Abstract
The biological fate of nanoparticles (NPs) taken up by organisms from their environment is a crucial issue for assessing ecological hazard. Despite its importance, it has scarcely been addressed due to the technical difficulties of doing so in whole organism in vivo studies. Here, by using transmission electron microscopy and energy dispersive X-ray spectroscopy (TEM-EDS), we describe the key aspects that characterize the interaction between an aquatic organism of global ecological and economic importance, the early larval stage of the Japanese oyster (Crassostrea gigas), and model gold NPs dispersed in their environment. The small size of the model organism allowed for a high-throughput visualization of the subcellular distribution of NPs, providing a comprehensive and robust picture of the route of uptake, mechanism of cellular permeation, and the pathways of clearance counterbalancing bioaccumulation. We show that NPs are ingested by larvae and penetrate cells through alimentary pinocytic/phagocytic mechanisms. They undergo intracellular digestion and storage inside residual bodies, before excretion with feces or translocation to phagocytic coelomocytes of the visceral cavity for potential extrusion or further translocation. Our mechanistically-supported findings highlight the potential of oyster larvae and other organisms which feature intracellular digestion processes to be exposed to man-made NPs and thus any risks associated with their inherent toxicity.
1. Introduction
After more than a decade of intense research into the potential environmental impact of nanotechnologies, there remain uncertainties about the extent to which biota is exposed to nanoparticles (NPs) released into water bodies. Not only is there a lack of evidence of the environmental concentrations of manufactured NPs, which is still dominated by modeled predictions, but also of the processes by which NPs pass across biological barriers, enter cells and are subject to biological processes (Hou, Westerhoff, and Posner Citation2013; Baalousha et al. Citation2016; Nowack Citation2017). This last point is crucial for assessing the risks they pose, since "NP cellular internalization" is analogous to "bioaccumulation" for dissolved chemicals, i.e. the essential condition for solid particles to manifest their size- and surface- dependent reactivity against cellular components. Without strong evidence of cellular internalization, the environmental impact of nanotechnology could be limited to only a few classes of NPs whose toxicity is not strictly related to their particle form (e.g. dissolving NPs).
Most knowledge of how NPs are taken up into cells comes from biomedical studies based on in vitro experiments with different cell lines and from in vivo exposures of laboratory animal models through instillation or inhalation (Albanese, Tang, and Chan Citation2012; Canton and Battaglia Citation2012; Zhang, Gao, and Bao Citation2015). In spite of its indisputable scientific value, there are obvious limitations in extrapolating this body of knowledge to realistic environmental scenarios where NP fate is highly influenced by the characteristics of the receiving environment (e.g. solution chemistry, composition of solid matrices) and of the organism (i.e. biological-ecological features driving the mode of interaction with nano-sized particles).
Aquatic environments are considered one of the final sinks of many released NPs, but the impact of NPs on marine biota remains virtually unknown. In spite of its pivotal importance, the assessment of the biological fate of NPs taken up by aquatic organisms from their environment is rarely studied because of the analytical difficulty in tracing nanosized particles in biological samples (Baker, Tyler, and Galloway Citation2014; Selck et al. Citation2016).
Studies which have attempted to trace the pathways of in vivo NP uptake have used a wide range of aquatic organisms (including algae, vertebrate and invertebrate species at different developmental stages), nanomaterials (i.e. carbonaceous, metal, polymeric), and analytical methods (mainly imaging approaches) (Ma and Lin Citation2013; Schultz et al. Citation2015). These studies depict contrasting scenarios, suggesting differences in the abilities of organisms to interact with NPs. Technical limitations have to date hindered a comprehensive understanding of the mechanisms of cellular penetration, trafficking, and translocation of NPs. For instance, among the most widely used bioimaging approaches, advanced light microscopic techniques (e.g. hyperspectral imaging, confocal microscopy, near infrared fluorescence microscopy) typically cannot resolve individual particles and/or the cellular structures where the particle-associated signal is detected, leaving knowledge gaps on the transportation mechanisms employed and on the actual fate of NPs inside cells (Meyer et al. Citation2010; Unrine et al. Citation2010; Hull et al. Citation2011; Kwok et al. Citation2012; Ambrosone et al. Citation2014; Schultz et al. Citation2015). In contrast, the higher resolution transmission electron microscopy (TEM), which enables a detailed view of NP intracellular localization, is generally confined to a limited number of sections or samples, and may lack insight into the magnitude and the overall consistency of the permeation and translocation phenomenon, when applied to whole organism (Renault et al. Citation2008; Joubert et al. Citation2013; Schultz et al. Citation2015; Wray and Klaine Citation2015).
Here, we investigated how early-life stage oysters interact with manufactured NPs dispersed in their environment, using TEM analysis supported by energy dispersive X-ray spectroscopy (EDS). Early larval stages of bivalves have not been widely used until now to study the uptake and impact of NPs (Ringwood et al. Citation2009; Kadar et al. Citation2010; Ringwood et al. Citation2010; Noventa et al. Citation2018a, Citation2018b), despite their ability to bioconcentrate solid particles and the bio-imaging advantage offered by their transparency and small size (approximately 70 µm). Early veliger larvae (alias D-shell shaped larvae, prodissoconch I) actively feed on suspended food particles, processing them through a well-developed digestive apparatus (Yonge Citation1926b; Millar Citation1955; Galtsoff Citation1964; Elston Citation1980a; Waller Citation1981; Bayne Citation2017) (). Food particles (i.e. bacteria, algae, organic matter (Douillet Citation1993)), captured through the velum, are moved toward the mouth by the shorter velar cilia and carried into the stomach by crossing the ciliated esophagus. The stomach, a barrel-shaped organ in communication with the digestive gland and structurally differentiated into gastric shield and style sac, is the site of particle sorting (Gallager Citation1988). Here, ingested particles can be directed toward the digestive diverticula (for intracellular digestion and assimilation) or the intestine–anus via intestinal groove (for excretion into the mantle cavity).
