Abstract
Background: Multi-wall carbon nanotubes (MWCNTs) are manufactured nanomaterials to which workers and the general population will be increasingly exposed in coming years. Little is known about potential human health effects of exposure to MWCNTs, but effects on the lung and the immune system have been reported in animal and mechanistic studies.
Objectives: We conducted a cross-sectional study to assess the association between occupational exposure to MWCNTs and effects on lung health and the immune system.
Methods: We assessed 51 immune markers and three pneumoproteins in serum, complete blood cell counts (CBC), fractional exhaled nitric oxide (FENO), and lung function among 22 workers of a MWCNT producing facility and 39 age- and gender-matched, unexposed controls. Measurements were repeated four months later among 16 workers also included in the first phase of the study. Regression analyses were adjusted for potentially confounding parameters age, body mass index, smoking, and sex, and we explored potential confounding by other factors in sensitivity analyses.
Results: We observed significant upward trends for immune markers C-C motif ligand 20 (p = .005), basic fibroblast growth factor (p = .05), and soluble IL-1 receptor II (p = .0004) with increasing exposure to MWCNT. These effects were replicated in the second phase of the study and were robust to sensitivity analyses. We also observed differences in FENO and several CBC parameters between exposed and non-exposed, but no difference in lung function or the pneumoproteins.
Conclusions: We observed indications of early effects of occupational exposure to MWCNTs on lung health and the immune system.
Introduction
Multi-wall carbon nanotubes (MWCNTs) are a type of manufactured nanomaterials that have many potential (industrial, medical) applications due to their unique physicochemical properties (Stark & Stoessel, Citation2015). Although production is currently generally small scale, increased production of MWCNTs is expected in the coming years which will increase exposure to both producers (workers involved in manufacture and application) and users of MWCNTs (Bekker et al., Citation2015; Kuempel et al., Citation2016).
There is considerable evidence from animal and in vitro studies that MWCNTs induce inflammation, oxidative stress, pulmonary fibrosis, mesothelioma-like effects, and cardiovascular effects (Kim et al., Citation2015; Kuempel et al., Citation2017; NIOSH, Citation2013). Data in humans relating to biological perturbations involving the lungs and immune system due to exposure to MWCNTs are available from a handful of epidemiological studies conducted in occupationally exposed populations. Lee et al. (Citation2015) reported an effect of occupational exposure to MWCNT on oxidative stress markers (hydrogen peroxide, malondialdehyde, 4-hydroxy-2-hexenal, and n-hexanal) in exhaled breath condensate in a small Korean population (n = 18) (Lee et al., Citation2015). No effects on lung function or hematology were reported. Wu et al. (Citation2014) reported a null effect of occupational exposure to carbon nanotubes (type not further specified) on fractional exhaled nitric oxide (FENO; a marker of lung inflammation) based on a cross-sectional analysis of a Taiwanese population (n = 57). In further publications based on this population, but augmented with workers that were exposed to other engineered nanomaterials (n = 364), associations were reported with worsening of allergic dermatitis, increased levels of small airway damage marker (Club Cell Secretory Protein 16; CC16) and lung function test parameters (Liao et al. Citation2014a, Citationb). Fatkhutdinova et al. conducted a small scale pilot study among 11 workers with more than 1 year exposure to MWCNT and 14 non-exposed controls (Fatkhutdinova et al., Citation2013, Citation2016; Shvedova et al., Citation2016). The authors reported significant associations between exposure to MWCNT and levels of interleukins (IL)-1β, IL-4, IL-5, IL-6, IL-8, tumor necrosis factor α (TNF-α), and Krebs von den Lungen-6 (KL-6) measured in sputum and levels of IL-1β, IL-4, IL-10, and TNF- α measured in serum (Fatkhutdinova et al., Citation2016). The same authors also reported genome-wide differential expression in messenger-RNA and non-coding RNA between workers exposed to MWCNT and non-exposed controls (Shvedova et al., Citation2016).
Although suggestive, evidence from these studies is limited due to their size and the absence of quantitative exposure assessment and the heterogeneity of the study populations in terms of the engineered nanoparticles to which subjects were exposed.
We conducted a cross-sectional study among workers occupationally exposed to MWCNTs in a MWCNT production facility and non-exposed controls. We assessed the association between quantitative measures of MWCNTs (Kuijpers et al., Citation2015) and a set of markers of lung health and early perturbations of the immune system; complete blood cell counts (CBC), 51 circulating inflammation markers (Chaturvedi et al., Citation2011; Shiels et al., Citation2013), three pneumoproteins, lung function: forced expiration volume in 1 s and forced vital capacity (FEV1, FVC), and FENO. We were able to collect repeated measurements for a subset of markers.
