Abstract
Assessment of nanomaterial (NM) induced inflammatory responses has largely relied on rodent testing via measurement of leukocyte accumulation in target organs. Despite observations that NMs activate neutrophil driven inflammatory responses in vivo, a limited number of studies have investigated neutrophil responses to NMs in vitro. We compared responses between the human neutrophil-like HL-60 cell line and human primary neutrophils following exposure to silver (Ag), zinc oxide (ZnO), copper oxide (CuO) and titanium dioxide (TiO2) NMs. NM cytotoxicity and neutrophil activation were assessed by measuring cellular metabolic activity, cytokine production, respiratory burst, and release of neutrophil extracellular traps. We observed a similar pattern of response between HL-60 cells and primary neutrophils, however we report that some neutrophil functions are compromised in the cell line. Ag NMs were consistently observed to stimulate neutrophil activation, with CuO NMs inducing similar though weaker responses. TiO2 NMs did not induce a neutrophil response in either cell type. Interestingly, ZnO NMs readily induced activation of HL-60 cells but did not appear to activate primary cells. Our findings are relevant to the development of a tiered testing strategy for NM hazard assessment which promotes the use of non-rodent models. Whilst we acknowledge that HL-60 cells may not be a perfect substitute for primary cells and require further investigation regarding their ability to predict neutrophil activation, we recommend their use for initial screening of NM-induced inflammation. Primary human neutrophils can then be used for more focused assessments of neutrophil activation before progressing to in vivo models where necessary.
Introduction
Materials at the nanoscale often exhibit unique and beneficial functional properties which are not apparent in their larger counterparts. This has been exploited by various industrial and commercial sectors, and the manufacture of engineered nanomaterials (NMs) with tunable physicochemical characteristics has seen rapid growth in recent years. However, the novel, size-dependent properties of NMs raise concerns regarding their potential risk to human health and the environment.
Evidence linking carbon particles found in air pollution with adverse health effects in humans demonstrates that nanomaterials emitted by anthropogenic activities have the potential to be toxic (Seaton et al. Citation1995). Negative effects resulting from human exposure to engineered NMs have also been reported, for instance inhalation of zinc oxide NMs induces airway inflammation in healthy human volunteers (Monse et al. Citation2019). With the expanding nanotechnology industry, occupational and environmental incidences of human NM exposure are on the rise. As such, there is an urgent need to devise testing strategies that allow for the rapid and reliable screening of NM safety.
It is well established that inflammation is one of the key mechanisms underlying NM toxicity. Multiple in vivo rodent studies have used assessment of the infiltration of immune cells, such as neutrophils, into the target site of interest (e.g. lungs, liver) as an indicator of NM toxicity (Ma-Hock et al. Citation2009; Smulders et al. Citation2014; Gosens et al. Citation2015). Neutrophils are part of the front-line of host defence against intruding pathogens and foreign bodies and perform a multitude of protective functions including phagocytosis, degranulation, respiratory burst response, cytokine production, and the release of neutrophil extracellular traps (NETs) (reviewed by Johnston et al. Citation2018). However, whilst activation of neutrophils is a normal physiological response to foreign bodies and pathogens, excessive, prolonged neutrophil activation has the potential to inflict tissue damage.
While in vivo experiments provide a holistic understanding of the toxicity of NMs, in vitro methods allow for detailed and controlled mechanistic studies to be performed at the cellular level. Furthermore, cheaper and faster cell-based studies also support the effort to reduce, replace and refine the use of laboratory animals; the optimization and promotion of in vitro techniques should contribute toward the 3Rs principal (Russell and Burch Citation2005). Whilst several in vitro studies have investigated neutrophil responses, most of these have typically relied on the use of primary human cells and investigated a limited number of NMs and endpoints. As part of the effort to promote the more widespread adoption of alternative, non-rodent models for the study of NM toxicity, we compare the response of two in vitro neutrophil cell models to a panel of NMs: primary human blood neutrophils, and differentiated cells from the neutrophil-like HL-60 cell line.
Primary human neutrophils are practically challenging to work with owing to their short lifespan, health and safety concerns surrounding the use of human blood, susceptibility to priming and activation during isolation, and results that are typically subject to a high degree of inter-donor variability (Dransfield and Rossi Citation2004; Rossi, Ward, and Dransfield Citation2004). The use of immortalized HL-60 cells can overcome these issues; however, as a transformed cell line they may respond differently to primary human cells. The aim of this study was therefore to compare and validate responses between the HL-60 cell line and primary human neutrophils to identify the benefits and limitations of each model for nanotoxicology studies.
In the present study, primary human neutrophils and differentiated HL-60 cells were exposed to silver (Ag), zinc oxide (ZnO), copper oxide (CuO), and titanium dioxide (TiO2) NMs, and concentration-dependent responses measured for a selection of functions that are indicative of neutrophil activation. In the first instance, the impact of NMs on cell metabolic activity was used to rank NM toxicity, and to determine sub-lethal NM concentrations for which to study additional neutrophil responses. We then investigated the release of selected cytokines (interleukin (IL)-8, macrophage chemotactic protein (MCP)-1, growth regulated oncogene alpha (GRO-α), IL-1 receptor antagonist (IL-1Ra) in response to NM exposure. Cytokines are signaling proteins, some of which can be released by activated neutrophils during inflammation, and play a crucial role in modulating the inflammatory response (Tecchio, Micheletti, and Cassatella Citation2014; Tamassia et al. Citation2018). IL-8, MCP-1, and Gro-α are chemotactic cytokines that are primarily involved in the activation and recruitment of cell populations (e.g. neutrophils, macrophages) to the site of inflammation, while IL-1Ra inhibits pro-inflammatory activity of IL-1 cytokines and is therefore involved in dampening and resolving the inflammatory response. Activation of a respiratory burst and the generation of reactive oxygen species (ROS) is a hallmark of neutrophil activation, and we therefore measured the extracellular release of superoxide anion (O2•−) following NM exposure. The release of NETs is a process in which activated neutrophils eject web-like structures comprised of DNA, histones, and granular antimicrobial proteins, designed to trap and destroy extracellular pathogens. This novel form of programmed cell death termed NETosis is a relatively recent discovery in the field of neutrophil biology, yet there is already emerging evidence to show that NET release plays a role in NM-mediated inflammation (Yang et al. Citation2019). Given that literature regarding NM-induced NETosis is limited, we also explored the capacity of the investigated NM panel to trigger NET release in vitro, as this information may inform screening strategies for neutrophil responses to NMs in the future. NET formation by HL-60 cells is more challenging to investigate due to their suspension nature, and thus only the response of primary cells was explored. Finally, to compare the phagocytic function between HL-60 cells and primary neutrophils, we investigated the uptake of fluorescently labeled NMs.
We hypothesize that we will observe a similar pattern of NM-induced toxicity and neutrophil activation in our in vitro models as has been reported by in vivo rodent studies of neutrophilic inflammation following NM exposure. It is envisioned that our findings will inform model and endpoint selection for future studies which investigate neutrophil responses to NMs in vitro as part of a tiered testing strategy.
Materials and methods
All materials were purchased from Sigma-Aldrich (Poole, UK), unless stated otherwise.