Figure 1. Crassostrea gigas D-shaped shell larvae (48 h post fertilization, hpf). Anatomy of the main organs constituting the digestive apparatus as visible through the clear shell by inverted light microscopy (Zeiss Axio Observer Inverted Microscope). (A) Oyster larva under protruded body position. (B) Oyster larva under retracted body position (position taken before death by all organisms). The black spot in the style sac area is a dense accumulation of ingested NPs. A: anus; E: esophagus; DG: digestive gland; I: intestine; M: mouth; MC: mantle cavity; S: stomach; SS: style sac; V: velum; VC: visceral cavity.
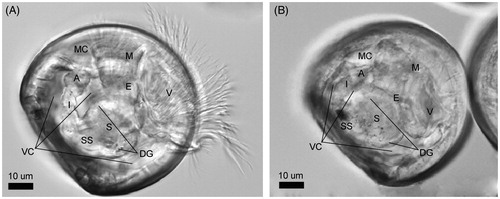
In the present study, we exposed oyster larvae to spherical gold NPs (100 nm diameter), an electron dense nanomaterial highly suitable for TEM imaging, and traced the internalization pathway and endocytic process in vivo. Our results illustrate the extensive biodistribution of NPs in the digestive tract and in the coelomocytes (i.e. free- phagocytic cells present in the visceral cavity) and the pathway of NP movement along the alimentary route through endocytic–exocytic processes.
2. Materials and methods
2.1. Nanoparticle supply and seawater suspensions
The tested 100-nm gold NPs were DiagNano™ Alexa Fluor 488 Labeled (GFL-100-488), Creative Diagnostics (https://www.cd-pioparticles.com/p/Fluorophore-Gold-Nanoparticles_258_259_271_3108.html). The surface was coated with a non-reactive methyl polymer improving their stability in dispersion media. The stock solution was provided in a low ionic strength buffer (zeta potential of −18 eV). For in vivo exposure for 48 h of oyster embryos, NP suspensions were prepared freshly before use by diluting the gold NP stock suspension in artificial seawater (ASW; Tropic Marin® SEA SALT, salinity 30.0 ± 0.2 ppt; final volume 25 mL). At the start of the experiment, stock solution (previously bath-sonicated for 5 min) was added into each exposure chamber, at the final concentration of 5 mg L−1. The exposure concentration was chosen to maximize the performance of the TEM, rather than to mimic the natural environment. This high concentration maximized the likelihood that NPs would be present and visible in ultrathin sections using TEM. As gold is not subject to oxidation and dissolution, this concentration did not lead to larval toxicity. Negative controls (i.e. ASW only) were also carried out.
2.2. Oyster fertilization and in vivo exposure of embryos
Ripe oysters (Crassostrea gigas) were collected in Starcross (estuary of river Exe, Devon, UK; low tide time) during their reproductive season (August 2017). The ICES protocol n°54 (Leverett Citation2013) was followed to carry out the in vitro fertilization. Briefly, embryos were obtained from three pairs of parental adults. Male and female gametes were recovered by gently cutting the gonads with a sharp scalpel, and the recovered egg suspension was fertilized by adding a few milliliters of sperm. Once the polar body was visible on 80–90% of the eggs, the fertilized eggs were incubated for 2 h at 24 °C, in dark conditions and without aeration. At the achievement of the 16–32 cell stage, embryos were transferred at a density of 250 embryos mL−1 into the exposure chambers.
The in vivo exposure was carried out under static conditions (ASW at 30 ppt salinity, pH = 8.1 ± 0.05; 12:12-h light/dark cycle, no aeration) and over 48 h. At the end of the exposure, larvae were filtered through a 25-µm mesh sieve and aliquots were purposely fixed for TEM analysis, as described below.
2.3. Sample preparation for the transmission electron microscopy (TEM) analysis
Larvae were fixed with 3%glutaraldehyde/2% paraformaldehyde in 0.1-M Pipes buffer, pH 7.2. After post-fixation with 1% osmium tetroxide (reduced with 1.5% potassium hexacyanoferrate in DIW), samples were washed in deionized water before being dehydrated through a graded ethanol series. The samples were embedded in Spurr resin (TAAB, Aldermaston, UK). Around 70-nm ultrathin sections were collected on pioloform-coated 100 mesh copper TEM grids and for contrasting stained with lead citrate whenever necessary to improve the identification of cellular structures. The grids were analyzed using a JEOL JEM 1400 transmission electron microscope operated at 120 kV and images taken using a digital camera (ES 100 W CCD, Gatan, Abingdon, UK).
2.4. TEM-based method for the identification of the internalized NPs and method validation via EDS
The identification of bioaccumulated NPs using TEM visualization is based on the greater electron density of nanogold compared to that of the surrounding biological matrix. The NPs were visually isolated from the biological background by minimizing the contrast of the micrographs. By decreasing the contrast, all biological structures progressively disappear, including potential false-positive electron dense elements (i.e. black spots of organic nature having similar round shape and size of the tested NPs). Once discriminated, NPs were mapped over larvae' body structures by resetting the contrast (see Supplementary Figure S1).
The reliability of the TEM-based method was assessed via energy dispersive X-ray spectroscopy (EDS). A subgroup of NPs, selected randomly among those visually discriminated, was characterized for their chemical composition by the use of a TEM-EDS instrument (JEOL JEM-2100 LaB6 200 kV TEM with Oxford INCA EDS). The gold composition of the NPs was established based on the relative atomic abundance of gold over biological elemental constituents (i.e. Au to O percentage ratio). The results of TEM-EDS analysis are reported in the supplementary information (Supplementary Figures S2–S4).
3. Results
3.1. Alimentary uptake of NPs and cellular internalization via endocytic mechanism
Oyster embryos (C. gigas; 2 h post-fertilization, hpf) were exposed for 48 h to 5 mg L−1 of gold NPs. At the end of the exposure, larvae were recovered via filtration and processed for TEM analysis of NP biodistribution and cellular internalization.