Methods
Study population
We conducted a cross-sectional study among 21 workers of a MWCNT producing facility and 29 age- and gender-matched, unexposed controls. Controls were selected from four different locations in the vicinity of the MWCNT producing facility among which three locations were at companies not involved in the production or use of MWCNTs (a consumer electronics store, a chemical plant, and an occupational health services company). The fourth location was a department at the MWCNT facility not involved in producing or handling of MWCNTs. The study was approved by the Commission for Medical Ethics of UZ Leuven (reference number S54607) and conducted in two phases. During phase 1 (June 2013) previously synthesized MWCNTs were bagged and incorporated into coatings, dispersions, and plastics. During the phase 2 (October 2013), in addition to these activities, MWCNTs were also actively synthesized. In phase 2, a subset of the study population (10 exposed individuals and six matched controls) were included.
Assignment of MWCNT exposure
The collection of the exposure measurements that formed the basis of the MWCNT exposure assignment in this study has been described before (Kuijpers et al., Citation2015). Briefly, breathing zone measurement of inhalable particulate matter was taken from workers in different parts of the production and research & development process across seven days. MWCNT mass was estimated by determining elemental carbon (EC) levels in the collected particulate matter. The procedure is described in Tromp et al. (Citation2017) and is summarized in the Supplementary material. Based on exposure measurements and individual task patterns, workers at the MWCNT production and laboratory facility were divided into three exposure groups: operators, lab personnel with relatively high exposure (lab high), and lab personnel with relatively low exposure (lab low). Exposure to MWCNTs was assigned using an exposure score (i.e. 1, 2, and 3) and by estimating the geometric mean (GM) MWCNT mass concentration for each exposure group using a mixed model.
Assessment of health outcomes
Questionnaires
All individuals participating in the study completed a questionnaire that was previously validated within the ELON study (Rijcken et al., Citation1996). The questionnaire was used to acquire information on general demographic information, health history, respiratory health, asthma and allergies, complaints of the circulatory system, lifestyle factors including smoking, and alcohol consumption, radiation exposure history, family medical history and work history. This baseline questionnaire was distributed once per individual included in the study during phase 1. Study participants also completed a questionnaire that was used to acquire information on smoking, alcohol consumption, health, and medication use (among other factors) in the 24 h before the biological samples were collected.
Complete blood cell counts, immune markers, and pneumoproteins
Whole blood was collected in the morning hours (before midday) by standard phlebotomy of venipuncture of forearm veins in a sitting position. CBC were determined in fresh blood at the clinical laboratory of the University Hospitals Leuven, Gasthuisberg, Belgium. Circulating blood cytokines, interleukins, and chemokines were determined by Luminex (Austin, TX) multi-analyte profiling kits according to a procedure described in Shiels et al. (Citation2013). Markers were selected to reflect several key components of inflammation, including acute-phase proteins, pro- and anti-inflammatory cytokines, chemokines, growth factors, and angiogenesis factors (Shiels et al., Citation2013) and based on their performance and reproducibility in multiplexed assays (Chaturvedi et al., Citation2011). To combine the information available from independent immune markers, we calculated two inflammation scores based on four independent markers (CRP, BCA-1/CXCL13, MDC/CCL22, and IL-RA; and CRP, SAA, CXCL9, and sTNFRII) that were reported to be significantly predictive of lung cancer risk (Shiels et al., Citation2013, Citation2015). Risk scores were calculated by summing the z-scores of the independent markers. We assessed the impact of adding white blood cell count measurements to the risk scores. Serum samples were also assayed for pneumoproteins CC16, SP-A, and SP-D using standard ELISA kits from R&D Systems (Minneapolis, MN) according to the instructions of the manufacturer and quantified using SoftMaxPro 5.4.5 ELISA analysis software (Molecular Devices, Sunnyvale, CA). All markers in blood were analyzed in duplicate and the average concentration was used for further statistical analysis. To assess technical variability in the assessment of these markers we calculated the intraclass correlation coefficient (ICC) for each marker, using duplicate split samples (ICCdup).
Lung function
Lung function was performed using the EasyOne electronic spirometer (ndd Medizintechnik, Zurich, Switzerland), which meets standards by the European Respiratory Society (ERS) and American Thoracic Society (ATS) (Miller et al., Citation2005). Tests were done in sitting positions, repeated until at least three technically correct maneuvers were obtained, and were validated by a certified lung function technician. The best value from the technically correct maneuvers was selected according to the maximum value method of the European Respiratory Society (Quanjer et al., Citation1993). Individuals for which not at least two acceptable and reproducible tests were collected were excluded from the analysis. We measured FEV1 and FVC and calculated the percentage of predicted values for these measures using European Respiratory Society equations (Quanjer et al., Citation1993).
Fractional exhaled nitric oxide (FENO)
FENO was measured in ppb using the NIOX MINO (Aerocrine, Solna, Sweden). Subjects inhaled filtered air through the monitor until reaching full lung capacity. Next the subject exhaled through the device at an approximate flow of 50 mL/s. FENO was measured with an electrochemical sensor.
Statistical analyses
To assess the volatility of markers measured in blood over time, we calculated an ICC based on marker measurements from phase 1 and phase 2 (ICCrep). Analyses of the association between markers measured in blood, FENO, and lung function parameters (FEV1, FVC, and FEV1/FVC) and exposure were conducted using multiple linear regression.
