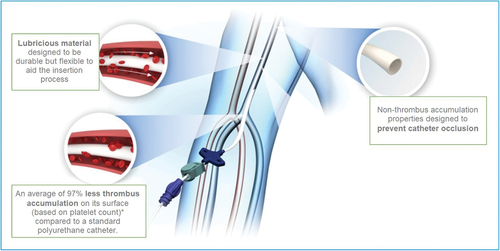
Hydrophilic biomaterial (HBM) is a type of hydrogel composite polymer that absorbs water, making the material very biocompatible while creating a highly lubricious catheter surface that, among other things, allows for ease of insertion and minimal vessel inflammatory impact [Citation10]. One of the biggest challenges with using hydrogels for the construction of intravenous catheters is the lack of mechanical strength. This hydrogel composite material is unique because it possesses the strength that provides many of the same beneficial properties of polyurethane catheters while maintaining the hydrogel-related benefits [Citation11]. The bulk-material hydrophilicity makes it inherently non-thrombogenic and reduces friction on the catheter surface, making insertion smoother and less likely to cause irritation or damage while lessening the impact of the foreign material on the vein [Citation6,Citation12]. The hydrophilic surface is described as the smoothest surface for a catheter, with smoothness attributed to the water-absorbing gel-like layer of the material [Citation13–15]. This smooth layer reduces friction for insertion and removal of the catheter, making the process much easier and less painful for the patient, and resists thrombus formation, potentially reducing thrombophlebitis and other clotting and inflammatory processes related to the body’s response to foreign materials.
Normal clotting processes related to a foreign material are reduced with HBM catheters. The hydrated super lubricious surface resists the sticking of proteins and blood cells, reducing the risk of thrombotic-related complications of venous thrombosis and catheter occlusion. Reduced cellular adherence on the inner and outer surfaces of the catheter also discourages bacterial attachment and biofilm growth, thus minimizing catheter-related infections [Citation16–19]. These material characteristics have the potential to reduce the common catheter complications of thrombosis, occlusion and infection that limit the catheter function and contribute to patient morbidity and mortality.
1.2. Catheter technologies and unresolved problems
Despite improvements in polyurethane catheter materials, current PICC and midline catheters made of thermoplastic polyurethane continue to have complications contributing to catheter failure within a range of 1–30% [Citation20]. Studies comparing traditional PICCs with anti-microbial and anti-thrombotic catheter materials demonstrated no association for reductions of catheter-related infection or with reduced incidence of thrombosis and occlusion [Citation20]. Significant and serious complications with PICC and midline catheters impact the patient’s health, the ability of clinical staff to manage and the healthcare system’s cost with delayed or added treatment and extended hospital stays. Improvements in catheter materials are necessary to mitigate complications, and protect the patient, saving time and cost for the healthcare staff and facility.
The aim of this review is to provide a synopsis and clinical profile of a new type of catheter material, the current evidence, and the suggested benefits of clinical use with application in PICCs and midline catheters.
2. Introduction to HBM
A novel hydrogel composite material PICC and midline catheter was created, comprised of a porous poly-vinyl alcohol (PVA) integrated with poly-acrylic acid (PAA), heat treated to establish a physically cross-linked high-strength hydrogel material. In a hydrated state, HBM establishes a hydrophilic steric barrier surface to repel protein adsorption [Citation11,Citation21]. This new type of catheter material was developed into HydroPICC™ and HydroMID™ (Access Vascular, Inc. Billerica, MA, U.S.A.) with indications for intravenous access with short or long-term peripheral and central venous access for the administration of solutions, medications, parenteral nutrition, laboratory blood sampling, power injection of contrast media, and central venous pressure monitoring [Citation22]. The HydroPICC and HydroMID catheters consist of an HBM shaft combined with a luer-locking hub, resulting catheter surface designed to reduce the accumulation of blood and bacterial cells. The radiopaque polyol catheters have a polyurethane extension tube and suture wing without radiopaque additive. These unique catheters combine the mechanical properties and advantages of polyurethane with the antithrombotic features of hydrogels into a single catheter material [Citation22].
2.1. How the HBM catheter performs
In the clinical setting, the hydrophilic nature of the composite material establishes a saturated surface that is composed of 35% water with a negligible contact angle [Citation11,Citation21]. The super lubricious, slippery surface makes the catheter easier to insert, with recent data indicating up to 9X less force when inserting a hydrophilic catheter [Citation12]. These hydrophilic properties are created from the hydrogel component’s high affinity for water, allowing the catheter to absorb and maintain surface moisture and prevent bacterial and blood adherence [Citation23]. The HBM features are attributed to the three-dimensional cross-linking network structure of synthetic and/or natural hydrophilic polymers and the composite mixture of PVA cross-linked with hydrophilic heat-treated chains that provide durability and added strength.
The slippery, hydrated surface of the catheter allows it to mimic the body’s natural chemistry and avoid the common foreign body response of cellular attachment and fibrin formation designed to engulf and isolate the foreign material. By avoiding this normal body reaction, this catheter is engineered to bypass those catheter complications associated with this foreign body response. Most catheters with coatings have only short-term reductions, but the HBM catheter lumen is entirely composed of the hydrogel composite, which allows it to continue to perform in the same non-reactive manner for months. The durability and surface features are long-lasting, with testing of surface functionality for over 162 days [Citation11]. The smooth and hydrated surface limits the body’s host response and resists cellular adherence for the long term [Citation11,Citation15,Citation24].