As presented in , NPs were found simultaneously in all organs of the digestive tract, with the largest abundance observed inside the lumen of the stomach and in the digestive gland (). This ubiquitous presence is to some extent a consequence of the high concentration used; at a lower exposure concentration the distribution along the digestive tract might be different although the general pathway of uptake is preserved.
Figure 2. Ingestion of NPs and their internalization in the absorptive cells of the digestive gland. (A) Overview of the distribution of NPs inside the stomach lumen and the adjoined digestive gland. (B) Ingested NPs and bacteria in the proximity of the edge between the gastric shield and the style sac. (C) Entry of an ingested bacterium into an absorptive cell via phagocytosis. (D) Primary phagosome whose marked multi-lobe shape suggests recent fusion of pinosomes. (E) Progressive maturation of the endocytic bodies along their migration from the apical side to the basal side of an absorptive cell (i.e. pinosomes, early heterophagosomes, rounded-shape mature heterophagosomes). Pictures were taken from a grid stained with lead citrate. Arrows indicate NPs. ib: internalized bacterium; b: bacterium; m: mitochondrion; hp: heterophagosome; c: cilium; ld: lipid droplet; rer: rough endoplasmic reticulum.
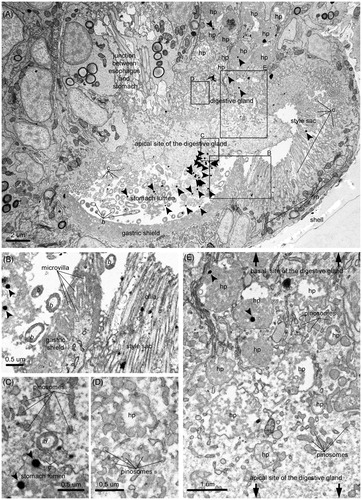
Figure 3. Intracellular digestion of NPs. (A) Electron micrograph showing a section of the digestive gland and a portion of the circular section of the densely ciliated esophagus. The digestive cells are sectioned at their mid-/basal- side, featuring an abundance of heterolysosomes with NPs inside the majority of them. The filling of these endocytic bodies with bacteria at different levels of decomposition and flocculent electron-dense material of heterogenous texture suggest stages in the digestive process, as exemplified by the detailed pictures B–E. More precisely, (B) Initial stage of intracellular decomposition, (C) Intermediate stage, (D) Late stage (E) Final stage, i.e. NP containing residual body storing indigestible material. Sections were imaged without prior lead citrate contrasting. Arrows point to NPs. e: endosome; ib: internalized bacterium; b: bacterium; m: mitochondrion; hp: heterophagosome; hl: heterolysosome; rer: rough endoplasmic reticulum; ld: lipid droplet.
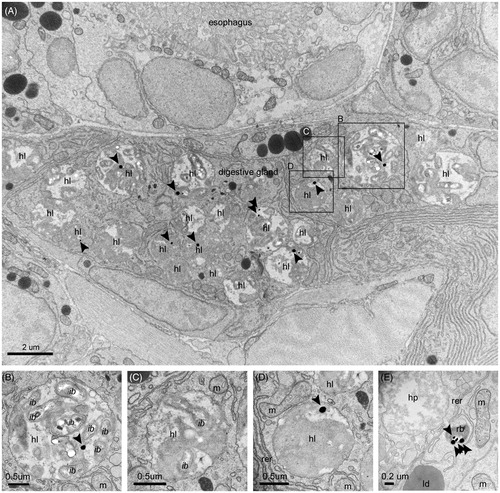
Figure 4. NP excretion. (A) NPs inside the lumen of the intestine and the residual bodies of epithelial cells. In the intestinal lumen, NPs are mixed with bacteria and the fecal laden mucous string. (B) Intestinal section containing bacteria, NPs, and feces. (C) NP containing residual bodies in the process of discarding their cargo in the lumen of the intestine. Pictures were taken from a grid stained with lead citrate. Arrows indicate NPs. hl: heterolysosome; b: bacterium; m: mitochondrion; rb: residual body; n: nucleus; c: cilium.
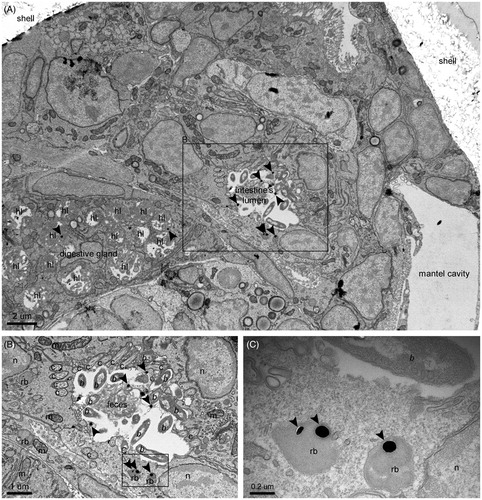
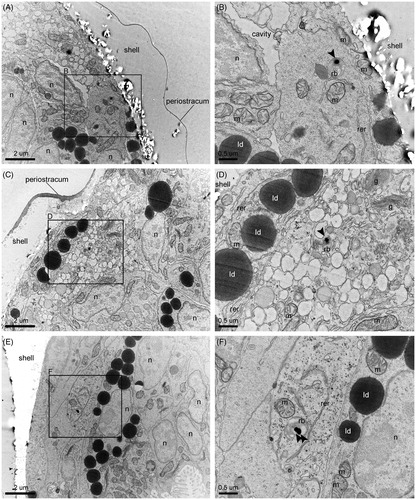
Figure 5. NP translocation. (A, C, E) NP containing residual bodies in cells near or lining the shell, identified as phagocytic coelomocytes. (B, D, F) Detailed images of the framed areas of pictures A, C, and E, respectively. Sections were imaged without prior lead citrate contrasting. Arrows point to NPs. m: mitochondrion; rb: residual body; n: nucleus; ld: lipid droplet; g: Golgi apparatus; rer: rough endoplasmic reticulum.