Preparation of NM suspensions
A panel of NMs with different physico-chemical characteristics was selected for investigation. Ag (NM-300K), ZnO (NM-110), and TiO2 (NM-105) NMs were sourced from the Joint Research Center (JRC) nanomaterials repositoryFootnote1 and supplied by the Fraunhofer Institute (Munich, Germany). CuO NMs from Plasma Chem (Berlin, Germany) were kindly gifted from the Sustainable Nanotechnologies (SUN) EU project. All NMs were supplied as powders, with the exception of Ag which was supplied as a 10.16% w/w aqueous dispersion containing stabilizing agents (4% glycerol trioleate and 4% Tween 20). The physico-chemical properties of the selected NMs have been reported previously and are summarized in .
Table 1. Characteristics of the investigated NM panel.
NMs were prepared using the dispersion protocol devised as part of the ENPRA project (Jacobsen et al. Citation2010). Briefly, NMs were made up as 1 mg/mL suspensions in 2% fetal calf serum (FCS) in sterile H2O. Samples were vortexed, sonicated for 16 min in an ultrasonic bath, and placed on ice. NMs were then diluted to the required concentrations in complete cell culture medium and used immediately.
HL-60 cell culture
The human acute myeloid leukemia suspension cell line, HL-60, was used as a neutrophil model. HL-60 cells were cultured in culture medium (RPMI 1640 containing 2 mmol/L l-glutamine, 100 U/mol penicillin streptomycin) supplemented with 10% heat-inactivated FCS, and maintained at 37 °C in an atmosphere of 5% CO2. Cells were differentiated to a neutrophil-like phenotype by incubating with 1 µM all-trans-retinoic acid for 48 h at a density of 2 × 105 cells/mL, as previously described (Brown et al. Citation2010). In preparation for assays, differentiated HL-60 cells (dHL-60) were seeded in multi-well plates and incubated for 1 h prior to treatment to allow cells to settle at the bottom of the wells. All dHL-60 cell incubations were performed at 37 °C in 5% CO2, unless stated otherwise.
Isolation of primary neutrophils from human blood
Neutrophils were obtained from human blood donated by consenting, healthy volunteers, conducted in accordance with, and approved by, the Research Ethics Committee of Heriot-Watt University (Edinburgh, UK). Peripheral blood was drawn from the antecubital vein of donors and collected into tubes containing 3.8% sodium citrate to prevent coagulation. Neutrophils were isolated using the percoll density gradient centrifugation method, as described previously (Haslett et al. Citation1985). Cytocentrifuge preparations of harvested leukocytes were stained with Diff-Quik (Rapid Romanowsky stain) for the morphological assessment of neutrophil purity, and only populations containing ≥ 95% neutrophils were used. From this point forward, neutrophils isolated from human blood will be referred to as primary neutrophils. In preparation for assays, primary neutrophils were seeded in multi-well plates in serum-free culture medium (RPMI 1640 containing 2 mmol/L l-glutamine and 100 U/mol penicillin streptomycin) and incubated for 1 h at 37 °C in 5% CO2 to allow cell adhesion. Unless stated otherwise, all subsequent incubations with neutrophils were performed using cell culture medium containing 10% autologous donor serum, at 37 °C and 5% CO2.
Cell metabolic activity
dHL-60 cells and freshly isolated primary neutrophils were seeded in 96-well plates at a density of 1 × 105 cells/well (50 µL/well). NMs were added to wells (50 µL/well) at final concentrations ranging between 0.98 and 125 µg/mL. Untreated, negative control cells were incubated with culture medium only, and cells treated with Triton X-100 (0.1%) were included as a positive control for cell death. Cells were exposed to treatments for 20 h, after which time the supernatants were removed and reserved for subsequent analysis (stored at −80 °C). Cells were washed in PBS, and cellular metabolic activity assessed using the alamarBlue assay; a 0.01 mg/mL resazurin solution was prepared in cell culture medium and added to cells at a volume of 100 µL/well. Following incubation for 3.5 h, supernatants were transferred to a fresh 96-well plate and fluorescence intensity measured on a Tecan Spark microplate reader (560/590 nm excitation/emission wavelength). Cellular metabolic activity was expressed as a percentage of the negative, untreated control cells. In subsequent assays, NMs were tested at a range of sub-lethal concentrations centered around the inhibitory concentration 20% (IC20, the concentration required to reduce the metabolic activity of cells by 20%). NMs that did not impact metabolic activity (TiO2 in both cell types, ZnO in primary neutrophils) were tested at the upper end of the original concentration range i.e. up to 125 µg/mL.
Assessment of cell morphology using light microscopy
The morphology of dHL-60 cells and primary neutrophils was assessed using light microscopy following exposure to Ag, CuO, TiO2 and ZnO NMs. Primary neutrophils were adhered onto glass coverslips in 24-well plates at a density of 5.8 × 105 cells/well, and exposed to NMs for 4 h at a range of concentrations based on their individual IC20 values. Cells were then washed with PBS, fixed and stained using Diff-Quik, and finally mounted onto glass slides using dibutylphthalate polystyrene xylene (DPX) mounting medium, ready for imaging. dHL-60 cells in suspension do not adhere to glass or plastic surfaces and were therefore added to 24-well plates without coverslips and treated as described for primary neutrophils. Following treatment and washing, dHL-60 cells were cytocentrifuged onto slides, stained with Diff-Quik, and sealed with coverslips using DPX mounting medium ready for imaging.
Cytokine production
GRO-α, MCP-1, IL-8 and IL-1Ra cytokines were measured in the cell supernatants generated by the metabolic activity assays (20 h post-exposure to NMs), using Bio-Plex human cytokine kits in accordance with manufacturer guidelines (Bio-Rad, Hercules, CA, USA). As a positive control, cells were exposed to 0.1 µg/mL phorbol 12-myristate 13-acetate (PMA), and negative control cells were exposed to cell culture medium only. Cytokine levels were measured on a Bio-Plex MAGPIX multiplex reader, and expressed in pg/mL.
Extracellular superoxide anion production
The extracellular release of superoxide anions (O2•−) was measured using the cytochrome C reduction assay as previously described (Johnston et al. Citation2015). A reaction mixture containing cytochrome C, d-glucose and PBS was freshly prepared (1:2:1 ratio) and kept on ice in the dark prior to use. dHL-60 cells and primary neutrophils were seeded in 24-well plates at a density of 5.8 × 105 cells/well, (50 µL/well). Ag, ZnO, CuO and TiO2 NMs were added to wells at selected sub-lethal concentrations (50 µL/well), followed immediately by the addition of 900 µl reaction mixture to each well (final ratio of medium:NMs:reaction mix of 1:1:18). In order to adjust for any O2•− production by NMs, the assay was also performed in the absence of cells. Untreated control cells received reaction mix only, and positive control cells were exposed to 0.1 µg/mL PMA which is known to stimulate O2•− production. To confirm that the assay was measuring O2•− production, an additional control was performed by incubating PMA-treated cells with superoxide dismutase (SOD): an inhibitor of cytochrome C reduction. Following 2 h exposure, supernatants were transferred to fresh 96-well plates, and absorbance measured at 550 nm on a microplate reader. The level of cytochrome C reduction was calculated by subtracting the absorbance measured in the presence of cells from that in their absence, and data expressed as nMoles of O2•− production using a molar extinction coefficient for cytochrome C of 21.1 mM−1cm−1. CuO NMs were found to interfere with reagents of this assay and were therefore not included in the analysis.