We used Tobit regression models to account for left censoring caused by values below the detection limit for all markers (including, for consistency, markers that were not left censored). We excluded markers from statistical analysis if the percentage of concentrations below the limit of detection was higher than 60%. We specified a lognormal distribution for all continuous outcome markers in the Tobit regression. We used simple multiple linear regression to model FEV1 and FVC on a linear scale. All analyses were adjusted for potentially confounding parameters age, body mass index, smoking, and sex. Analyses were conducted separately for phases 1 and 2.
We conducted categorical analysis comparing three categories of exposed workers (operators, lab high, and lab low) to non-exposed controls. In addition, we conducted analyses assessing the trend across exposure categories (assigning values of 0–3 to the exposure categories based on their exposure ranking), and conducted linear regression using the assigned actual exposure estimates for each category (Kuijpers et al. Citation2015). Analysis of the risk scores (both on a linear scale and after natural log-transformation) was conducted in phase 1 only, following the same strategy as for individual markers, but using linear regression.
We conducted a series of sensitivity analyses for phase 1 to assess the robustness of our noteworthy findings by additionally correcting univariably for information from the baseline questionnaire: alcohol use (reported by 51 subjects), doctor diagnosed cardiovascular disease (n = 3), doctor diagnosed chronic disease (n = 24), doctor diagnosed inflammatory disease (n = 17), metabolic disease (n = 2), educational level, previous exposure to chemicals (n = 16), previous exposure to nanoparticles (n = 17), previous exposure to particulates (n = 6); the latter three categories were created based on reported jobs and tasks in the workers occupational history, for information from the questionnaire covering the 24 h before blood collection: self-reported ‘recent infection’ (n = 1), and for white blood cell count (as a marker of infection), excluding laboratory workers that potentially had previous exposure as an operator (n = 2), excluding the manager of the operators (n = 1; initially categorized as operator), jack-knifing controls by location, and excluding smokers and females.
For our most robustly associated markers we conducted a Kruskal–Wallis test to assess whether measurements from operators, lab workers, and controls could have originated from the same distribution and a Wilcoxon test to assess whether measurements from operators and controls were significantly different from each other.
A p value of .05 was used as cut-off value to declare statistical significance. Because we consider this an exploratory pilot study with limited statistical power, we do not report which of our findings survived a correction for multiple testing (Benjamini & Hochberg Citation1995). All statistical analyses were conducted in R version 3.03 (R Core Team, Citation2014).
Results
We included 22 workers exposed to MWCNTs and 39 non-exposed workers (controls) (phase 1). A subset of 10 exposed workers and six controls was assessed a second time (phase 2). We provide further details on the study population in . In phase 1, we assigned GM MWCNT mass concentrations of 1, 7, and 45 EC μg/m3 to exposure groups “lab low”, “lab high”, and “operators”, respectively. We measured higher exposure levels among operators during phase 2 as a result of the primary production process which was active in phase 2, resulting in an assigned GM MWCNT mass concentration of 57 μg/m3. No individual MWCNTs (diameter <10 nm, length >1 μm) were observed in the collected inhalable particulate matter samples. In general, the particle size of the MWCNT agglomerates ranged from 200 nm to 100 μm, indicating a modal distribution with a mode diameter between 650 and 1000 nm (Kuijpers et al. Citation2015). Although the exposure groups were generally matched on gender and age, the operators were on average slightly older than the rest of the study population and the only group that was exclusively male.
Table 1. Demographic characteristics of the study population. In between brackets characteristics of the subset of individuals that also participated in phase 2.
We measured 51 immunological markers in blood collected in phases 1 and 2 of the project. Five markers (IL-3, IL-33, thymic stromal lymphopoietin, thrombopoietin, and stem cell factor) were excluded from statistical analysis because the percentage of concentrations below the limit of detection was higher than 60%. Median ICCdup of the immunological markers in phase 1 was 0.82 (IQR: 0.63, 0.93) and in phase 2 0.95 (IQR: 0.83, 0.98). Median ICCrep was 0.51 (IQR: 0.36, 0.66). The median Pearson correlation between immunological markers measured among controls in phase 1 and phase 2 was 0.62.
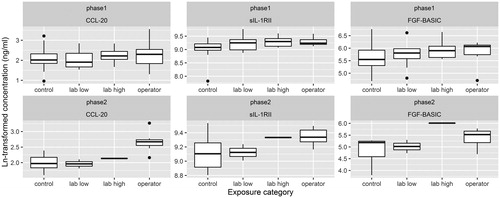
In phase 1, we observed trends in immune marker concentrations with exposure to MWCNTs for C–X–C motif chemokine 11 (CXCL11) [upwards; increasing with increasing exposure], C–C motif ligand 20 (CCL20) [upwards], Interleukin 16 (IL-16) [downwards], eskine and cutaneous T-cell-attracting chemokine (CTACK) [downwards], basic fibroblast growth factor (FGF-BASIC) [upwards], and soluble IL-1 receptor II (sIL-1RII) [upwards] ().