The hydrophilic properties of the material provide the main value to the patient and the catheter long life of thromboresistance. Catheter material research supports that the PVA/PAA hydrogel composite effectively resists platelet adhesion by 97%, validated through blood loop testing in comparison to thermoplastic polyurethane catheters (PowerPICCTM, Becton Dickinson, Franklin Lakes, NJ, U.S.A.). The HydroPICC has been evaluated using in vitro and in vivo model testing, demonstrating the reduction in thrombus blood cellular accumulation. Pre-clinical in vitro and in vivo evaluations, while predictive of performance outcomes, cannot verify clinical performance with respect to thrombus formation. HydroPICC and HydroMID catheters exhibit enhanced resistance to blood components (platelet and thrombus) accumulation with reduced cellular adherence [Citation11]. With reduced platelet adhesion to the catheter surface, catheter-related occlusion would be reduced or eliminated.
In 1994, Maki noted that central venous catheter bloodstream infections, at rates of 3–5%, posed a greater risk to patients than any other indwelling medical device [Citation25]. Cellular adherence to all intravenous catheters begins immediately upon insertion with a coating of platelets, plasma and tissue proteins [Citation26], trapping any bacteria that originate from the skin insertion site, the hub, or from touch contamination of healthcare providers inserting or performing catheter management [Citation27,Citation28]. Biofilm-producing bacteria have previously been confirmed on the surfaces of all central venous catheters associated with this cellular adherence [Citation28]. Catheters with hydrogel and hydrophilic materials reduce cellular adherence and attachment of bacteria. Through its ability to discourage biofilm formation, HBM catheters mitigate the risk of life-threatening complications in patients who require treatment via intravenous access.
The research by Maikranz and associates delves into the subject of bacterial attachment in relation to hydrophobic and hydrophilic catheter surfaces [Citation29]. Hydrophilic catheter surfaces tend to have low adhesion forces resulting in few bacterial molecules attaching, approximately four times lower than with hydrophobic surfaces, like those present on traditional polyurethane and silicone catheters. It is hypothesized that the smooth, negatively charged and well-hydrated surface of the hydrophilic hydrogel catheter further minimizes the adhesion of bacterial cells [Citation6,Citation11,Citation30–32]. Mehall and associates in vivo research found that bacteria bind to cellular formation and fibrin, and without a conditioning layer of blood cells and fibrin on the surface of a catheter, the opportunity for bacterial attachment and biofilm growth was greatly reduced [Citation33]. The performance of this hydrogel composite material creates a potential for this catheter to significantly reduce complications and replacement of traditional polyurethane and silicone PICC and midline catheters.
2.2. Cost effectiveness
Catheter complications and failure contribute significantly to the higher cost of care in hospitals. With estimates in the billions, catheter complications interrupt treatment, often require catheter replacement due to failure, and contribute to patient morbidity and mortality [Citation34]. Results compiled within an integrative review benchmarked the incidence of catheter complications for PICC thrombosis, deep vein thrombosis, thrombotic occlusion, and infection with a pooled total complication rate of 5.6% [Citation13]. The breakdown of cost per complication based on the Centers for Medicare and Medicaid Services (CMS) using 2022 ICD-10 codes per incident is $17,000 per PICC-related thrombosis, $26,000 per PICC-related deep vein thrombosis, and $1,100,000 for lost revenue related to central line-associated bloodstream infections (CLABSI). The cost projections for all complications at this incidence rate were $4.5 billion U.S.A. per annum for PICCs alone and $4.9 billion projected for both PICCs and midlines. Given even a conservative percentage reduction, this novel catheter material would reap significant savings for hospitals able to avoid complications. Greater confidence achieved with lower infection rates and catheter failures would allow PICC and midline catheters to be used without fear of catheter-related bloodstream infection or associated reimbursement-related concerns.
2.3. Evidence
A systematic review of studies and gray publications from 1980–2023 was performed with keywords of hydrogel and hydrophilic catheter materials, hydrophobic, biomaterial, HydroPICC/HydroMID, within MEDLINE/PubMed, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Scopus, EmBASE, and Cochrane review online sources [Citation13]. A total of 28 publications were selected, with one clinical study reporting outcomes of the HydroMID. Unpublished studies with in vitro laboratory and clinical results were included [Citation34–36].
The literature review identified publications outlining the properties of hydrophilic coatings, catheters, and hydrogel characteristics [Citation13]. Five identified publications were focused on hydrogel hydrophilic biomaterial (HBM) composite catheters () [Citation12,Citation22,Citation35,Citation37,Citation38]. Clinical publications evaluated HBM in comparison with thermoplastic polyurethane catheters for complications or specific occlusion rates [Citation35,Citation37]. The first study of 205 subjects with midline catheters reported one complication in the HBM group and 24 in the thermoplastic polyurethane group, a 6X reduction in complications [Citation33]. The second 2023 retrospective study of PICCs evaluated 121 subjects with an equal distributive comparison of HBM and thermoplastic catheters [Citation36]. There were no occlusions in the HBM group and 13 in the thermoplastic group. With the occlusion incidence of 7–35% reported in the literature, the HBM performance is atypical and warrants further study [Citation38–40].