Cellular uptake of fluorescent beads
The uptake of fluorescently labeled latex beads was used to determine whether dHL-60 cells and primary neutrophils varied in their ability to internalize NMs. Primary neutrophils were seeded in optical bottom 96-well plates at a density of 1 × 105 cells/well, and incubated with 0.1 µm red fluorescent carboxylate-modified microspheres (FluoSpheres™, 580/605 nm excitation/emission; ThermoFisher Scientific, Waltham, MA, USA) for 2 h. dHL-60 cells in suspension were incubated with microspheres in regular 96-well plates under the same conditions, and subsequently cytocentrifuged onto glass slides in preparation for counterstaining. Primary neutrophils and dHL-60 cells were then washed in PBS and fixed overnight in 4% methanol-free paraformaldehyde (PFA) at 4 °C. Following fixation, cells were washed, permeabilized for 10 min using 0.1% Triton X-100 and washed again. Cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated phalloidin for 30 min at room temperature for cytoplasmic actin staining. After a final wash step, cells were sealed with ProLong Diamond mounting medium formulated with DAPI (4′, 6-diamidino-2-phenylindole) for DNA staining. Z-stacks through cells were acquired on an Andor Revolution XDi spinning disk confocal microscope using a 100× oil immersion objective (Olympus, Tokyo, Japan). 405 nm (diode), 488 nm (Argon) and 561 nm (HeNe) laser lines were used to excite DAPI, FITC, and the fluorescently labeled NMs respectively, and images captured on an Andor EMCCD camera.
Neutrophil extracellular trap formation
Neutrophil Extracellular Trap (NET) release in response to NM exposure was visualized in primary neutrophils using antibodies directed against neutrophil elastase, a primary component of NETs. As a suspension cell line, dHL-60 cells require cytocentrifugation to adhere to glass surfaces. Since this process is likely to destroy NETs that may have formed during NM exposure, it was not possible to visualize NET release in this cell line using the methods described.
Primary neutrophils were seeded in optical glass-bottom 96-well plates at a density of 1 × 105 cells/well and exposed to Ag, ZnO, CuO and TiO2 NMs at a range of concentrations (31.25–125 µg/ml) for 2, 4 and 6 h, as previously described. All subsequent incubation and wash steps were performed gently to avoid damage to any NETs that had been released during NM exposure. Cells were washed in PBS and fixed overnight in 4% PFA at 4 °C. Following a PBS wash, cells were blocked for 30 min in UltraCruz blocking reagent (Santa Cruz Biotechnology, Dallas, TX, USA), and incubated with neutrophil elastase primary antibodies (1:100 dilution, mouse monoclonal IgG1; Santa Cruz Biotechnology) overnight at 4 °C. Cells were then washed and incubated with FITC-conjugated secondary antibodies (1:200 dilution, mouse IgGК BP-FITC; Santa Cruz Biotechnology) for 1 h at room temperature. Cells were given a final wash and sealed with ProLong Diamond mounting medium containing DAPI for DNA staining. Confocal z-stacks through cells were acquired on spinning disk confocal microscope using a 100× oil immersion objective and presented as maximum-intensity projections.
Data analysis and presentation
All results are presented as mean values determined from a minimum of 3 biological replicates, ± the standard error of the mean (SEM). All experiments were performed in triplicate and repeated on at least three separate days. Statistical analysis was performed using GraphPad Prism 5, and details are provided in the figure legends for individual experiments. Significance was set at p < 0.05.
Results
Impact of NMs on the metabolic activity of dHL-60 cells and primary neutrophils
We compared the metabolic activity of dHL-60 cells and primary neutrophils following a 20 h exposure to a panel of NMs (Ag, ZnO, TiO2, CuO) at concentrations ranging between 0.98 and 125 µg/mL. A decline in the mitochondrial metabolic activity of cells is indicative of reduced cell viability. Therefore, the measured metabolic activity of treated cells was expressed as a percentage of untreated controls and used as a proxy for cell viability. Results are shown in .
Figure 1. Metabolic activity of primary neutrophils and dHL-60 cells following NM exposure. Primary neutrophils (black lines) and dHL-60 cells (grey lines) were exposed to Ag (A), ZnO (B), CuO (C) and TiO2 (D) NMs for 20 h at concentrations ranging between 0.98 μg/mL and 125 μg/mL. Untreated control cells were exposed to cell culture medium only. Cellular metabolic activity was assessed using the alamarBlue assay and expressed as a percentage of the untreated controls (% cell metabolic activity) (n = 3–5). Error bars indicate SEM. Significant differences between dHL-60 cells and primary neutrophils are indicated by *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA followed by post-hoc Bonferroni test). To further assess the impact of Ag, ZnO, CuO and TiO2 NMs on cell viability, the morphology of cells was investigated. Untreated (UT) and NM-treated cells were fixed, stained and visualized using light microscopy. Representative images are shown of dHL-60 cells (upper panels) and primary neutrophils (lower panels) following a 4 h exposure to NMs (E). Scale bar = 100 µm. IC50 values were calculated and used to rank the toxicity of NMs for both cell types (F).
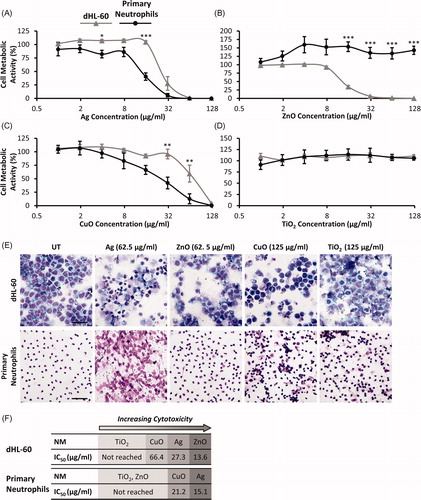
Exposure to Ag NMs had a significant impact on the metabolic activity of dHL-60 cells and primary neutrophils, with concentrations over 62.5 µg/mL resulting in complete cell death in both cell types (). dHL-60 metabolic activity was also impacted by ZnO NMs and reduced to 35% at a concentration of 15.6 µg/mL. Complete cell death was observed at ZnO NM concentrations ≥62.5 µg/mL. In contrast, primary neutrophil metabolic activity was not reduced by exposure to ZnO NMs (). CuO NMs were found to reduce the metabolic activity of both cell types in a concentration-dependent manner, though primary neutrophils were more sensitive to the effects of exposure, as shown in . For example at a concentration of 62.5 µg/mL CuO NMs reduced metabolic activity to 13% for primary neutrophils versus 59% in dHL-60 cells. TiO2 NMs had no effect on the metabolic activity of either cell type at the concentrations tested ().