Table 2. Difference in immunological marker concentration (ng/mL) between workers exposed to multi-walled carbon nanotubes and controls in phase 1Table Footnotea.
For CCL20 and sIL-1RII, estimates from trend analysis were significant regardless of the approach for exposure assessment (ranking: β = .1309; p = .0051 and β = .0975; p = .0004, respectively, and assignment: β = .0093; p = .0087 and β = .0057; p = .0079, respectively). For these two markers, we also observed significant differences in blood concentrations between operators or lab-workers and controls (with the exception of a non-significant decrease in the concentration of CCL20 among low-exposed lab workers). We observed no consistent association between exposure to MWCNT and the two inflammation scores (Supplemental Material, Table S1), although we did observe significant elevation of the inflammation score among higher exposed lab workers (lab high). After log-transformation the inflammation score reported in Shiels et al. (Citation2013) was no longer significantly elevated among higher exposed lab workers.
Among the markers that were significantly associated with MWCNT exposure in phase 1, we observed significant trends in blood concentration with exposure to MWCNT (either assigned exposure ranking, or assigned GM MWCNT) for CCL20 (upwards; blood concentrations among operators also significantly elevated), CTACK (upwards; blood concentrations among operators also significantly elevated), FGF-BASIC (upwards; blood concentrations among operators and higher exposed lab personnel also significantly elevated) in phase 2 (Supplemental material, Table S2). The effect for CTACK was in the opposite direction of what was observed during phase 1. For sIL-1RII, we observed a non-significant upward trend with exposure to MWCNT and we observed significantly elevated blood concentrations among operators and higher exposed lab personnel.
In , we show boxplots of the distributions of the markers that were most robustly associated with MWCNT exposure in phases 1 and 2 (CCL20, sIL-1RII, and FGF-BASIC). The effect of exposure to MWCNT on blood concentrations of sIL-1RII was robust (both in direction of effect and statistical significance) to all sensitivity analyses (Supplemental Material, Table S3). The effect of exposure to MWCNT on blood concentrations of CCL20 was generally stable across sensitivity analyses. While the direction of the effect for the three exposure categories remained unchanged in each sensitivity analysis, the effect for the highest exposure category “operator” lost its formal statistical significance after correction for alcohol use, previous exposure to particulates, and when we excluded females and smokers. Results for FGF-BASIC were robust to the sensitivity analyses in terms of the direction of the effect. The effects among higher exposed lab workers and operators became stronger after correction for educational level and when we excluded females and smokers, now reaching statistical significance. Our findings for sIL-1RII, CCL20, and FGF-BASIC were not (partially) explained by a strong correlation between these markers (Supplemental material, Figure S1). While FGF-BASIC was strongly correlated to several other immunological markers, the correlations with sIL-1RII and CCL20 were low in phase 1 (0.21 and 0.31, respectively) and slightly higher in phase 2 (0.25 and 0.64, respectively).
Figure 1. Boxplots showing the distribution of Ln-transformed concentrations of CCL20, sIL-1RII, and FGF-BASIC during phase 1 and phase 2, by exposure category. Kruskal–Wallis rank sum test p values for phase 1 were .6172, .02232, .1527, for CCL20, sIL-1RII, and FGF-BASIC, respectively. Wilcoxon rank sum test p values (operators versus controls) for phase 1 were .4352, .01087, and .1188. Kruskal–Wallis rank sum test p-values for phase 2 were .018, .2097, and .1018, for CCL20, sIL-1RII, and FGF-BASIC, respectively. Wilcoxon rank sum test p values (operators versus controls) for phase 2 were .0047, .1375, and .0734.
Median ICCrep for the 23 parameters that were measured as part of the CBC was 0.69 (IQR: 0.54, 0.79). We observed significant depression in neutrophils and significant elevation in monocytes, mean platelet volume, immature platelet fraction, and immature reticulocytes fraction with increasing exposure to MWCNT in phase 1 () and phase 2 (Supplemental material, Table S4). For neutrophils, monocytes, and mean platelet volume, the results were robust to all sensitivity analyses (Supplemental material, Table S5), with the exception of a correction for previous exposure to nanoparticles which resulted in a loss of formal significance for mean platelet volume (p = .1134). The result for immature platelet fraction and immature reticulocytes fraction was not robust in the sensitivity analyses. Neutrophils and white blood cells were elevated among the higher exposed lab workers in phase 1, but this effect was not replicated in phase 2. In phase 2, we observed several significant associations that were not observed in phase 1.
Table 3. Difference in complete blood cell counts between workers exposed to multi-walled carbon nanotubes and controls in phase 1Table Footnotea.
We observed significantly lower FENO among operators compared to controls during phase 1 (). This effect was robust (both in direction of effect and statistical significance) to all sensitivity analyses (Supplemental Material, Table S6), with the exception of a correction for previous exposure to nanoparticles, which resulted in a loss of formal significance (p = .069). We observed significant trends based on assigned GM MWCNT mass concentrations in the full population as well as among male non-smokers. Trends based on exposure ranking were in the same direction, but were not significant.