Bacterial adherence leading to infection is the most serious complication facing PICC and midline catheters in the clinical setting. A catheter material that resists bacterial attachment is beneficial in two ways: avoidance of the initial attachment leading to colonization and reduction of the development of biofilm produced by the bacteria after attachment. Microorganisms, and their by-products of biofilm, are the main cause of catheter-associated bloodstream infections. Biofilm growth on a catheter allows the bacteria to gain strength and virulence. Once biofilm-enabled bacterial colonies are released into the bloodstream, an infection develops, and higher dosages of antibiotics are needed to control the spread. The lubricity and anti-fouling features of the hydrogel catheter are inherently anti-microbial and, thus, biofilm-limiting [Citation41–43]. This novel catheter material with a surface hydration layer serves as protection against bacterial attachment and biofilm production.
Catheter surface modifications with hydrophilic hydrogel provide positive features that reduce catheter colonization and infectious complications [Citation44–47]. Two clinical studies have demonstrated outcomes for the HBM catheters [Citation35,Citation37]. The first retrospective study compared 4 French single-lumen PICCs, 60 HBM catheters and 61 thermoplastic polyurethane catheters [Citation35]. The results were measured for occlusion incidence. No occlusions were detected in the HBM group and 13 in the thermoplastic polyurethane group for a probability value of p < 0.0001 for total occlusions. In the second retrospective study, 4 French single-lumen midline catheters were evaluated [Citation37]. The study included 205 subjects with midline catheters, 104 in the HBM group and 101 in the thermoplastic polyurethane group. There was one occlusive event and four replacements in the HBM group and 17 occlusions and 22 replacements in the polyurethane group. Replacement reporting reflected catheter failure from any cause for 4.5% in the HBM group and 28.1% in the polyurethane group. Thrombotic complications with upper-extremity thrombosis were none in the HBM group and 7 in the polyurethane group. Therapy was completed in 98% of the HBM group and 69% of the polyurethane group. Each of these studies, while low-level evidence, supports the hydrogel hydrophilic catheters and this HBM PICC and midline catheter advantages of super-lubricious surfaces promoting thromboresistance, vessel protection and prevention of catheter occlusion. More robust, high-quality research is needed to validate the clinical outcomes and results of these HBM catheters. Small sample quasi-experimental studies without controls are not conclusive but rather suggestive, serving as pilot studies to guide future research of larger scale, prospective trials with randomization and controls.
2.4. Alternative devices
Silicone catheter materials have been available for central venous catheters since the 1960s, polyurethane since 1975 and surface modifications for PICCs and midlines since the 1980s. Medical-grade polyurethane and silicone catheters include differing chemical compositions with aliphatic polyether, aromatic polyether, aliphatic polycarbonate, and polydimethylsioxane [Citation6]. The hydrophobicity of silastic silicones and thermoplastic polyurethanes causes higher levels of adsorbing proteins with resultant thrombosis formation leading to catheter complications and functional device failure [Citation41]. The hydrophobic surface of polyurethane catheters suggests that they are more susceptible to catheter-related infections and thrombogenicity than other silicone-based catheters, thus emphasizing the need for new materials with antibacterial and antithrombotic features [Citation48,Citation49].
Surface modifications of antiseptics, antibiotics, anticoagulants, and anti-thrombogenic substances have had limited results [Citation20]. PICCs with modified surfaces and composition are antiseptic catheters of chlorhexidine silver sulfadiazine, antibiotic catheters with minocycline-rifampin, anticoagulant heparin coatings, and anti-thrombogenic catheters of fluoro-oligomer modified polyurethane. The weight of the clinical human evidence supporting the impact of outcome improvement of these surface compositions on complication reduction is minimal.
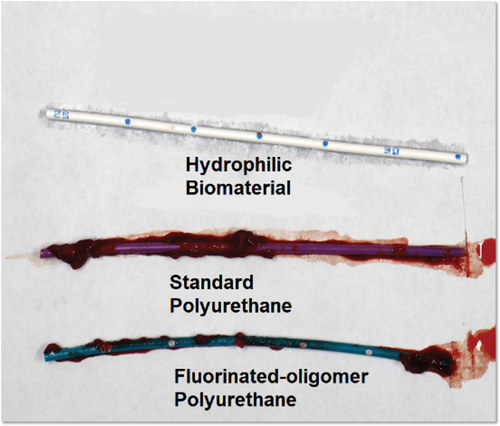
Anti-thrombogenic hydrophobic PICCs and hydrophilic HBM catheters were compared to the control of the thermoplastic polyurethane PICC in a study using in-vitro loop testing [Citation11]. Thrombotic accumulation on the HBM catheter’s inner and outer surface was reduced by 97%, and in the antithrombotic hydrophobic catheter by 64%, in comparison to the thermoplastic polyurethane PICC. The features of hydrogel, those most likely to reduce complications, center around the hydrated nature of the material, promoting greater biocompatibility with a low-level body response to the foreign body and a 35% hydrophilic hydrated slippery catheter surface. In comparison, thermoplastic polyurethane catheters are hydrophobic, with 2% hydrated surface repelling water and a higher cellular attachment rate than hydrogel catheters making them less favorable, in comparison to the HBM composite material catheters.