As suggested by the abundant co-presence of bacteria, larvae extensively capture NPs together with their food through the cilia of the velum. Then, the movement of the mouth and esophageal cilia carries the particles into the lumen of the stomach, the site of post-ingestive sorting. As shown in , the larval stomach is structurally divided into the ventral chamber and the style sac: the former contains the gastric shield, an amorphous, medium electron-dense matrix surrounding the microvilli, whereas the latter features long cilia extending from a distinctive region rich in mitochondria (). Here, ingested particles undergo mechanical disruption due to vigorous rotation against the gastric shield, activated by the transverse beating of the style sac's cilia. At this developmental stage, larvae lack of the crystalline style which secretes hydrolytic enzymes for extracellular digestion processes in the adult phase (Elston Citation1980a). Thus, the products of this preliminary processing are sorted for full digestion or ejection from the body.
Particles sorted for full digestion are taken up by the larval digestive gland, whose function is comparable to the digestive diverticula of adult oysters, in spite of its simpler form (Millar Citation1955). This H-shaped organ is in direct communication with the stomach (Elston Citation1980a). The internalization of NPs is carried out by specialized absorptive cells whose fine structure closely resembles that described for adult bivalves (Yonge Citation1926a; Owen Citation1970, Citation1972; Wikfors and Smolowitz Citation1995) ( and ). Their main distinctive feature, i.e. dense vacuolation due to a highly developed endo-lysosomal system, is concerned with their heterophagic function, which allows for the entry of exogenous materials via pinocytosis and phagocytosis. Thus, ingested particles are taken up at the apical side of the absorptive cells by pinosomes or macropinosomes, forming from invaginations of the cell membrane. The peristaltic movement of the digestive gland lobes forces nutrient materials into and out of this organ in larvae (Millar Citation1955; Elston Citation1980a). Likely because of the speed of this endocytotic step, no NP containing pinosomes were observed in the analyzed electron micrographs, despite these initial forms being well represented. shows a bacterium in the process of being wrapped by the apical membrane and enclosed inside a vesicle.
Thus, internalized NPs accumulate inside the heterophagosomes of the sub-apical regions (). These large single membrane bodies, collecting the content of the fusing pinosomes, accumulate exogenous material for digestion. NPs were regularly found in these cellular compartments, which change in size, morphology, and the extent/texture of their flocculent contents during the progression of the intradigestion process (Owen Citation1972). As displayed in , moving from the sub-apical region to a mid-region of the absorptive cell, the early heterophagosomes, characterized by irregular shape and generally filled by small amounts of granular moderate-electron dense materials, develop into more mature forms, featuring a regular round shape and abundant electron-dense content. Their subsequent fusion with primary lysosomes increases their digestability up to the mature stage, i.e. heterolysosomes, where their cargo is degraded by hydrolytic enzymes (Yonge Citation1926a). NPs were abundant in these mature structures. shows a section of digestive gland with prevalent heterolysosomes and one gold NP inside many of them (i.e. 9 over 23 displayed heterolysosomes). Here, NPs undergo full intracellular digestion together with the co-internalized food particles, as shown by the progressive degradation of the co-ingested bacteria ().
3.2. Clearance pathway of internalized NPs
Intracellular digestion ends with the formation of residual bodies that store indigestible materials. These structures are commonly characterized by the shrinkage of their vacuolar content due to assimilation (). Their ultrastructure differs from adult bivalves (Owen Citation1972), suggesting some differences in the exocytotic phase in larval and adult stages.
The residual bodies were not observed as “excreted spheres” in the lumen of the digestive organs, but they were mostly located inside cells in the digestive gland and intestine epithelium. Their role in the clearance process was indicated by the observation of exocytotic-like forms (). Here, the NP containing residual bodies appear in the process of discharging their cargo into the lumen of the intestine supporting the hypothesis that internalized NPs can be egested and excreted through the anus into the mantle cavity with the intestinal feces.
The presence of complete bacteria in the intestinal lumen together with NPs () suggested that not all NPs undergoing ejection are previously internalized into cells. Some of them are likely sorted for excretion in the stomach, taken into the intestinal groove (Elston Citation1980a), and transported toward the intestine together with fecal laden mucous strings.
3.3. Translocation of internalized NPs to phagocytic coelomocytes
Residual bodies containing NPs were frequently observed inside phagocytic coelomocytes, distinguished by their dense granular cytoplasm and abundant vacuoles, lining the shell or the mantle (). In contrast to the phagocytes of the adult stage, these cells have rarely been studied in the larval phases (Elston Citation1980a, Citation1980b; Wikfors and Smolowitz Citation1995) and their physiology and functions are not well known. They are suggested to be generated by the mantle tissue during the prodissoconch I stage (i.e. the larval phase studied here) and undergo differentiation while still incorporated within the mantle tissue or in the fluid of the visceral cavity. At their mature stage, they are found inside the visceral cavity, suspended in the fluid and attached to the luminal surface (Elston Citation1980a, Citation1980b) and the connective tissue around the digestive diverticula (Yonge Citation1926b), where they remove foreign material and host tissue debris absorbed by the digestive gland and released into the visceral cavity. The finding of NP containing residual bodies inside these cells unveils a crossroads in the pathway of internalized NPs. The NPs can be translocated here from the digestive gland to undergo further processing and distribution and/or to be eliminated via an alternative ejection route. In fact, obsolescent, dying or infected phagocytes are extruded from the body via diapedesis, a phenomenon occurring in the larval phases through the velum and associated membranes (Elston Citation1980b). More study is needed to clarify the role of these cells in the alimentary pathway and in the fate of non-nutritious particles.
3.4. Extent and magnitude of internalization
Our robust assessment of the internalization phenomenon was enabled thanks to the small size of larvae (approx. 70 um length), allowing a high number of larvae to be imaged on single TEM grids, sectioned as entire organisms. This allowed high-throughput imaging of the biodistribution of NPs at the subcellular level, a condition that is not usually attained by TEM-based studies tracing the biodistribution of NPs over larger individual organisms. The use of larger model organisms generally allows for the analysis of fewer frames carrying small tissue samples taken by biopsy, and hence a more limited insight into the extent of the phenomenon.