Table 4 Difference in fractional exhaled nitric oxide (ppb) between workers exposed to multi walled carbon nanotubes and controls in phase 1Table Footnotea,Table Footnoteb.
Acceptable spirometry data were collected from 55 individuals. We observed some indication for a larger (percentage of the predicted) FVC among operators compared to controls, but observed no significant trends with FVC, FEV1, and FEV1/FVC with exposure to MWCNTs, regardless of the approach for exposure assessment that we used (). Results were similar among non-smoking males (Supplemental Material, Table S7).
Table 5. Difference in lung function between workers exposed to multi-walled carbon nanotubes and controls in phase 1Table Footnotea.
ICCdup for the pneumoproteins was high (>0.94) and ICCrep for CC16, SP-A, and SP-D was 0.81, 0.77, and 0.48, respectively. We observed no significant trends in blood concentrations of the pneumoproteins with exposure to MWCNTs in phase 1, regardless of the approach for exposure assessment that we used (). This observation was confirmed by visual assessment of the variation in pneumoprotein concentrations across exposure categories (). We observed similar results when we restricted the dataset to male non-smokers and in phase 2 (results not shown).
Figure 2. Boxplots showing the distribution of Ln-transformed concentrations of CC-16, SP-A, and SPD during phase 1 and phase 2, by exposure category. Kruskal–Wallis rank sum test p values for phase 1 were .1388, .4333, .706, for CC-16, SP-A, and SPD, respectively. Wilcoxon rank sum test p values (operators versus controls) for phase 1 were .4, .8576, and .4529. Kruskal–Wallis rank sum test p values for phase 2 were .5381, .2996, .7483, for CC-16, SP-A, and SPD, respectively. Wilcoxon rank sum test p values (operators versus controls) for phase 2 were .5338, .2343, and .6282.
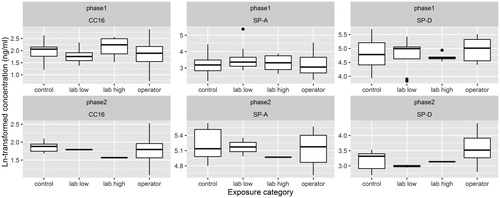
Table 6. Difference in pneumoproteins (ng/mL) between workers exposed to multi-walled carbon nanotubes and controls in phase 1Table Footnotea.
Discussion
We observed an indication for an effect of exposure to MWCNTs on selected immune markers (CCL20, sIL-1RII, FGF-BASIC), FENO, and selected blood parameters (neutrophils, monocytes, and mean platelet volume) in a small scale cross-sectional study in a population occupationally exposed to MWCNT.
The observed increase in CCL20, sIL-1RII, and FGF-BASIC may be indicative of an inflammatory reaction. CCL20 is a chemokine involved in antimicrobial activity that has been reported to be negatively associated with emphysema in patients with chronic obstructive pulmonary disease (Carolan et al., Citation2014; Crane-Godreau et al., Citation2009). CCL20 production and secretion was shown to be suppressed when cells were exposed to cigarette smoke (Crane-Godreau et al., Citation2009). Although the literature is scarce, these reports contradict our finding of elevated levels of CCL20 among MWCNT exposed workers, for which we would expect the effect to be in the same direction as for cigarette smoke. SIL-1RII is a decoy receptor that binds proinflammatory interleukin 1 (IL-1), reducing its activity (Aka et al., Citation2015). The observed increase in SIL-1RII might, therefore, be indicative of an IL-1-mediated response as a result of exposure to MWCNT. While we did not assess IL-1 in the current study, it has been reported to play a role in cigarette smoke induced inflammation (Pauwels et al., Citation2011). FGF-BASIC is a member of a family of proteins with growth, anti-apoptotic, and differentiation promoting activity (Powers et al., Citation2000). Tumor cell expression of FGF-BASIC has been reported as marker for cancer prognosis (Barclay et al., Citation2005; Rades et al., Citation2014), but we are not aware of any literature relating exposure to environmental agents to changes of FGF-BASIC in peripheral blood. Considering the small number of identified markers we refrain from the formal identification of enriched pathways. Two of the inflammatory markers included in our study were also measured in serum by Fatkhutdinova et al. (Citation2016): IL-8 and TNF-α. We did not replicate the significant association between exposure to MWCNT and TNF-α reported in that study, though the non-significant effect we observed was in the same direction (upregulation) (Fatkhutdinova et al., Citation2016). Eleven of the immune markers we assessed in our study have been reported to be predictive of lung cancer risk (CRP, SAA, sTNFRII, IL-1RA, IL-7, TGF-A, ENA 78/CXCL5, MIG/CXCL9, BCA-1/CXCL13, TARC/CCL1, MDC/CCL22) (Shiels et al., Citation2013, Citation2015). These markers and two risk scores based on these markers were not consistently associated to exposure to MWCNTs in our study. We, therefore, did not observe indirect evidence of a potential increased risk of lung cancer due to exposure to MWCNTs. CRP is also an established marker of acute phase inflammation and a risk factor for cardiovascular disease. CRP has been reported to be significantly affected by ambient particle exposure (including nano-sized particles) (Li et al. Citation2012). We did not replicate this finding in our study.