Cellular and protein adsorption conditioning of catheter surfaces is a normal response to the insertion of a foreign material into the body [Citation34]. The host produces a cellular and thrombotic response designed to attach to, engulf and destroy the invading agent. A qualitatively large amount of thrombus was observed on the tip of every conventional thermoplastic polyurethane catheter device, while only a minimal amount of thrombus accumulation was observed on the anti-thrombogenic catheter and the HBM composite hydrogel tips () [Citation11]. PICC and midline complications of infection, thrombosis and occlusion are related to the buildup of blood cells, fibrin and bacteria adhering to the surface of the catheter. Hydrogel biocompatible catheters minimize or eliminate the foreign body cellular response by preventing the functions of attachment and cellular engulfment, such as with fibrin sleeves and cellular chemicals designed to dissolve the catheter. Thermoplastic polyurethane catheters can resist the chemical response but cannot resist the protein and cellular attachment and engulfment that leads to catheter complications.
Figure 2. Representative images from in vitro blood loop model studies demonstrating differences in bacterial adhesion on HBM vs. polyurethane catheters. Presented at vascular access conference in 2023. Used with permission from Access Vascular, Inc.
Catheter materials that resist or repel bacteria and blood adherence have beneficial properties that, when applied to medical applications of Foley catheters, guidewire coatings, and endovascular devices, result in complication avoidance and longer catheter function [Citation44,Citation50]. Foley catheter hydrogel coatings promote smoother insertions and enhance patient comfort [Citation50,Citation51]. When considering the ideal characteristics of a PICC or midline catheter material, the HBM catheters display a low-level host response, have low friction and surface resistance, and are able to maintain the highly lubricious hydrated surface that reduces the normal cellular buildup on catheters (). The highly lubricious surface of the hydrogel catheter is a strong feature making the catheter low impact on the vessel during insertion and removal. The slippery nature of the catheter promotes a smooth and atraumatic insertion [Citation12]. These smooth surfaces are likely kinder to vessels upon insertion, with reduced injury/inflammation to the vein walls. Unlike catheters with hydrogel or hydrophilic coatings, the composite material features and lubricity demonstrated durability in-vitro for over 162 days [Citation11]. The low insertion force needed for trackability results in minimal friction and vessel irritation. Compared to thermoplastic polyurethane, the slippery high-strength HBM exhibited an 84% ± 25% reduction in average insertion force. All these features work together to provide ease of insertion without vessel irritation, biocompatible dwell, and slippery hydrophilic surfaces that continue to repel cellular attachment and minimize or prevent complications [Citation52].
2.5. Regulatory and contraindications
compares the features of the two currently available HBM catheters. US FDA-cleared HydroPICC and HydroMid are available according to these specifications () [Citation22,Citation36]:
Figure 4. Hydrophilic biomaterial used in the HydroPICC and HydroMID. Used with permission of Access Vascular, Inc.
Table 2. Comparison of available HBM catheters.
Device Description: The HydroPICC peripherally inserted central catheters (PICC) are 4 French and 5 French, single and dual lumen catheters comprised of a radiopaque hydrophilic catheter material with a suture wing, Luer lock hubs, and extension tubes made from materials commonly used in the manufacture of catheters. Catheters are provided packaged in kit configurations with the appropriate accessories for placement in the appropriate clinical environments. The maximum power injection flow rate for each lumen is indicated on each extension tube clamp.
The HydroPICC is indicated for intravenous access with short or long-term peripheral and central venous access for the administration of solutions, medications, parenteral nutrition, laboratory blood sampling, power injection of contrast media (maximum power injection flow rate is 3.5 mL/sec), and for central venous pressure monitoring. The HydroPICC Catheter magnetic resonance (MR) rating for non-clinical testing was conditional such that patients may be safely scanned in an MR system with a static magnetic field of 3.0 Tesla or less and a maximum spatial field gradient of 3,000 Gauss/cm or less with whole-body specific absorption rate averaging 4.0 W/kg for scan of 15 minutes.
Trade Name: HydroPICC
Manufacturer: Access Vascular, Inc.
510(k) Reference: K193015
Common Name: Intravascular Catheter
Regulation Number: 21CFR§880.5970
Regulation Name: Percutaneous, Implanted, Long-Term Intravascular Catheter Regulatory Class: Class II
Trade/Device Name: HydroPICC 5F Dual Lumen Catheter
Manufacturer: Access Vascular, Inc.
510(k) Reference K213550
Regulation Number: 21 CFR 880.5970
Regulation Name: Percutaneous, Implanted, Long-Term Intravascular Catheter Regulatory Class: Class II
Device Description: The HydroMID is a radiopaque, hydrophilic midline catheter used for short-term (< 30 days) intravenous access as a peripheral venous access device. The HydroMID is available with a suture wing and Luer lock hub in a single 4F outer diameter lumen, 20 cm in length. Placement of midline catheters may be in any peripheral vein, most commonly in the upper arm veins of the basilic, brachial, or cephalic. The terminal tip of the catheter is at the level of the axillary line for upper arm placement. The longer midline catheter provides an alternative to short peripheral IVs and avoids the use of central PICCs when no indication for central catheterization is present. Maximum power injection for the HydroMID is at a flow rate of 6mL/sec. Indications for the HydroMID midline catheter are for the intravenous infusion of solutions and medications considered isotonic and not irritating (pH between 5 and 9; osmolarity less than 600mOsm/L). Continuous vesicant infusions or parenteral nutrition hyperosmolar solutions are not appropriate for a midline catheter.