Here, the distinctive signs of NP cellular internalization were observed in the majority of larval profiles present on random sections (81.5%, n = 27 larval profiles sectioned); up to 15 particles were counted inside the cellular compartments of a single larva in a 70 nm-thick section. This highlighted a high level of consistency among exposed organisms and the strong potential of oyster larvae to internalize NPs via the alimentary endocytic route.
4. Discussion
This study illustrates the potential of oyster larvae to ingest NPs and internalize them via an alimentary endocytic pathway (). In addition to this evident potential for uptake, oyster larvae showed an intrinsic capacity to deal with internalized NPs, thanks to their evolution within mineral colloid-rich environments. Both ingested NPs and those internalized into cells were excreted from the body with feces. The observation of NP- containing residual bodies inside coelomocytes of the visceral cavity highlighted additional routes for NP trafficking, opening questions around NP translocation and ejection.
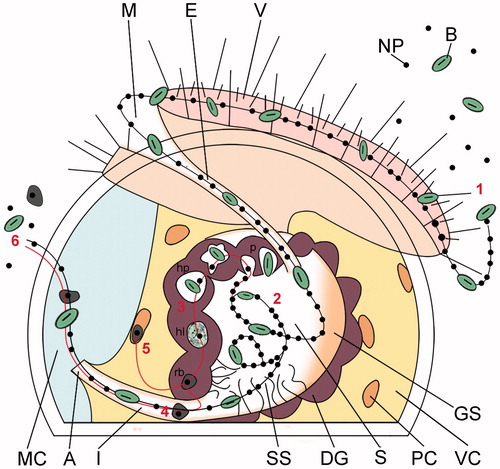
Figure 6. Schematic illustrating the proposed alimentary uptake pathway for NPs in Crassostrea gigas early veliger larvae. Sequential steps. (1) Capture and ingestion. Suspended particles are captured by the cilia of the velum, driven into the mouth and esophagus. (2) Post-ingestive sorting. Once inside the stomach, the ingested particles undergo vigorous swirling by the cilia of the style sac and post-ingestive sorting. Particles are directed toward the digestive gland for their cellular internalization, or toward the intestinal groove for excretion (see ). (3) Cellular uptake and intracellular digestion. Particles are taken up by the absorptive cells of the digestive gland via pinocytosis-macropinocytosis, gathered into heterophagosomes, and digested inside heterolysosomes; NPs, together with the residuals of the intradigestion process, are stored inside residual bodies (). (4) Clearance. NPs and non-nutritious material accumulated inside residual bodies are driven to the intestine for excretion with feces, along with non-absorbed particles sorted for excretion in the stomach (). (5) Translocation to phagocytic coelomocytes. Insoluble residuals of intracellular digestion, including NPs, are translocated to the phagocytic coelomocytes lining the inner membranes of the visceral cavity for processing or ejection via diapedesis (). (6) Excretion. Residuals of intradigestion and post-ingestive sorting are mixed into the fecal laden mucus string and ejected through the anus into the mantle cavity and eliminated from the larval body. A: anus; E: esophagus; DG: digestive gland; GS: gastric shield; I: intestine; M: mouth; MC: mantle cavity; PC: phagocytic coelomocyte; S: stomach; SS: style sac; V: velum; VC: visceral cavity; B: bacterium; NP: nanoparticle; p: pinocyte/macropinocyte; hp: heterophagosome; hl: heterolysosome; rb: residual body.
While some aspects described here may be considered peculiar to larval oysters, the larval digestive system is broadly similar to that reported for adult bivalves which feature a similar filter feeding alimentary strategy and intercellular digestion mechanism, allowing for NP cellular internalization at the digestive gland barrier (Koehler et al. Citation2008; Renault et al. Citation2008; Joubert et al. Citation2013). The main difference is that adult bivalves have a more complex open blood system, pre- and post-ingestive sorting processes and digestive apparatus than larvae (e.g. presence of the crystal style in the style sac, which releases extracellular enzymes assisting the intracellular digestion), and hence it is possible that the overall biodistribution pattern and fluxes of NPs may also be more complex. Larvae are easier to image due to their smaller body dimensions which makes tracing studies less challenging. This, combined with the relative simplicity of their digestive system, makes them a viable model for studying NP biodistribution, allowing toxicological data to be viewed alongside robust evidence of NP cellular uptake.
The ability of the larvae to internalized NPs into cells appears due to the retention of an intracellular digestion mechanism for the processing of particulate food. The larval digestive gland is not simply an assimilation organ for the absorption of the soluble products of the enzymatic digestion occurring in the stomach, as in the case of organisms with extracellular digestion mechanisms. Rather, it serves to absorb and assimilate entire food particles, enabling their internalization, their enzymatic degradation inside the digestive vacuoles (i.e. heterolysosomes), and finally the assimilation of any nutritious component. Thus, this physiological feature makes the larval absorptive cells highly accessible to NPs. This strongly supports the susceptibility of early veliger larvae, and potentially other organisms which rely on intracellular digestion mechanisms for food processing (e.g. Porifera, Cnidaria, Turbellaria, Mollusca, and Echinoidea (Yonge Citation1926a, Citation1931), to be exposed to any NPs that reach their living environment, more so than organisms featuring extracellular digestion or those exposed to NPs via alternative routes. With the exception of Mollusca, these taxa have, however, only rarely been used in in vivo eco-nanotoxicology studies, which more frequently opt for established model animals, such as crustaceans, annelids, and fish (Baker et al. Citation2014). For Porifera and Turbellaria, there are no data concerning the cellular uptake pathways of nanomaterials reported in the literature, according to the best of our knowledge. With respect to cnidarians and echinoderms, NP cellular internalization has been documented only for a few species, such as the freshwater polyp Hydra vulgaris (Marchesano et al. Citation2013), the sea anemone Nematostella vectensis (Ambrosone et al. Citation2014), and larvae and juveniles of the sea urchin Strongylocentrotus droebachiensis (Magesky, Ribeiro, and Pelletier Citation2016). This leaves an important research gap, given the location of these taxa at the base of the aquatic food chain, with important implications in terms of trophic transfer.