Given the short lifespan of primary neutrophils in culture (>50% apoptotic cell death following 20 h in culture; Taylor et al. Citation2007), it is important to note that a high proportion of primary cells in the untreated control group will have undergone apoptosis following the 20 h assay duration. Shorter time-points (e.g. 12 h) were not compatible with this assay due to practical considerations, specifically the cumulative time required for neutrophil isolation, NM incubation and alamarBlue treatment. In light of this, cell morphology was visualized 4 h post-exposure to NMs to confirm the findings of metabolic activity assays and further assess impacts of NMs on cell viability. Results are shown in . In dHL-60 cells, exposure to Ag, ZnO and CuO NMs resulted in a loss of cells compared with untreated controls, which is indicative of cell death. For these treatments the majority of remaining viable cells showed signs of damage. TiO2 NMs did not affect the number or morphology of dHL-60 cells. Primary neutrophils exposed to Ag NMs showed severe morphological changes, and the appearance of DNA in the extracellular environment suggests that these NMs may stimulate NET release. The remaining treatments did not affect the number of primary neutrophils or their morphology.
The inhibitory concentration 50% (IC50, the concentration required to reduce the metabolic activity of cells by 50%) was determined for each NM, and used to rank the toxicity of the NM panel for both cell types (). With the exception of ZnO NMs the overall ranking of NM toxicity was comparable in both cell types (), though primary neutrophils were typically more sensitive to the effects of NM exposure i.e. they responded to lower NM concentrations than dHL-60 cells. Accordingly, for both cell types, Ag and CuO NMs were found to be toxic, whilst TiO2 NMs were nontoxic. ZnO NMs were toxic to the cell line but not the primary cells. Data from cell metabolic activity assays were used to calculate sub-lethal NM concentrations with which to investigate responses that are indicative of neutrophil activation or NM-mediated toxicity to neutrophils in subsequent assays.
Release of pro- and anti-inflammatory cytokines by dHL-60 cells and primary neutrophils in response to NMs
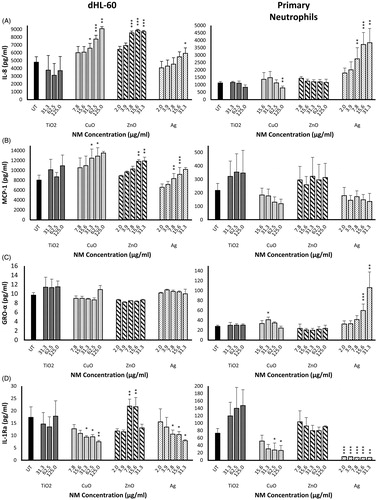
We measured the production of pro-inflammatory cytokines IL-8, MCP-1 and GRO-α, and the anti-inflammatory cytokine IL-1Ra by primary neutrophils and dHL-60 cells exposed to sub-lethal concentrations of Ag, ZnO, CuO and TiO2 NMs for 20 h. Results are shown in . Compared with untreated controls, there was no change in the production of cytokines in either cell type following exposure to TiO2 NMs. Exposure to Ag and CuO NMs resulted in a concentration-dependent increase in the release of pro-inflammatory cytokines in both dHL-60 cells (IL-8 and MCP-1) and primary neutrophils (IL-8 and GRO-α), alongside a concentration-dependent reduction in the secretion of the anti-inflammatory IL-1Ra (). Similarly, ZnO NMs stimulated a concentration-dependent increase in the production of IL-8 and MCP-1 by dHL-60 cells (), and we also observed an increase in IL-1Ra levels at ZnO NM concentrations of 7.8 and 15.6 µg/mL (). In contrast, we did not observe any change in the levels of pro- or anti-inflammatory cytokines released by primary neutrophils exposed to ZnO NMs. Gro-α levels in dHL-60 cells () and MCP-1 levels in primary neutrophils () were not affected in response to any of the tested NMs, when compared with untreated controls.
Figure 2. Production of pro-inflammatory cytokines IL-8, MCP-1 and GRO-α, and the anti-inflammatory cytokine IL-1Ra by primary neutrophils and dHL-60 cells 20 h post-exposure to NMs. dHL-60 cells (left panel) and primary neutrophils (right panel) were exposed to Ag, ZnO, CuO or TiO2 NMs for 20 h. Untreated (UT) control cells were exposed to cell culture medium only. Cell supernatants were analyzed for the presence of IL-8 (A), MCP-1 (B), GRO-α (C) and IL-Ra (D) using Bio-Plex human cytokine kits. Data are expressed as mean cytokine levels of production (pg/mL) (n = 3–5). Error bars indicate SEM. Significant differences with untreated controls are indicated by *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA followed by post-hoc Bonferroni test).
To ensure cytokine responses observed in primary neutrophils were not the result of atypical cells surviving the 20 h experimental duration, cytokine levels were measured following a 6 h exposure to Ag, ZnO and CuO NMs, revealing a similar pattern of production (Supplementary Figure 1).
NM-mediated activation of respiratory burst in dHL-60 cells and primary neutrophils
We investigated the respiratory burst response in dHL-60 cells and primary neutrophils following exposure to Ag, ZnO, and TiO2 NMs. CuO NMs have been shown to generate ROS (Angelé-Martínez et al. Citation2017), and we were unable to distinguish between superoxide anion (O2•−) released by cells from that produced by the CuO NMs themselves. Due to this assay interference, CuO NMs were not included in the analysis. The extracellular release of O2•− by cells was measured 2 h post-exposure to sub-lethal NM concentrations. Results are shown in .
Figure 3. Extracellular superoxide anion production by primary neutrophils and dHL-60 cells 2 h post-exposure to NMs. Primary neutrophils (left panel) and dHL-60 cells (right panel) were exposed to either Ag (A), ZnO (B) or TiO2 (C) NMs at sub-lethal concentrations. O2•− production was measured using the cytochrome C reduction assay. Results are expressed as the average level of O2•− production (nMoles/mL) (n = 3–5). Error bars indicate SEM. Significant differences with untreated (UT) controls are indicated by *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA followed by post-hoc Bonferroni test).
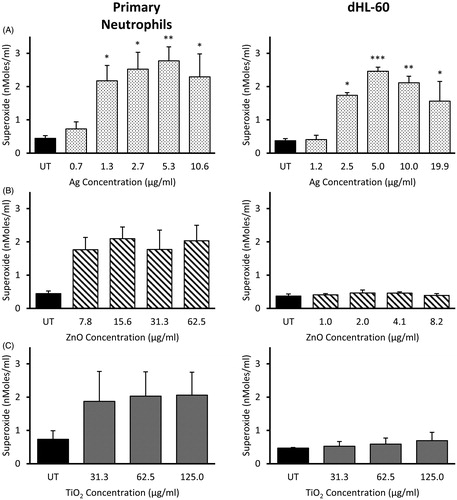
The positive PMA control induced a respiratory burst response in both dHL-60 cells and primary neutrophils (data not shown). We did not observe any increase in O2•− release by either primary neutrophils or dHL-60 cells following a 2 h exposure to TiO2 or ZnO NMs, when compared with untreated controls (). Ag NM exposure resulted in a concentration-dependent increase in O2•− production, detectable from 1.3 µg/mL in primary neutrophils and 2.5 µg/mL in dHL-60 cells and peaking at ∼5 µg/mL in both cell types ().