HydroMID catheter has demonstrated the same reduction of thrombus accumulation as specified in the device description for the HydroPICC.
Trade Name: HydroMID
Common Name: Intravascular Catheter
Regulation Number: 21CFR§880.5200
Regulation Name: Catheter, Intravascular, Therapeutic, Short-Term Less Than 30 days Regulatory Class: Class II
Both the HydroPICC and HydroMID catheters have demonstrated a reduction in catheter thrombus accumulation with in vitro and in vivo models, however, pre-clinical evaluations are not necessarily predictive of actual clinical performance.
No contraindications were listed for either device.
2.6. Catheter material conclusion
In conclusion, both polyurethane and hydrophilic hydrogel catheter materials have their own unique properties and advantages that make them suitable for use in the production of intravenous catheters. Both types of catheter materials are considered biocompatible, making them safe for long-term use. Polyurethane catheters are known for their durability and resistance to kinking, while hydrophilic hydrogel catheter materials are known for their ability to reduce friction and the risk of thrombosis. Composite hydrophilic hydrogel catheter materials are lubricious, less likely to cause irritation or damage, and more resistant to protein adsorption that leads to thrombotic complications and catheter occlusion. Performance demonstrated within pre-clinical in vitro and in vivo studies of thrombus formation evaluation may not reflect actual clinical performance [Citation22].
3. Expert opinion
This expert report investigates a catheter material type as a potential solution for reducing intravascular catheter complications. As stated by Ullman et al. in an expert commentary on anti-thrombogenic PICCs, ‘With around 30% of PICCs developing serious complications, there is little doubt that improvements in technologies are needed … ’ [Citation20]. Hydrogel and hydrophilic intravascular catheter characteristics have the potential for significant improvement and reduction in complications. This researcher suggests that the level of biocompatibility, protein adsorption resistance and reduced bacterial attachment positively impact the reduction of thrombotic-related complications common to intravenous catheters and specific to PICCs and midlines. With complications of thermoplastic polyurethane PICC and midline catheters reported at rates up to 33%, the impact of any level of reduction is beneficial to patients.
PICCs are the most common central venous catheters used with patients to establish a reliable form of intravenous access for the delivery of medical treatment. With more than 3 million PICCs purchased each year, any level of risk is significant. PICC and midline catheters, while reliable forms of access, do have frequent complications, including occlusion, thrombosis and infection, each related to cellular adherence to the catheter material. Biofouling or anti-biofouling are terms that apply to the body’s foreign material response with surface contamination, conditioning and adherence of cells. Thrombotic formation on the surface of a catheter is responsible for reduced catheter function through partial and complete catheter occlusion. Catheter occlusion is a frequent occurrence that hinders a patient’s ability to receive treatment, requires additional staff time for management, and escalates costs due to the necessity for thrombolytics to dissolve clots [Citation13,Citation39]. Hydrophilic catheter coatings and composites have been developed to mitigate these thrombotic complications, reduce adherence of blood and bacterial cells to catheters and provide greater patient safety in using these devices.
A catheter material that reduces catheter occlusion not only enhances patient outcomes but has the potential to revolutionize the catheter product market, prompting customers to expect similar advancements from all catheter manufacturers. Barriers to the uptake and use of newer catheters exist with customers familiar with and committed to one catheter and brand. PICCs and midlines are typically inserted by specially trained clinicians, with nurses being the primary users. In many cases, nursing vascular access teams have traditionally utilized the same catheter brand for an extended period. These experienced nurses may exhibit resistance to change, often pointing to discrepancies in catheter kits, components, and minor insertion variations as reasons for their hesitance.
Moreover, the adoption of newer materials and catheters introduces additional costs that can deter both users and healthcare facilities from embracing these innovations. Nevertheless, it’s essential to recognize that the upfront increase in cost is offset by the long-term benefits of reduced complications, extended catheter dwell times, and a reduction in the frequency of failed catheters necessitating replacement. This results in overall cost savings, making the transition to new catheter technology a wise investment in the long run. The clinical significance of hydrogel hydrophilic catheters extends to the realm of healthcare by significantly diminishing complications and lowering costs. This reduction in complications related to PICC and midline catheters has wide-reaching effects, benefiting both patients and medical staff, as it leads to decreased risks and less time spent by healthcare professionals in managing catheter-related issues.
The real-world impact and advantages of hydrogel materials for clinical applications are readily discernible through their exceptional lubricity, biocompatibility, and anti-biofouling properties. The smooth and hydrophilic surfaces of catheters made from hydrogel composite materials offer the potential for enhanced comfort for patients, reducing friction during insertion and removal procedures.
Additionally, reduced blood cell attachment has implications for infection prevention; bacteria that produce biofilm have reduced protection and fewer sources of nutrition when blood cells are not available. Hydrogel materials exhibit remarkable biocompatibility, effectively mitigating cellular adhesion and inhibiting biofilm formation on surfaces. Their highly slippery and hydrated surfaces actively deter bacterial attachment, causing bacterial cells to remain suspended in the bloodstream, rendering them more vulnerable to the body’s natural immune response and preempting bacterial biofilm production. When bacteria cannot adhere, biofilm formation is prevented.