We previously observed endocytotic forms similar to those illustrated here in oyster larvae exposed to manganese dioxide (MnO2) NPs, but were unable to trace them throughout the larval body at this level of resolution due to their lower electron density and irregular shape (Noventa et al. Citation2018a, Citation2018b). By considering the substantial diversity of these two nanomaterials (i.e. gold NPs: 100 nm size, spherical, coated by a non-reactive methyl polymer; MnO2 NPs: irregular shape and size, uncoated), this suggests that internalization can proceed in a similar fashion for particles of varied type and thus oyster larvae could theoretically internalize a wide variety of engineered NPs reaching the marine environment. This goes against the generally accepted view of cellular uptake as a selective process highly dependent on shape and surface proprieties of NPs (Kettler et al. Citation2014), and stresses the key importance of the barrier-epithelia in regulating the uptake of NPs. In contrast, this observation agrees with results presented for the cnidarian H. vulgaris, where different physico-chemical proprieties of NPs strongly influenced the efficiency of the internalization process (e.g. by favoring the contact with the absorptive membrane), but did not control the mechanism itself (Marchesano et al. Citation2013).
The gold NPs did not undergo aggregation thanks to a coating that enhanced their stability in seawater. However, for a vast number of uncoated manufactured NPs, the high ionic strength of seawater induces strong aggregation, which can modify their interaction with organisms and thus their effective internalization under in vivo conditions. We observed internalization by absorptive cells of relatively big food particles (i.e. entire bacteria, up to 1–2 µm), suggesting that the range of size of NPs from single particles to aggregates formed under high salinity conditions could be internalized. It is interesting to note that ingested NP aggregates underwent vigorous swirling by the cilia covering the digestive apparatus, leading to the detachment of single particles and hence re-transforming them to a non-aggregated state (see Supplementary video).
The observation of extensive NP internalization by oyster larvae has implications for the ecological risk of the huge variety of NPs that may be released into the environment. Oyster larvae are evidently able to process internalized NPs and eject them (via excretion and diapedesis). Acidic pH and low electrolyte concentrations such as are found within the cellular digestive system can reverse the aggregation of NPs and the adsorption of seawater ions from their surface, typical transformations occurring in the marine environment which can mitigate the toxicological potential of many NPs (Peijnenburg et al. Citation2015). The endo-lysosomal system of the molluskan digestive gland is a noted target of aquatic pollutants, sensitive to a variety of environmental stressors and xenobiotics that are taken up by digestive cells and bioaccumulated (Marigómez et al. Citation1996). This suggests that changes to the morphology and functioning of these cellular organelles, that are used as biomarkers of environmental stress in adult bivalves (i.e. lysosome abundance, alterations to membrane stability, lipofuscin accumulation (Etxeberria, Cajaraville, and Marigomez Citation1995; Domouhtsidou and Dimitriadis Citation2000; Moore et al. Citation2007; Rocha et al. Citation2016)) could have similar utility in larvae for evaluating the cytotoxicity of internalized NPs.
5. Conclusion
In summary, we provide a comprehensive visualization of the toxicokinetics of NPs ingested by oyster larvae, inclusive of mechanistic details of the cellular internalization process and novel translocation scenarios. We conclusively show that the early veliger larvae of oysters can internalize NPs dispersed in the surrounding environment into cells of the digestive gland via intracellular digestive mechanisms. We described the route of NP uptake and the pathways for excretion through which larvae can counterbalance the bioaccumulation of NPs and reduce their stay in the body.
Our mechanistically-supported findings have wider implications for the ecological fate of NPs released to the environment. The endocytic mechanism responsible for the cellular internalization of NPs is conserved in many phyla retaining intracellular digestion mechanisms, and it appears to be effective for the entry of particles of different nature, shape, and size. This suggests a remarkable potential for exposure to manufactured NPs of a wide group of marine organisms (including animals near the base of the aquatic food web) and re-emphasizes the ecological risk posed by NPs reaching the environment for the inhabiting populations.
Supplementary Video
Download MP4 Video (32.2 MB)Supplementary Figures S1-S4
Download MS Word (789.3 KB)Acknowledgements
The authors thank Dylan Windell for his kind support in the experimental work and Hong Chang for technical support in TEM-EDS.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albanese, A., P. S. Tang, and W. C. W. Chan. 2012. “The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems.” Annual Review of Biomedical Engineering 14 (1): 1–16. doi:10.1146/annurev-bioeng-071811-150124.
- Ambrosone, A., V. Marchesano, V. Mazzarella, and C. Tortiglione. 2014. “Nanotoxicology Using the Sea Anemone Nematostella vectensis: From Developmental Toxicity to Genotoxicology.” Nanotoxicology 8 (5): 508–520. doi:10.3109/17435390.2013.802386.
- Baalousha, M., G. Cornelis, T.A.J. Kuhlbusch, I. Lynch, C. Nickel, W. Peijnenburg, and N. W. Van Den Brink. 2016. “Modeling Nanomaterial Fate and Uptake in the Environment: Current Knowledge and Future Trends.” Environmental Science: Nano 3 (2): 323–345. doi:10.1039/C5EN00207A.
- Baker, T. J., C. R. Tyler, and T. S. Galloway. 2014. “Impacts of Metal and Metal Oxide Nanoparticles on Marine Organisms.” Environmental Pollution 186: 257–271. doi:10.1016/j.envpol.2013.11.014.
- Bayne, B. L. 2017. “Reproduction.” Chap. 9 in Biology of Oysters, edited by B. Bayne, 565–701. San Diego, CA: Elsevier.
- Canton, I., and G. Battaglia. 2012. “Endocytosis at the Nanoscale.” Chemical Society Reviews 41 (7): 2718–2739.