NET release by primary neutrophils in response to NM exposure
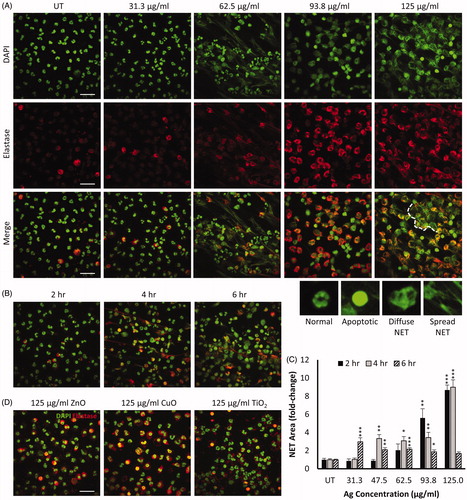
To determine whether NM exposure resulted in NET formation, primary neutrophils were treated with increasing concentrations of Ag, CuO, ZnO and TiO2 NMs, and subsequently stained for nuclear DNA and the NET-marker neutrophil elastase. Immunostained cells were then imaged using confocal fluorescence microscopy, and identified qualitatively as healthy, apoptotic, or NETs which were further sub-categorized as having either ‘diffuse’ or ‘spread’ morphology (Yan et al. Citation2012).
Qualitative assessment of primary neutrophils following 4 h exposure to Ag NMs revealed a concentration-dependent release of NETs (). At the lowest concentration tested (31.3 µg/mL), the majority of neutrophils retained their characteristic multi-lobed nuclear morphology as seen in untreated control neutrophils (). Upon exposure to Ag NMs at a concentration of 62.5 µg/mL, we observed the presence of NETs, the majority of which had a spread morphology. Neutrophils with either intact or apoptotic nuclei were also observed. All neutrophils had lost their multi-lobed nuclear structure following exposure to Ag NMs at a concentration of 93.7 µg/mL and appeared to have formed diffuse NETs. At the highest tested concentration of 125 µg/mL Ag NMs, NET production was widespread and larger, aggregate structures had formed. NET production was quantified in ImageJ based on the neutrophil elastase signal, which confirmed a concentration-dependent increase in NET formation in response to Ag NMs (). Time-dependent NET formation was also observed in response to Ag NMs (47.5 µg/mL) as shown in and Supplementary Figure 2, with the peak of the response observed at 4 h. We did not observe any evidence of NET production in primary neutrophils exposed to ZnO, CuO or TiO2 NMs, at the concentrations and time-points tested ().
Figure 4. NM-induced release of neutrophil extracellular traps (NETs) in primary neutrophils immunostained for nuclear DAPI (green) and neutrophil elastase (red). Representative images of primary neutrophils following 4 h exposure to increasing concentrations of Ag NMs are presented (A). Fixed and stained cells were imaged using confocal microscopy and qualitatively categorized as either normal (multi-lobed nuclei), apoptotic (condensed nuclei), or NETs which were further sub-categorized as having either ‘diffuse’ or ‘spread’ morphologies. Examples of neutrophil morphologies are shown. Aggregation of NETs resulting in the formation of larger structures is visible when primary neutrophils were exposed to 125 µg/mL Ag NMs, as indicated by white dashed line. Primary neutrophils exposed to 46.5 µg/mL Ag showed increase NET formation over time (B). Concentration and time-dependent NET release in primary neutrophils exposed to Ag NMs was quantified based on neutrophil elastase signal and expressed as a fold-change relative to untreated (UT) controls (n = 3–5) (C). Error bars indicate SEM. Significant differences with untreated controls are indicated by *p < 0.05, **p < 0.01, ***p < 0.001 (ANOVA followed by post-hoc Dunn’s multiple comparison test). NET formation was not observed in cells exposed to ZnO, CuO or TiO2 NMs at any of the tested concentrations (31.25–125 µg/ml) (D). Scale bar = 30 µm.
NM uptake by primary neutrophils and dHL-60 cells
To investigate the uptake of NMs by primary neutrophils and dHL-60 cells, cells were incubated with 100 nm fluorescent beads for 2 h, and subsequently fixed and counterstained for nuclei and cytoplasmic actin filaments. Cells were imaged by confocal fluorescence microscopy and results presented in .
Figure 5. Uptake of 100 nm fluorescently labeled NMs by primary neutrophils and dHL-60 cells. Primary neutrophils (A) and dHL-60 cells (B) were incubated with 100 nm fluorescent carboxylate-modified NMs (FluoSpheres™) (red) for 2 h, fixed and counterstained with phalloidin (green) and DAPI (blue) for visualization of cell nuclei and cytoplasmic actin respectively. Confocal Z-stacks were acquired, and orthogonal (XZ and YZ) projections used to confirm the internalization of particles by cells, highlighted by white arrows. Images shown are representative. Scale bar = 10 µm.
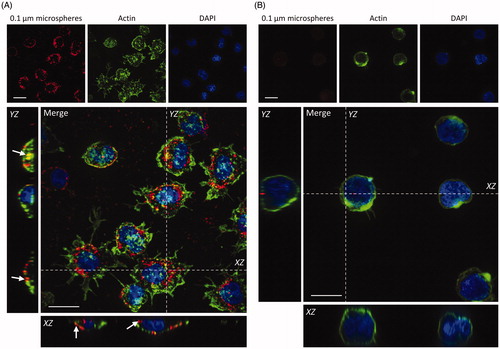
NMs were internalized into the cytoplasm of primary neutrophils, as shown in . This was confirmed by the co-localization of fluorescence signal from NMs and cytoplasmic actin, which is visible in the orthogonal projections of confocal z-stacks. Conversely, we observed minimal uptake of NMs by differentiated HL-60 cells following a 2 h incubation ().
Discussion
With the increasing ubiquity of NMs in our environment, it is essential that the generation of toxicology data regarding NM safety matches the pace of the developing nanotechnology industry. To date, numerous studies have demonstrated that one of the primary mechanisms underlying NM toxicity is the activation of inflammatory responses.
Inflammation is our fundamental defence mechanism against harmful stimuli, and involves an orchestrated response between several different cell types to protect and restore the site of injury. Neutrophils are our most abundant circulating leukocytes and are amongst the first responders to inflammation. Upon activation, neutrophils can elicit a broad spectrum of functions designed to capture and neutralize the invading pathogen, such as phagocytosis, degranulation, respiratory burst response, cytokine production, and the release of NETs. However, neutrophils have been described as the ‘double-edged swords’ of immunity given that they have the potential to inflict tissue damage if inflammation is not resolved (Parkos Citation2016).
Much of our knowledge regarding the inflammogenicity of NMs is derived from in vivo rodent studies, however their ability to predict human effects is limited due to species differences. Furthermore, there is a drive within the scientific community to seek out alternative, non-rodent models to align toxicology testing with the principles of the 3Rs i.e. the Replacement, Reduction & Refinement of animal testing (Russell and Burch Citation2005; Holmes, Creton, and Chapman Citation2010; Burden et al. Citation2015). Given the growing number of NMs with diverse physico-chemical characteristics (e.g. particle size, shape, charge, surface chemistry and aggregation), the use of in vitro models is especially appealing as they offer high-throughput and rapid testing solutions for the assessment of NM toxicity.