A catheter constructed from hydrophilic biomaterial, such as a hydrogel composite, will significantly decrease thrombotic complications in PICC and midline catheters, thereby enhancing catheter performance and improving patient outcomes. The ultimate outcome is a reduced risk of various thrombotic and infectious complications associated with PICC and midline catheters when employing hydrophilic hydrogel catheter materials.
Drawing from the substantial body of research showcasing the favorable outcomes associated with hydrophilic hydrogel catheters and the evolving clinical evidence surrounding HBM catheters, the future appears promising in terms of reducing complications for patients requiring PICC or midline catheters. As more research findings come to light, the next five to ten years hold great potential for substantial advancements in traditional polyurethane catheters. This progress aligns with the vision set forth by the Alliance for Vascular Access Teaching and Research (AVATAR) of ‘Making Catheter Complications History.’ The long-standing aspiration of eliminating complications can potentially become a reality through the inherent benefits of the hydrated surfaces offered by hydrophilic hydrogel catheters.
The future of intravenous catheter research aimed at complication reduction hinges on a thorough exploration of material science and its clinical impact, particularly in terms of the newer materials’ ability to mitigate the foreign body response. Research on catheter materials predominantly centers on in vitro studies that identify crucial material attributes and positive characteristics essential for promoting biocompatibility. The ultimate goal of catheter material research is to illustrate the tangible outcomes of complication prevention, thereby establishing a direct correlation between material advancements and clinical results. To achieve this, high-level in vivo studies become imperative, serving to validate the in vitro findings and measure their real-world impact on patient populations.
This author concluded that well-hydrated, biocompatible catheters with anti-biofouling anti-bacterial properties known to be beneficial in medical applications are better for patients. These catheters play a pivotal role in reducing the foreign body response by preventing cellular attachment, thereby enhancing patient well-being. It is noteworthy that while the literature generally reports a 2% reduction in infections with the use of antimicrobial catheters, the potential for a remarkable 50% or greater reduction in complications with HBM hydrogel catheters holds great promise. The resultant cost savings from reduced complications are expected to more than compensate for any increase in catheter costs, thus incentivizing their widespread adoption. The overarching goal to ‘Make Complications History’ is a shared aspiration where all stakeholders, including patients, stand to benefit, with an unwavering commitment to prioritizing patient safety and delivering high-quality healthcare. While additional clinical research is required to substantiate these findings and draw more robust conclusions regarding the performance outcomes associated with this material, the hydrogel hydrophilic HBM catheter exhibits significant potential.
4. Limitations
This is an expert opinion review of catheter materials with a specific evaluation of hydrogel material improvements as they apply to PICC and Midline catheters. This focus of intravenous catheter materials on a novel hydrophilic biomaterial was limited to available in vitro and in vivo research without the benefit of higher-grade studies or randomized controlled trials. The quality of the research was a primary limitation for conclusions. While the information was suggestive of improvement and the potential impact on patient outcomes, these findings require continued investigation.
5. Plain summary
With over 80% of patients admitted to acute care necessitating intravenous access, PICC and Midline catheters become indispensable for therapies extending beyond a few days. The spectrum of complications linked to catheter failure can range from 30–69%. In contrast, hydrogel hydrophilic catheter materials exhibit exceptional biocompatibility, effectively thwarting cellular and bacterial adherence and subsequent formation of biofilms. These slippery, hydrophilic catheter surfaces not only facilitate smoother and potentially more comfortable insertions for patients but also reduce the risk of vessel injury and thrombotic complications, enhancing catheter performance, and reducing interruptions in therapy while ensuring a more seamless patient experience. By preventing complications associated with PICC and midline catheters, patient risk is diminished, and substantial cost savings may be realized across all healthcare facilities.
Article highlights
Complications associated with PICC and midline catheters occur frequently.
Hydrogel materials are highly biocompatible and have been effective in reducing thrombus accumulation, preventing cellular adherence and the formation of biofilms on surfaces.
Hydrophilic super-hydrated catheter surfaces make insertions smoother and potentially more comfortable for the patient and may reduce the risk of vessel injury and inflammation.
Reducing cellular adherence and thrombotic catheter complications leading to occlusions in PICC and midline catheters would represent significant cost savings for all healthcare facilities by promoting longer catheter function without complications.
Declaration of interest
NM is employed by PICC Excellence, Inc., an education service provider with consulting, research and speaker bureau support provided to Access Vascular, 3M, Accuvein, Bedal, Chiesi U.S.A., General Electric Healthcare Technologies, Helmier, Javelin Health, Linear Health Sciences, Nexus Medical, Parker Laboratories, Prytime Medical, and Teleflex.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The author wishes to extend special thanks to Stephen Babcock and Aisha Cobbs for their editorial assistance.
Additional information
Funding
References
- iData Research. US Market Report Suite for Vascular Access Devices. 2023. Available from: https://idataresearch.com/product/vascular-access-devices-market-united-states/
- Chopra V, Anand S, Krein SL, et al. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012 Aug 1;125(8):733–741. doi: 10.1016/j.amjmed.2012.04.010
- Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015 May 1;38(3):189–203.
- Liem TK, Yanit KE, Moseley SE, et al. Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg. 2012 Mar 1;55(3):761–767.