Depression in FENO as result of exposure to MWCNT would suggest that MWCNT exposure may have an inhibitory effect on NO synthase in the airways (Malinovschi et al., Citation2006). Depression of FENO has been reported in response to cigarette smoke (a source of particulate exposure) (Malinovschi et al., Citation2006). However, this effect might be related to the high concentrations of NO in cigarette smoke itself (inducing a negative feedback loop resulting in downregulation of NO synthase) (Malinovschi et al., Citation2006), an effect that we would not expect as result of exposure to MWCNTs. Our results are in contrast with the study by Wu et al. (Citation2014), in which no significant effect of exposure to carbon nanotubes (type not further specified) on FENO was observed, while exposure to nanosized titanium dioxide significantly increased the risk of elevated FENO levels (>35 ppb) (Wu et al., Citation2014). Other literature generally reported elevation in FENO (e.g. in response to air pollution; Van Amsterdam et al., Citation1999; Zuurbier et al., Citation2011) as potential indicator of eosinophilic inflammation. In our study, eosinophil concentrations measured in peripheral blood in phase 1 were non-significantly elevated in operators compared with controls and laboratory workers, and therefore did not corroborate our finding for FENO. The clinical relevance of our finding for FENO is unclear. A FENO greater than 50 ppb has been suggested as a clinically relevant cut point to indicate that eosinophilic inflammation is likely, while levels smaller than 25 ppb are considered an indication that eosinophilic inflammation is less likely (Dweik & Boggs Citation2011). In our study, five individuals had a FENO greater than 50 ppb (none of them were exposed to high levels of MWCNTs), while the majority (n = 51) had levels lower than 25 ppb. While all blood counts were within clinical reference ranges, we observed significant depression of neutrophils and significant elevation of monocytes and mean platelet volume among operators compared to controls. Neutrophils play a role in inflammation and have been reported to increase after exposure to particulates (Salvi et al. Citation1999). Decreases in neutrophil counts can originate from viral infections, drug use, and exposure to certain solvents, among other causes (Gibson & Berliner Citation2014), but have not been reported to be lower in relation to exposure to particulates and are therefore not likely explained by exposure to MWCNTs. Lee et al. (Citation2015) reported that no noticeable abnormalities were observed in hematology and blood biochemical marker measurements among workers exposed to MWCNT, although did not analyze subclinical changes in these parameters. Interestingly, neutrophils are involved in the production of NO in the airways (Ricciardolo, Citation2003) which corresponds to our observation of decreased FENO. Monocytes also play a role in inflammation and have been shown to increase after exposure to particulates (Chaudhuri et al., Citation2012; Goto et al., Citation2004; Ishii et al., Citation2005), providing a suggestion of biological plausibility of this finding. Elevated mean platelet volume would indicate a high number of larger, younger platelets in the blood, resulting from upregulated bone marrow production and release of platelets into circulation and has been suggested as marker of platelet activation (Shah et al., Citation2012). Platelet activation has been associated in the literature with exposure to particulates in the form of ambient air pollution, but the direction of the effect has been inconsistent (Frampton et al., Citation2012; Strak et al., Citation2013).
We observed no effect of exposure to MWCNT on lung function. The non-significantly increased FVC among operators compared to controls might be attributable to insufficient correction for the effect of sex on FVC (although we did correct for sex both the regression models and in the predicted values). Our study did not have sufficient statistical power to detect subtle effects of MWCNT on lung function.
The null effect of exposure to MWCNTs on pneumoproteins CC16, SP-A, and SP-D that we observe in our current study contradicts with previously published in vitro studies that demonstrated an effect of exposure to MWCNTs on the production of these pneumoproteins (Chen et al., Citation2015; Han et al., Citation2010). Potential explanations include limited statistical power in our study (a false negative finding), differences between the in vitro studies and ours with regards to the levels and patterns of exposure that lung cells incurred and the types of MWCNTs that were used.
Strengths of our study include a relatively high contrast in exposure to MWCNTs (Liou et al., Citation2015) within the study population, quantitative exposure assessment, and detailed assessment of potential confounding factors. Even though our study is currently the largest that has evaluated the biological effects of MWCNT exposure in a human population, an important limitation is still its modest sample size. We conducted a series of sensitivity analyses to assess the influence of confounding on the noteworthy findings in the main analysis. To avoid identifying too many false positive findings, we did not explore the impact of negative-confounding on the markers for which we did not observe an association with MWCNT exposure in the main analysis.
Replication of our findings in an independent study population exposed to MWCNT is crucial. Setting up sufficiently large studies to allow the assessment of subtle health effects due to exposure to MWCNT is a challenge. In an inventory of engineered carbonaceous nanomaterial manufactures in the USA, the average number of workers per company that handled engineered carbonaceous nanomaterial was 10 (Schubauer-Berigan et al., Citation2011). Furthermore, the type of MWCNTs that are produced across companies, and within companies over time varies considerably. Therefore, large-scale collaborations between research groups and companies are needed to be able to study the early health effects of MWCNT exposure with sufficient statistical precision.