In spite of their pivotal role in inflammation, research dedicated to the neutrophil response to NMs in vitro is relatively sparse in contrast with other cell types such as macrophages (Johnston et al. Citation2018; Keshavan et al. Citation2019). Working with neutrophil-like cell lines can address many of the challenges surrounding the use of human primary neutrophils (e.g. short lifespan, donor pool limitations, ethical concerns), and typically are more accessible, cost-effective, comparatively easier to work with and more amenable to high-throughput testing strategies. However, there are concerns regarding the physiological relevance of cell lines, and as such it is important to demonstrate that they exhibit functional features that resemble the target cell type as closely as possible. Here, we compare NM-mediated responses between primary neutrophils isolated from human blood, and neutrophil-like cells derived from the human leukemia HL-60 cell line, for a selected panel of NMs.
Inflammatory responses by dHL-60 cells and primary neutrophils to the investigated NM panel
In the first instance, the cytotoxicity of the NM panel to both cell types was investigated via assessment of changes in metabolic function. Overall, our data have shown a similar patten of NM toxicity between primary neutrophils and dHL-60 cells (Ag > CuO > TiO2), with ZnO NMs being a notable exception. Similar rankings in the toxicity of these NMs have been reported by other studies (Bondarenko et al. Citation2013; Kermanizadeh et al. Citation2013; Smulders et al. Citation2014; Giovanni et al. Citation2015; Brzicova et al. Citation2019), most frequently of the order Ag > ZnO > CuO > TiO2.
Though TiO2 has long been considered a biologically inert material with low toxicity, studies have shown that exposure to TiO2 NMs can elicit toxic responses such as inflammation, apoptosis, and oxidative stress (Skocaj et al. Citation2011; Baranowska-Wójcik et al. Citation2020). Of interest to this study is the fact that increased numbers of neutrophils have been reported in the bronchoalveolar lavage fluid (BALF) of rodents following pulmonary exposure to TiO2 NMs, indicating pulmonary toxicity (Sager, Kommineni, and Castranova Citation2008; Ma-Hock et al. Citation2009). Our results showed that TiO2 NMs did not have any significant impact on the metabolic activity of primary neutrophils or dHL-60 cells, and did not induce pro-inflammatory responses with respect to cytokine secretion, respiratory burst, or NET release. These findings align with existing in vitro studies that have shown TiO2 NMs have no effect on the viability of human primary neutrophils (Hedenborg Citation1988; Gonçalves, Chiasson, and Girard Citation2010). Further investigation by Gonçalves, Chiasson, and Girard (Citation2010) revealed that TiO2 NMs in fact delayed apoptosis of human primary neutrophils; an effect with potentially harmful consequences in vivo, if persisting neutrophils release toxic substances into the surrounding tissue.
Owing to their broad range of applications, Ag NMs are amongst the most widely used NMs in commercial and industrial products, and accordingly many studies have investigated their cytotoxic potential (Akter et al. Citation2018). In vivo, Ag NMs have been reported to cause inflammatory effects in multiple organs and tissues, including the lung (Arai, Miyayama, and Hirano Citation2015; Gan et al. Citation2020), liver (Gaiser et al. Citation2013; Roda et al. Citation2019), heart (Ferdous et al. Citation2019), and brain (Xu et al. Citation2015). In vitro, the cytotoxicity of Ag NMs has been largely attributed to oxidative stress, DNA damage, and pro-inflammatory responses (reviewed by Stensberg et al. Citation2011; Kumar, Sharma, and Maitra Citation2017; Akter et al. Citation2018; Cameron, Hosseinian, and Willmore Citation2018, Johnston et al. Citation2018). In the present study, Ag NMs were overall the most potent inducers of pro-inflammatory responses by primary neutrophils and dHL-60 cells across the endpoints tested, echoing those findings reported within the literature. Complete cell death of both cell types occurred at Ag NM concentrations ≥62.5 µg/mL, and we observed peak levels of increased superoxide anion production at a concentration of ∼5 µg/mL. Elevated secretion of pro-inflammatory cytokines was accompanied by a significant decrease in anti-inflammatory IL-1Ra, indicating that Ag NMs are likely to stimulate a prolonged inflammatory response with delayed resolution. Furthermore, within the investigated NM panel, only Ag NMs were found to trigger NET release by primary neutrophils. The mechanisms underlying NET release are still under investigation, however, our findings would appear to agree with current knowledge in this field, as it has been established that NET release is dependent on the generation of ROS through activation of NADPH oxidase, triggered by pro-inflammatory mediators (Stoiber et al. Citation2015). Overall, our findings regarding the pro-inflammatory effects of Ag NMs draw a strong parallel to those reported by existing in vitro and in vivo studies.
CuO NMs have been shown to induce pulmonary inflammation in vivo (Pettibone et al. Citation2008; Kim et al. Citation2011; Gosens et al. Citation2016), and we observed pro-inflammatory responses in both primary neutrophils and dHL-60 cells following CuO NM exposure, though responses were less pronounced than those resulting from Ag NMs. Cellular metabolic activity was significantly reduced, and primary neutrophils were notably more sensitive to the effects of exposure. Cytokine analyses revealed increased production of pro-inflammatory cytokines and a decrease in the anti-inflammatory cytokine IL-1Ra. We were unable to measure superoxide production in cells exposed to CuO NMs, due to significant assay interference. Future studies should prioritize alternative techniques to study extracellular ROS production such as chemiluminescence assays, or consider measuring intracellular ROS (Griendling et al. Citation2016). In contrast to observations made in Ag NMs, we did not observe NET release in primary neutrophils exposed to CuO NMs.
An unexpected result of this study was the striking difference in ZnO NM-mediated responses between dHL-60 cells and primary neutrophils. Our cytotoxicity studies revealed that exposure to ZnO NMs caused a significant reduction in dHL-60 cell metabolic activity, though did not appear to impact the metabolic activity of primary neutrophils at any of the tested concentrations. While we did not detect any significant change in cytokine production by primary neutrophils, ZnO NMs stimulated the secretion of pro-inflammatory cytokines IL-8 and MCP-1 by dHL-60 cells. Superoxide anion release was not affected in either cell type. Our findings in primary neutrophils are in contrast to those observed in other cell types where ZnO NMs have been shown to exert cytotoxic effects driven by the generation of ROS and/or pro-inflammatory mediators, for example in hepatocytes (Kermanizadeh et al. Citation2013) lung epithelial cells (Huang et al. Citation2010; Lai et al. Citation2015) and macrophages (Wilhelmi et al. Citation2013). There is also ample in vivo evidence supporting ZnO NM induced inflammation (reviewed by Singh Citation2019), such as neutrophil infiltration in the lungs of exposed animals (Sayes, Reed, and Warheit Citation2007; Gosens et al. Citation2015; Morimoto et al. Citation2016).
Though literature regarding the impact of ZnO NMs on human primary immune cells in vitro is limited, there are studies that have described similar results to our own. Silva and Girard (Citation2016) reported that exposure to ZnO NMs (100 µg/mL) inhibited apoptosis of isolated human eosinophils and had no effect on ROS production, though they did detect an increase in the release of pro-inflammatory cytokines. Goncalves and Girard (Citation2014) also observed delayed apoptosis of human primary neutrophils in response to ZnO NM exposure, with no changes in either extracellular superoxide or intracellular ROS production, however changes in morphology indicated that the cells were in an activated state. The authors were careful to point out that this lack of apoptosis did not rule out ZnO NMs as an agonist of inflammation; on the contrary, delayed apoptosis and the prolonged presence of neutrophils could lead to tissue damage as a result of unresolved inflammation. Within the limited time-frame investigated in the present study, and lacking in vivo context, we cannot ascertain whether the responses observed in primary neutrophils would ultimately have deleterious effects in vivo.