- Domouhtsidou, G. P., and V. K. Dimitriadis. 2000. “Ultrastructural Localization of Heavy Metals (Hg, Ag, Pb, and Cu) in Gills and Digestive Gland of Mussels, Mytilus galloprovincialis (L.).” Archives of Environmental Contamination and Toxicology 38 (4): 472–478. doi:10.1039/c2cs15309b.
- Douillet, P. 1993. “Bacterivory in Pacific oyster Crassostrea gigas larvae”. Marine Ecology Progress Series 98: 123–134.
- Elston, R. 1980a. “Functional Anatomy, Histology and Ultrastructure of the Soft Tissues of the Larval American Oyster, Crassostrea virginica.” Proceedings of the National Shellfisheries Association 70: 65–93.
- Elston, R. 1980b. “Functional Morphology of the Coelomocytes of the Larval Oysters (Crassostrea virginica and Crassostrea gigas).” Journal of the Marine Biological Association of the United Kingdom 60: 947–957. doi:10.1017/S0025315400042004.
- Etxeberria, M., M. P. Cajaraville, and I. Marigomez. 1995. “Changes in Digestive Cell Lysosomal Structure in Mussels as Biomarkers of Environmental Stress in the Urdaibai Estuary (Biscay Coast, Iberian Peninsula).” Marine Pollution Bulletin 30 (9): 599–603. doi:10.1016/0025-326X(94)00248-8.
- Gallager, S. M. 1988. “Visual Observations of Particle Manipulation during Feeding in Larvae of a Bivalve Mollusc.” Bulletin of Marine Science 43: 344–365.
- Galtsoff, P. 1964. “Larval Development and Metamorphosis.” Chap. 16 in The American oyster, Crassostrea virginica Gmelin, 355–380. Washington, USA: Fishery Bulletin of the U.S. Fish Wildlife Service–4.
- Hou, W.-C., P. Westerhoff, and J. D. Posner. 2013. “Biological Accumulation of Engineered Nanomaterials: A Review of Current Knowledge.” Environmental Science: Processes and Impacts 15 (1): 103–122. doi:10.1039/C2EM30686G.
- Hull, M. S., P. Chaurand, J. Rose, M. Auffan, J.-Y. Bottero, J. C. Jones, I. R. Schultz, and P. J. Vikesland. 2011. “Filter-Feeding Bivalves Store and Biodeposit Colloidally Stable Gold Nanoparticles.” Environmental Science & Technology 45 (15): 6592–6599. doi:10.1021/es200809c.
- Joubert, Y., J.-F. Pan, P.-E. Buffet, P. Pilet, D. Gilliland, E. Valsami-Jones, C. Mouneyrac, and C. Amiard-Triquet. 2013. “Subcellular Localization of Gold Nanoparticles in the Estuarine Bivalve Scrobicularia plana after Exposure through the Water.” Gold Bulletin 46 (1): 47–56.
- Kadar, E., F. Simmance, O. Martin, N. Voulvoulis, S. Widdicombe, S. Mitov, J. R. Lead, and J. W. Readman. 2010. “The Influence of Engineered Fe2O3 Nanoparticles and Soluble (FeCl3) Iron on the Developmental Toxicity Caused by CO2-Induced Seawater Acidification.” Environmental Pollution 158 (12): 3490–3497. doi:10.1016/j.envpol.2010.03.025.
- Kettler, K., K. Veltman, D. Van De Meent, A. Van Wezel, and A. J. Hendriks. 2014. “Cellular Uptake of Nanoparticles as Determined by Particle Properties, Experimental Conditions, and Cell Type.” Environmental Toxicology and Chemistry 33 (3): 481–492. doi:10.1002/etc.2470.
- Koehler, A., U. Marx, K. Broeg, S. Bahns, and J. Bressling. 2008. “Effects of Nanoparticles in Mytilus edulis Gills and Hepatopancreas – A New Threat to Marine Life?” Marine Environmental Research 66 (1): 12–14. doi:10.1016/j.marenvres.2008.02.009.
- Kwok, K. W. H., M. Auffan, A. R. Badireddy, C. M. Nelson, M. R. Wiesner, A. Chilkoti, J. Liu, S. M. Marinakos, and D. E. Hinton. 2012. “Uptake of Silver Nanoparticles and Toxicity to Early Life Stages of Japanese Medaka (Oryzias latipes): Effect of Coating Materials.” Aquatic Toxicology 120–121: 59–66. doi:10.1016/j.aquatox.2012.04.012.
- Leverett D, T. J. 2013. “Oyster Embryo-Larval Bioassay (Revised).” ICES Techniques in Marine Environmental Sciences No (54): 34.
- Ma, S., and D. Lin. 2013. “The Biophysicochemical Interactions at the Interfaces between Nanoparticles and Aquatic Organisms: Adsorption and Internalization.” Environmental Science: Processes and Impacts 15 (1): 145–160. doi:10.1039/C2EM30637A.
- Magesky, A., CaO. Ribeiro, and É. Pelletier. 2016. “Physiological Effects and Cellular Responses of Metamorphic Larvae and Juveniles of Sea Urchin Exposed to Ionic and Nanoparticulate Silver.” Aquatic Toxicology (Amsterdam, Netherlands) 174: 208–227. doi:10.1016/j.aquatox.2016.02.018.
- Marchesano, V., Y. Hernandez, W. Salvenmoser, A. Ambrosone, A. Tino, B. Hobmayer, J. M De La Fuente, and C. Tortiglione. 2013. “Imaging Inward and Outward Trafficking of Gold Nanoparticles in Whole Animals.” ACS Nano 7 (3): 2431–2442. doi:10.1021/nn305747e.
- Marigómez, I., A. Orbea, I. Olabarrieta, M. Etxeberria, and M. P. Cajaraville. 1996. “Structural Changes in the Digestive Lysosomal System of Sentinel Mussels as Biomarkers of Environmental Stress in Mussel-Watch Programmes.” Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology 113 (2): 291–297. doi:10.1016/0742-8413(95)02100-0.