- Swaminathan L, Flanders S, Horowitz J, et al. Safety and outcomes of midline catheters vs peripherally inserted central catheters for patients with short-term indications: a multicenter study. JAMA Intern Med. 2022 Jan 1;182(1):50–58. doi: 10.1001/jamainternmed.2021.6844
- Di Fiore A. Clinical and engineering considerations for the design of indwelling vascular access devices: materials and product development overview. JAVA. 2005;10(1):24–27. doi: 10.2309/java.10-1-2
- Ullman AJ, August D, Kleidon T, et al. Peripherally inserted central catheter iNnovation to reduce infections and clots (the PICNIC trial): a randomised controlled trial protocol. BMJ Open. 2021 Apr 1;11(4):e042475. doi: 10.1136/bmjopen-2020-042475
- Schults JA, Kleidon T, Petsky HL, et al. Peripherally inserted central catheter design and material for reducing catheter failure and complications. Cochrane Database Syst Rev. 2019 Jul;2019(7). Art. No.: CD013366. doi: 10.1002/14651858.CD013366
- Slaughter E, Kynoch K, Brodribb M, et al. Evaluating the impact of central venous catheter materials and design on thrombosis: a systematic review and meta‐analysis. Worldviews Evid Based Nurs. 2020 Oct;17(5):376–384. doi: 10.1111/wvn.12472
- Aswathy SH, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020 Apr 1;6(4):e03719.
- Mannarino MM, Bassett M, Donahue DT, et al. Novel high-strength thromboresistant poly (vinyl alcohol)-based hydrogel for vascular access applications. J Biomater Sci Polym Ed. 2020 Mar 23;31(5):601–621. doi: 10.1080/09205063.2019.1706148
- LeRoy KJ, Donahue DT. Trackability of a high-strength thromboresistant hydrogel catheter: an in vitro analysis comparing venous catheter forces in a simulated use pathway. J Mech Behav Biomed Mater. 2023 Mar 1;139:105670. doi: 10.1016/j.jmbbm.2023.105670
- Moureau NL. Integrative review: complications of Peripherally Inserted Central Catheters (PICC) and midline catheters with economic analysis of potential impact of hydrophilic catheter material. Int J Nurs Health Care Res. 2022;5(10):17.
- Tripathi S, Kumar S, Kaushik S. The practice and complications of midline catheters: a systematic review. Crit Care Med. 2021;49(2):e140–e150. doi: 10.1097/CCM.0000000000004764
- Greenhalgh R, Dempsey-Hibbert NC, Whitehead KA. Antimicrobial strategies to reduce polymer biomaterial infections and their economic implications and considerations. Int Biodeterior Biodegrad. 2019;136:1–14. doi: 10.1016/j.ibiod.2018.10.005
- Donlan RM. Biofilms and device-associated infections. Emerging infectious diseases. Emerg Infect Dis. 2001 Mar;7(2):277. doi: 10.3201/eid0702.010226
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbio Rev. 2002 Apr;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002
- Morris NS, Stickler DJ, Winters C. Which indwelling urethral catheters resist encrustation by proteus mirabilis biofilms? Br J Urol. 1997;80(1):58–63. doi: 10.1046/j.1464-410X.1997.00185.x
- Stickler DJ, King J, Nettleton J, et al. The structure of urinary catheter encrusting bacterial biofilms. Cells Mat. 1993;3:315–319.
- Ullman AJ, Bulmer AC, Dargaville TR, et al. Antithrombogenic peripherally inserted central catheters: overview of efficacy and safety. Expert Rev Med Devices. 2019 Jan 2;16(1):25–33.
- Correa S, Grosskopf AK, Lopez Hernandez H, et al. Translational applications of hydrogels. Chem Rev. 2021 May 3;121(18):11385–11457. doi: 10.1021/acs.chemrev.0c01177
- Data on file at Access Vascular. IFU for HydroPICC, HydroPICC Dual Lumen, and HydroMID catheters, Access Vascular Inc. Available from: http://www.accessvascularinc.com/avi-story-and-file:///E:/Manufacturers/Access%20Vascular/Hydropicc%20Care%20Team%20Guidance.pdf
- Xu H, Huang Y, Jiao W, et al. Hydrogel-coated ventricular catheters for high-risk patients receiving ventricular peritoneum shunt. Medicine. 2016;95(29):e4252. doi: 10.1097/MD.0000000000004252
- Mitra D, Kang ET, Neoh KG. Polymer-based coatings with integrated antifouling and bactericidal properties for targeted biomedical applications. ACS Appl Polym Mater. 2021 Apr 20;3(5):2233–2263.
- Maki DG. Infections caused by intravascular devices used for infusion therapy: pathogenesis, prevention, and management, p 155–212. Infections associated with indwelling medical devices. 2nd ed. Washington (DC): ASM Press; 1994.
- Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893–898. doi: 10.1016/S0140-6736(97)10006-X
- Sousa C, Henriques M, Oliveira R. Mini-review: antimicrobial central venous catheters–recent advances and strategies. Biofouling. 2011 Jun 22;27(6):609–620.