Reasons as to why the responses of the dHL-60 cells may differ from those of the primary neutrophils are explored below, as well as discussion of the limitations of each cell model.
Differences in responses between dHL-60 cells and primary neutrophils
Established in 1977 (Collins, Gallo, and Gallagher Citation1977), the HL-60 cell line was widely employed to study neutrophil differentiation and migration, although its use has since been expanded to study neutrophil functions across a variety of research fields including NM-mediated inflammatory responses (Johnston et al. Citation2015).
Interestingly, several studies have reported significant differences between the behavior of HL-60 cells and primary neutrophils. Yaseen et al. (Citation2017) reported that ROS production and NET release in response to a bacterial pathogen was markedly impaired in HL-60 cells compared with primary neutrophils. Babatunde et al. (Citation2019) observed differences in the chemotactic behavior between the two cell types, notably that dHL-60 cells migrated at a slower pace and in smaller groups. Furthermore, Pelletier, Savoie, and Girard (Citation2000) have shown that the pollutant Na2SO3 is an inflammatory agonist of primary neutrophils, but not to dHL-60 cells.
Overall, we have found that the pattern of inflammatory responses between the HL-60 cell line and primary human neutrophils exposed to our NM panel was comparable, with the exception of ZnO NMs. For example, Ag NM-induced inflammatory responses were characterized by cytotoxicity, increased production of pro-inflammatory cytokines, decreased production of anti-inflammatory cytokines, and increased superoxide production. Meanwhile, TiO2 NMs had no significant impact on either cell type. However, there remain key differences in the specificities of each response between the two cell types e.g. differences in specific cytokines produced, amplitude of response and phagocytic function. These differences demonstrate that HL-60 cells can respond differently to primary neutrophils, highlighting limitations of this cell line. Furthermore, the suspension nature of HL-60 cells presents additional practical challenges, for instance we were unable to investigate NET release using immunofluorescent antibody staining in fixed cells.
We hypothesized that differences in NM-mediated responses may have been at least partly related to differences in the phagocytic function of these two cell types i.e. primary neutrophils and dHL-60 cells may vary in their capacity to internalize NMs. We therefore investigated the uptake of 100 nm carboxylated fluorescent NMs over a 2 h incubation period. We found that primary neutrophils readily internalized particles in contrast with minimal uptake by dHL-60 cells. These findings demonstrate that there may be distinct differences in NM uptake between the two cell types, and we propose that the increased sensitivity of primary neutrophils to NM exposure may arise from enhanced NM uptake. However, it is important to note that the phagocytic capability of neutrophils is influenced by a variety of factors, including particle size, composition and dispersion medium. Therefore, further studies should visualize and quantify the uptake of the tested NM panel, to better understand the role of NM uptake in the observed cellular responses. Bisso et al. (Citation2018) have investigated the effects of human serum albumin on NM internalization, reporting that neutrophil uptake of polystyrene and liposomal NMs was respectively abolished or reduced in culture medium containing 10% human serum, whereas the uptake of poly(lactic-co-glycolic acid) (PLGA) particles was enhanced. These findings were echoed by (Kelley et al. Citation2018) in differentiated HL-60 cells, observing a significant increase in the uptake of both PEGylated (polyethylene glycol-conjugated) and carboxylated NMs in the presence of human serum, in contrast with conventional culture medium containing FCS. The use of autologous human serum in neutrophil cell lines for NM toxicology may therefore offer greater physiological relevance, bridging the gap between cell lines and in vivo systems and should be explored in the future.
Ultimately, there are limitations of the HL-60 cell line which may compromise their ability to act as a substitute for primary cells when assessing neutrophil responses to NMs. Accordingly, we have outlined below a set of recommendations to guide future investigations of neutrophil responses in vitro using these two cell models.
Recommendations for future testing: model and endpoint selection
It is important to establish where the strengths and limitations of in vitro models lie to better understand how they can be applied to nanotoxicology testing. The findings from our study may inform future testing strategies which assess neutrophil responses to NMs in vitro, as a guide to both model and endpoint selection. Furthermore, our research is potentially relevant to the investigation of a wider panel of substances and pathogens, and not limited to the field of nanotoxicology. For future studies, we propose that the responses of HL-60 cells are screened before proceeding with primary cells, where a wider range of endpoints that cannot be investigated in HL-60 cells can be explored. Below are our recommendations for prioritization of endpoints.
Firstly, we recommend that the response of HL-60 cells and primary cells is screened via assessment of cellular metabolic activity, allowing the toxic potency of different NMs to be compared and enabling identification of sub-lethal NM concentrations for subsequent investigation. Assessment of cytokine production is a common measure of NM toxicity in vitro, and can be measured in supernatants collected during cytotoxicity studies thereby maximizing the amount of data that can be gathered from a single experiment; this is particularly attractive when working with primary cells. We have observed that the types of cytokine released vary between the two cell types in response to NM exposure. Specifically, IL-8 production was observed in both cell types, whereas GRO-α release was only observed in primary cells and similarly MCP-1 production was only detected in HL-60 cells. We therefore suggest that assessment of IL-8 production is prioritized as a marker of cell activation in future studies of neutrophil responses in vitro. Of benefit is that throughout the literature, the release of the pro-inflammatory cytokine IL-8 in response to NM exposure has consistently been reported in numerous cell types such as macrophages, lung and intestinal epithelial cells, and hepatocytes (Brown et al. Citation2010; Gaiser et al. Citation2013; Johnston et al. Citation2015; Ude et al. Citation2017).
Assessment of respiratory burst activation via measurement of superoxide anion production provided a rapid assessment of neutrophil activation by NMs, with results obtained 2 h post-exposure in both cell types. Whilst primary neutrophils produced a stronger response, this assay is applicable to both cell types and can provide a good indication of cell activation by NMs. However, the assay we selected was not suitable for CuO NMs due to problems with NM interference. This issue has been reported for other NMs and assays (Ong et al. Citation2014), demonstrating that assessment of NM interference is a key component of nanotoxicity testing and ultimately dictates which endpoints are applicable in vitro.
We observed Ag-NM induced NET formation in primary cells in vitro using immunofluorescent antibody labeling in fixed cells, however we found this assay was not reproducible in HL-60 cells due to their suspension nature (data not shown). Previous studies have demonstrated NET release by HL-60 cells (Kawakami et al. Citation2014; Nakayama and Saitoh Citation2015); therefore, we would propose that future studies should consider techniques for NET detection that are compatible in both cell types such as the use of intravital stains (e.g. Sytox live-cell exclusion DNA dye, combined cell membrane permeable/impermeable dyes). These techniques which use live cells offer the additional benefit of allowing for visualization of NETosis kinetics.
Whilst in vitro studies may indicate whether NMs are likely to stimulate a pro-inflammatory response, it is challenging to determine whether inflammation is resolved within an in vitro setting. Information regarding inflammation resolution is critical in the assessment of NM toxicity, to determine whether responses are physiological or pathological in nature. Measurement of the anti-inflammatory cytokine IL-Ra proved useful in identifying NMs that may hinder the resolution of neutrophilic inflammation. However, in vivo studies are required to confirm the activation of persistent inflammatory responses by NMs. We have advocated previously that zebrafish may help bridge the gap between in vitro and rodent models in nanotoxicology, by allowing us to explore the activation and resolution of inflammatory responses following NM exposure (Johnston et al. Citation2018).