In conclusion, in this molecular cross-sectional study, we observed some indications of early biological perturbations associated with exposure to MWCNTs. We view this as an exploratory study and, therefore, a false discovery rate correction was not conducted. We have assessed the robustness of our findings in a series of sensitivity analyses and by conducting a repeat assessment of selected markers among the highest exposed workers. However, considering our modest sample size and our cross-sectional study design, influence of selection biases cannot fully be excluded. Our findings warrant follow-up in other MWCNT exposed populations incorporating personal exposure estimates. In addition, studies are needed that assess the potential impact of exposure to MWCNT on human health including outcomes such as non-malignant respiratory disease, oxidative stress, and cardiovascular disease.
Supplemental material
Download MS Word (265.3 KB)Acknowledgements
The authors thank Annemarie Melis and Marylene de Zeeuw for contribution to the fieldwork and Kai Chahal for his contribution to the analysis of the pneumoproteins.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Aka PV, Kemp TJ, Rabkin CS, Shiels MS, Polizzotto MN, Lauria C, et al. 2015. A multiplex panel of plasma markers of immunity and inflammation in classical kaposi sarcoma. J Infect Dis 211:226–9.
- Barclay C, Li AW, Geldenhuys L, Baguma-Nibasheka M, Porter GA, Veugelers PJ, et al. 2005. Basic fibroblast growth factor (FGF-2) overexpression is a risk factor for esophageal cancer recurrence and reduced survival, which is ameliorated by coexpression of the FGF-2 antisense gene. Clin Cancer Res 11:7683–91.
- Bekker C, Kuijpers E, Brouwer DH, Vermeulen R, Fransman W. 2015. Occupational exposure to nano-objects and their agglomerates and aggregates across various life cycle stages; a broad-scale exposure study. Ann Occup Hyg 59:681–704.
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300.
- Carolan BJ, Hughes G, Morrow J, Hersh CP, O’Neal WK, Rennard S, et al. 2014. The association of plasma biomarkers with computed tomography-assessed emphysema phenotypes. Respir Res 15:127.
- Chaturvedi AK, Kemp TJ, Pfeiffer RM, Biancotto A, Williams M, Munuo S, et al. 2011. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol Biomarkers Prev 20:1902–11.
- Chaudhuri N, Jary H, Lea S, Khan N, Piddock KC, Dockrell DH, et al. 2012. Diesel exhaust particle exposure in vitro alters monocyte differentiation and function. PLoS One 7:e51107.
- Chen R, Zhang L, Ge C, Tseng MT, Bai R, Qu Y, et al. 2015. Subchronic toxicity and cardiovascular responses in spontaneously hypertensive rats after exposure to multiwalled carbon nanotubes by intratracheal instillation. Chem Res Toxicol 28:440–50.
- Crane-Godreau MA, Maccani MA, Eszterhas SK, Warner SL, Jukosky JA, Fiering S. 2009. Exposure to cigarette smoke disrupts CCL20-mediated antimicrobial activity in respiratory epithelial cells. Open Immunol J 2:86–93.
- Dweik R, Boggs P. 2011. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 184:602–15.
- Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, et al. 2016. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl Pharmacol 299:125–31.
- Fatkhutdinova LM, Khaliullin TO, Vasilyeva OL, Zalyalov RR, Valeeva IK, Mustafin IG, et al. 2013. Pilot cross-sectional study for potential fibrogenic risk assessment in real multiwalled carbon nanotube aerosol exposure at the workplaces. Kazan meditsinskiy Z 94:770–4.
- Frampton MW, Bausch J, Chalupa D, Hopke PK, Little EL, Oakes D, et al. 2012. Effects of outdoor air pollutants on platelet activation in people with type 2 diabetes. Inhal Toxicol 24:831–8.
- Gibson C, Berliner N. 2014. How we evaluate and treat neutropenia in adults. Blood 124:1251–8. quiz 1378.
- Goto Y, Ishii H, Hogg JC, Shih C-H, Yatera K, Vincent R, et al. 2004. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med 170:891–7.
- Han SG, Andrews R, Gairola CG. 2010. Acute pulmonary response of mice to multi-wall carbon nanotubes. Inhal Toxicol 22:340–7.
- Ishii H, Hayashi S, Hogg JC, Fujii T, Goto Y, Sakamoto N, et al. 2005. Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Respir Res 6:87.
- Kim JS, Song KS, Yu IJ. 2015. Multiwall carbon nanotube-induced DNA damage and cytotoxicity in male human peripheral blood lymphocytes. Int J Toxicol 35:27–37.