- Raad I, Costerton W, Sabharwal U, et al. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993;168(2):400–407. doi: 10.1093/infdis/168.2.400
- Maikranz E, Spengler C, Thewes N, et al. Different binding mechanisms of staphylococcus aureus to hydrophobic and hydrophilic surfaces. Nanoscale. 2020;12(37):19267–19275. doi: 10.1039/D0NR03134H
- Tebbs SE, Sawyer A, Elliott TS. Influence of surface morphology on in vitro bacterial adherence to central venous catheters. Br J Anaesth. 1994;72(5):587–591. doi: 10.1093/bja/72.5.587
- Kohren W, Jansen B. Polymer materials for the prevention of catheter-related infection. Zentralblatt für Bakteriologie. 1995;283(2):175–186. doi: 10.1016/S0934-8840(11)80199-4
- Gatter N, Kohnen W, Jansen B. In vitro efficacy of a hydrophilic central venous catheter loaded with silver to prevent microbial colonization. Zentralbl Bakteriol. 1998;287(1–2):157–169. doi: 10.1016/S0934-8840(98)80162-X
- Mehall JR, Saltzman DA, Jackson RJ, et al. Catheter materials affect the incidence of late blood-borne catheter infection. Surg Infect. 2001;2(3):225–230. doi: 10.1089/109629601317202704
- Ryder M. The role of biofilm in vascular catheter-related infections. N Dev Vasc Dis. 2001;2:15–25.
- Bunch J. A retrospective assessment of midline catheter failures focusing on catheter composition. J Infus Nurs. 2022 Sep 1;45(5):270–278. Available from: https://www.accessvascularinc.com/news/new-access-vascular-retrospective-data-review-demonstrates-midline-catheters-composed-of-advanced-biomaterials-may-improve-dwell-times
- Data on file. AVI data for reduction of thrombus accumulation was evaluated using in vitro and in vivo models. Pre-clinical in vitro/in vivo evaluations do not necessarily predict clinical performance with respect to thrombus formation.
- Bunch J, Hanley B, Donahue D. A retrospective, comparative, clinical study of occlusion rate of peripherally inserted central catheters fabricated of poly(vinyl alcohol)-based hydrogel composite. J Mater Sci Mater Med. 2023;34(7):34. doi: 10.1007/s10856-023-06736-0
- Hawthorn A, Bulmer AC, Mosawy S, et al. Implications for maintaining vascular access device patency and performance: application of science to practice. J Vasc Access. 2019 Sep;20(5):461–470. doi: 10.1177/1129729818820200
- Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: the 3P-O study. J Vasc Interv Radiol. 2017;28(5):749–756.e2. doi: 10.1016/j.jvir.2017.02.005
- Johnston AJ, Streater CT, Noorani R, et al. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates-the ‘ELeCtriC’study. J Vasc Access. 2012 Oct;13(4):421–425. doi: 10.5301/jva.5000071
- Ngo Brian Khai D, Grunlan MA. Protein resistant polymeric biomaterials. ACS Macro Lett. 2017 Sep 19;6(9):992–1000. Epub 2017 Aug 29. PMID: 35650885. doi: 10.1021/acsmacrolett.7b00448
- Hoffman AS. Non-fouling surface technologies. J Biomater Sci Polym Ed. 1999 Jan 1;10(10):1011–1014.
- Ostuni E, Grzybowski BA, Mrksich M, et al. Adsorption of proteins to hydrophobic sites on mixed self-assembled monolayers. Langmuir. 2003 Mar 4;19(5):1861–1872. doi: 10.1021/la020649c
- Liu L, Shi H, Yu H, et al. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater Sci. 2020;8(15):4095–4108. doi: 10.1039/D0BM00659A
- Lai NM, Chaiyakunapruk N, Lai NA, et al. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults, edited by Cochrane emergency and critical care group. Cochrane Database Syst Rev. 2018;(12):CD007878. doi: 10.1002/14651858.CD007878.pub3
- Casimero C, Ruddock T, Hegarty C, et al. Minimising bloodstream infection: developing new materials for intravascular catheters. Medicines. 2020 Aug 26;7(9):49.
- Zhang F, Hu C, Yang L, et al. A conformally adapted all-in-one hydrogel coating: towards robust hemocompatibility and bactericidal activity. J Mat Chem B. 2021;9(11):2697–2708. doi: 10.1039/D1TB00021G
- Braun U, Lorenz E, Weimann C, et al. Mechanic and surface properties of central-venous port catheters after removal: a comparison of polyurethane and silicon rubber materials. J Mech Behav Biomed Mater. 2016;64:281–291. doi: 10.1016/j.jmbbm.2016.08.002
- Gharibi R, Agarwal S. Polyurethanes from hydrophobic elastic materials to hydrogels with potent nonleaching biocidal and antibiofilm activity. ACS Appl Polym Mater. 2021 Aug 30;3(9):4695–4707.
- Niemczyk A, El Fray M, Franklin SE. Friction behaviour of hydrophilic lubricious coatings for medical device applications. Tribol Int. 2015;89:54–61. doi: 10.1016/j.triboint.2015.02.003
- Yang SH, Lee YSJ, Lin FH, et al. Chitosan/Poly (vinyl alcohol) blending hydrogel coating improves the surface characteristics of segmented polyurethane urethral catheters. J Biomed Mater Res B Appl Biomater. 2007;83(2):304–313. doi: 10.1002/jbm.b.30796
- Pollard D, Allen D, Irwin NJ, et al. Evaluation of an integrated amphiphilic surfactant as an alternative to traditional polyvinylpyrrolidone coatings for hydrophilic intermittent urinary catheters. Biotribology. 2022 Dec 1;32:100223. doi: 10.1016/j.biotri.2022.100223