- Meyer, J. N., C. A. Lord, X. Y. Yang, E. A. Turner, A. R. Badireddy, S. M. Marinakos, A. Chilkoti, M. R. Wiesner, and M. Auffan. 2010. “Intracellular Uptake and Associated Toxicity of Silver Nanoparticles in Caenorhabditis elegans.” Aquatic Toxicology 100 (2): 140–150. doi:10.1016/j.aquatox.2010.07.016.
- Millar, R. H. 1955. “Notes on the Mechanism of Food Movement in the Gut of the Larval Oyster, Ostrea edulis.” Quarterly Journal of Microscopical Science 96: 539–544.
- Moore, M. N., A. Viarengo, P. Donkin, and A. J. S. Hawkins. 2007. “Autophagic and Lysosomal Reactions to Stress in the Hepatopancreas of Blue Mussels.” Aquatic Toxicology 84 (1): 80–91. doi:10.1016/j.aquatox.2007.06.007.
- Noventa, S., C. Hacker, D. Rowe, C. Elgy, and T. Galloway. 2018a. “Dissolution and Bandgap Paradigms for Predicting the Toxicity of Metal Oxide Nanoparticles in the Marine Environment: An in Vivo Study with Oyster Embryos.” Nanotoxicology 12 (1): 63–78.
- Noventa, S., D. Rowe, and T. Galloway. 2018b. “Mitigating Effect of Organic Matter on the in vivo Toxicity of Metal Oxide Nanoparticles in the Marine Environment.” Environmental Science: Nano. doi:10.1039/C8EN00175H.
- Nowack, B. 2017. “Evaluation of Environmental Exposure Models for Engineered Nanomaterials in a Regulatory Context.” NanoImpact 8: 38–47.
- Owen, G. 1970. “The Fine Structure of the Digestive Tubules of the Marine Bivalve Cardium edule.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 258 (822): 245–260.
- Owen, G. 1972. “Lysosomes, Peroxisomes and Bivalves.” Science Progress 60 (239): 299–318.
- Peijnenburg, W. J. G. M., M. Baalousha, J. Chen, Q. Chaudry, F. Von Der Kammer, T.A.J. Kuhlbusch, J. Lead, et al. 2015. “A Review of the Properties and Processes Determining the Fate of Engineered Nanomaterials in the Aquatic Environment.” Critical Reviews in Environmental Science and Technology 45 (19): 2084–2134.
- Renault, S., M. Baudrimont, N. Mesmer-Dudons, P. Gonzalez, S. Mornet, and A. Brisson. 2008. “Impacts of Gold Nanoparticle Exposure on Two Freshwater Species: A Phytoplanktonic Alga (Scenedesmus subspicatus) and a Benthic Bivalve (Corbicula fluminea).” Gold Bulletin 41 (2): 116–126.
- Ringwood, A. H., N. Levi-Polyachenko, and D. L. Carroll. 2009. “Fullerene Exposures with Oysters: Embryonic, Adult, and Cellular Responses.” Environmental Science and Technology 43 (18): 7136–7141.
- Ringwood, A. H., M. Mccarthy, T. C. Bates, and D. L. Carroll. 2010. “The Effects of Silver Nanoparticles on Oyster Embryos.” Marine Environmental Research 69: S49–S51.
- Rocha, T. L., S. M. T. Sabóia-Morais, and M. J. Bebianno. 2016. “Histopathological Assessment and Inflammatory Response in the Digestive Gland of Marine Mussel Mytilus galloprovincialis Exposed to Cadmium-Based Quantum Dots.” Aquatic Toxicology 177: 306–315.
- Schultz, C., K. Powell, A. Crossley, K. Jurkschat, P. Kille, A. J. Morgan, D. Read, et al. 2015. “Analytical Approaches to Support Current Understanding of Exposure, Uptake and Distributions of Engineered Nanoparticles by Aquatic and Terrestrial Organisms.” Ecotoxicology 24 (2): 239–261.
- Selck, H., R. D. Handy, T. F. Fernandes, S. J. Klaine, and E. J. Petersen. 2016. “Nanomaterials in the Aquatic Environment: An EU-USA Perspective on the Status of Ecotoxicity Testing, Research Priorities and Challenges Ahead.” Environmental Toxicology and Chemistry 35 (5): 1055–1067.
- Unrine, J. M., O. V. Tsyusko, S. E. Hunyadi, J. D. Judy, and P. M. Bertsch. 2010. “Effects of Particle Size on Chemical Speciation and Bioavailability of Copper to Earthworms (Eisenia fetida) Exposed to Copper Nanoparticles.” Journal of Environmental Quality 39: 1942–1953.
- Waller, T. R. 1981. “Functional Morphology and Development of Veliger Larvae of the European Oyster, Ostrea edulis Linné.” Washington, USA: Smithsonian Institution Press.
- Wikfors, G. H., and R. M. Smolowitz. 1995. “Experimental and Histological Studies of Four Life-History Stages of the Eastern Oyster, Crassostrea virginica, Exposed to a Cultured Strain of the Dinoflagellate Prorocentrum minimum.” The Biological Bulletin 188 (3): 313–328.
- Wray, A. T., and S. J. Klaine. 2015. “Modeling the Influence of Physicochemical Properties on Gold Nanoparticle Uptake and Elimination by Daphnia magna.” Environmental Toxicology and Chemistry 34 (4): 860–872.
- Yonge, C. M. 1926a. “XV.—the Digestive Diverticula in the Lamellibranchs.” Transactions of the Royal Society of Edinburgh 54 (03): 703–718.
- Yonge, C. M. 1926b. “Structure and Physiology of the Organs of Feeding and Digestion in Ostrea edulis.” Journal of the Marine Biological Association of the United Kingdom 14 (02): 295–386.
- Yonge, C. M. 1931. “Digestive Processes in Marine Invertebrates and Fishes.” J. du Conseil Internat. pour L'Explor. de La Mer 6 (2): 175–212.
- Zhang, S., H. Gao, and G. Bao. 2015. “Physical Principles of Nanoparticle Cellular Endocytosis.” ACS Nano 9 (9): 8655–8671.