- Kuempel E, Jaurand M, Møller P, Morimoto Y, Kobayashi N, Pinkerton K, et al. 2017. Evaluating the key evidence and data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit Rev Toxicol 47:1–58. doi: 10.1080/10408444.2016.1206061
- Kuijpers E, Bekker C, Fransman W, Brouwer DH, Tromp P, Vlaanderen J, et al. 2015. Occupational exposure to multi-walled carbon nanotubes during commercial production synthesis and handling. Ann Occup Hyg 60:305–17.
- Lee JS, Choi YC, Shin JH, Lee JH, Lee Y, Park SY, et al. 2015. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology 9:802–11.
- Li Y, Rittenhouse-Olson K, Scheider WL, Mu L. 2012. Effect of particulate matter air pollution on C-reactive protein: a review of epidemiologic studies. Rev Environ Health 27:133–49.
- Liao H-Y, Chung Y-T, Lai C-H, Lin M-H, Liou S-H. 2014a. Sneezing and allergic dermatitis were increased in engineered nanomaterial handling workers. Ind Health 52:199–215.
- Liao H-Y, Chung Y-T, Lai C-H, Wang S-L, Chiang H-C, Li L-A, et al. 2014b. Six-month follow-up study of health markers of nanomaterials among workers handling engineered nanomaterials. Nanotoxicology 8:100–10.
- Liou S-H, Tsai CSJ, Pelclova D, Schubauer-Berigan MK, Schulte PA. 2015. Assessing the first wave of epidemiological studies of nanomaterial workers. J Nanopart Res 17:413.
- Malinovschi A, Janson C, Holmkvist T, Norbäck D, Meriläinen P, Högman M. 2006. Effect of smoking on exhaled nitric oxide and flow-independent nitric oxide exchange parameters. Eur Respir J 28:339–45.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. 2005. General considerations for lung function testing. Eur Respir J 26:153–61.
- NIOSH. 2013. Occupational Exposure to Carbon Nanotubes and Nanofibers.
- Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, et al. 2011. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J 38:1019–28.
- Powers CJ, McLeskey SW, Wellstein A. 2000. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7:165–97.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. 1993. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir Eur Respir J Suppl 16:5–40.
- R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rades D, Seibold ND, Gebhard MP, Noack F, Bruchhage KL, Schild SE. 2014. Fibroblast growth factor 2 is of prognostic value for patients with locally advanced squamous cell carcinoma of the head and neck. Strahlentherapie Onkol Organ Dtsch Röntgengesellschaft [Et Al] 190:68–74.
- Ricciardolo FLM. 2003. Multiple roles of nitric oxide in the airways. Thorax 58:175–82.
- Rijcken B, Kerkhof M,D, Graaf A, Boezen HM, Droste JHJ, Kremer AM. 1996. Europees Luchtweg Onderzoek Nederland. Groningen: Rijksuniversiteit Groningen.
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, et al. 1999. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 159:702–9.
- Schubauer-Berigan MK, Dahm MM, Yencken MS. 2011. Engineered carbonaceous nanomaterials manufacturers in the United States: workforce size, characteristics, and feasibility of epidemiologic studies. J Occup Environ Med 53:S62–S7.
- Shah B, Sha D, Xie D, Mohler ER, Berger JS. 2012. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999–2004. Diabetes Care 35:1074–8.
- Shiels MS, Katki HA, Hildesheim A, Pfeiffer RM, Engels EA, Williams M, et al. 2015. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J Natl Cancer Inst 107:djv199. doi: 10.1093/jnci/djv199
- Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park J-H, et al. 2013. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 105:1871–80.
- Shvedova AA, Yanamala N, Kisin ER, Khailullin TO, Birch ME, Fatkhutdinova LM. 2016. Integrated analysis of dysregulated ncRNA and mRNA expression profiles in humans exposed to carbon nanotubes. PLoS One 11:e0150628.
- Stark W, Stoessel P. 2015. Industrial applications of nanoparticles. Chem Soc Rev 44:5793–805.
- Strak M, Hoek G, Godri KJ, Gosens I, Mudway IS, van Oerle R, et al. 2013. Composition of PM affects acute vascular inflammatory and coagulative markers – the RAPTES project. PLoS One 8:e58944.
- Tromp P, Kuijpers E, Bekker C, Godderis L, Lan Q, Jedynska A, et al. 2017. A new approach combining analytical methods for workplace exposure assessment of inhalable multi-walled carbon nanotubes. Ann Occup Hygiene. (Under revision).
- Van Amsterdam JG, Verlaan BP, Van Loveren H, Elzakker BG, Vos SG, Opperhuizen A, et al. 1999. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch Environ Health 54:331–5.
- Wu W-T, Liao H-Y, Chung Y-T, Li W-F, Tsou T-C, Li L-A, et al. 2014. Effect of nanoparticles exposure on fractional exhaled nitric oxide (FENO) in workers exposed to nanomaterials. Int J Mol Sci 15:878–94.
- Zuurbier M, Hoek G, Oldenwening M, Meliefste K, van den Hazel P, Brunekreef B. 2011. Respiratory effects of commuters' exposure to air pollution in traffic. Epidemiology 22:219–27.