Conclusion
Despite the prominent role neutrophils play in NM-induced inflammation in rodents in vivo, it is surprising that only a limited number of studies have investigated neutrophil responses to NMs in vitro. Whilst our results indicate that HL-60 cells may not be a perfect substitute for primary cells and require further investigation regarding their ability to predict neutrophil responses, we suggest that in vitro neutrophil studies of NM exposure are more routinely used to assess NM safety, and propose the more widespread use of both the neutrophil-like HL-60 cell line and primary human neutrophils. Beyond the present study, a wider panel of NMs possessing diverse physico-chemical properties should be tested across both cell models to further determine whether or not the HL-60 cell line is predictive of primary human cell responses. However, based on our current findings we advocate that a tiered testing strategy incorporating a variety of model systems is adopted to study neutrophil responses to NMs. In the first instance, we recommend that the HL-60 cell line is used to screen for various neutrophil functions before progressing with primary human cells. Regarding the selection of endpoints, we would advise that cytotoxicity, cytokine production, and activation of respiratory burst are considered in both cell types. We propose that transgenic zebrafish larvae (not protected by EU legislation up to 5 days post-fertilization) that allow for direct visualization of neutrophil activity and inflammatory resolution should be considered as the next step before progressing to rodent studies (if deemed necessary). This overall approach will better align nanotoxicology testing with the 3Rs principles.
Supplementary figure2.png
Download PNG Image (7.2 MB)Supplementary figure1.png
Download PNG Image (287 KB)Acknowledgements
We would like to thank the volunteers who donated their blood for our research, our colleagues in the nano-safety research group and Vincent Bombail for advice regarding statistical analyses. We would also like to thank our anonymous reviewers, selected by the journal editor, for their constructive and valuable feedback of our manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
References
- Akter, M., M. T. Sikder, M. M. Rahman, A. K. M. A. Ullah, K. F. B. Hossain, S. Banik, T. Hosokawa, et al. 2018. “A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives.” Journal of Advanced Research 9: 1–16. doi:10.1016/j.jare.2017.10.008.
- Angelé-Martínez, C., K. V. T. Nguyen, F. S. Ameer, J. N. Anker, and J. L. Brumaghim. 2017. “Reactive Oxygen Species Generation by Copper(II) Oxide Nanoparticles Determined by DNA Damage Assays and EPR Spectroscopy.” Nanotoxicology 11 (2): 278–288. doi:10.1080/17435390.2017.1293750.
- Arai, Y., T. Miyayama, and S. Hirano. 2015. “Difference in the Toxicity Mechanism Between Ion and Nanoparticle Forms of Silver in the Mouse Lung and in Macrophages.” Toxicology 328: 84–92. doi:10.1016/j.tox.2014.12.014.
- Babatunde, K. A, X. Wang, A. Hopke, P. -Y. Mantel, D. Irimia. 2019. “Chemotaxis and Swarming in Differentiated HL60 Neutrophil-like Cells.” bioRxiv 714345. doi:10.1101/714345.
- Baranowska-Wójcik, E., D. Szwajgier, P. Oleszczuk, and A. Winiarska-Mieczan. 2020. “Effects of Titanium Dioxide Nanoparticles Exposure on Human Health – A Review.” Biological Trace Element Research 193 (1): 118–129. doi:10.1007/s12011-019-01706-6.
- Bisso, P. W., S. Gaglione, P. P. G. Guimarães, M. J. Mitchell, and R. Langer. 2018. “Nanomaterial Interactions with Human Neutrophils.” ACS Biomaterials Science & Engineering 4 (12): 4255–4265. doi:10.1021/acsbiomaterials.8b01062.
- Bondarenko, o., K. Juganson, A. Ivask, K. Kasemets, M. Mortimer, and A. Kahru. 2013. “Toxicity of Ag, CuO and ZnO Nanoparticles to Selected Environmentally Relevant Test Organisms and Mammalian Cells In Vitro: A Critical Review.” Archives of Toxicology 87 (7): 1181–1200. doi:10.1007/s00204-013-1079-4.
- Brown, D. M., C. Dickson, P. Duncan, F. Al-Attili, and V. Stone. 2010. “Interaction Between Nanoparticles and Cytokine Proteins: Impact on Protein and Particle Functionality.” Nanotechnology 21 (21): 215104. doi:10.1088/0957-4484/21/21/215104.
- Brzicova, T., E. Javorkova, K. Vrbova, A. Zajicova, V. Holan, D. Pinkas, V. Philimonenko, et al. 2019. “Molecular Responses in THP-1 Macrophage-Like Cells Exposed to Diverse Nanoparticles.” Nanomaterials (Basel, Switzerland) 9 (5): 687. doi:10.3390/nano9050687.
- Burden, N., K. Chapman, F. Sewell, V. Robinson. 2015. “Pioneering Better Science Through the 3Rs: An Introduction to the National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs).” Journal of the American Association for Laboratory Animal Science. American Association for Laboratory Animal Science 54 (2): 198–208.
- Cameron, S. J., F. Hosseinian, and W. G. Willmore. 2018. “A Current Overview of the Biological and Cellular Effects of Nanosilver.” International Journal of Molecular Sciences 19 (7): 2030. doi:10.3390/ijms19072030.
- Collins, S. J., R. C. Gallo, and R. E. Gallagher. 1977. “Continuous Growth and Differentiation of Human Myeloid Leukaemic Cells in Suspension Culture.” Nature 270 (5635): 347–349. doi:10.1038/270347a0.
- Dransfield, I., and A. G. Rossi. 2004. “Granulocyte Apoptosis: Who Would Work with a “Real” Inflammatory Cell?” Biochemical Society Transactions 32 (Pt3): 447–451. in doi:10.1042/BST0320447.
- Ferdous, Z., S. Al-Salam, Y. E. Greish, B. H. Ali, and A. Nemmar. 2019. “Pulmonary Exposure to Silver Nanoparticles Impairs Cardiovascular Homeostasis: Effects of Coating, Dose and Time.” Toxicology and Applied Pharmacology 367: 36–50. doi:10.1016/j.taap.2019.01.006.
- Gaiser, B. K., S. Hirn, A. Kermanizadeh, N. Kanase, K. Fytianos, A. Wenk, N. Haberl, et al. 2013. “Effects of Silver Nanoparticles on the Liver and Hepatocytes in Vitro.” Toxicological Sciences: An Official Journal of the Society of Toxicology 131 (2): 537–547. doi:10.1093/toxsci/kfs306.
- Gan, J., J. Sun, X. Chang, W. Li, J. Li, S. Niu, L. Kong, et al. 2020. “Biodistribution and Organ Oxidative Damage Following 28 Days Oral Administration of Nanosilver with/Without Coating in Mice.” Journal of Applied Toxicology: Jat 40 (6): 815–831. doi:10.1002/jat.3946.
- Giovanni, M., J. Yue, L. Zhang, J. Xie, C. N. Ong, and D. T. Leong. 2015. “Pro-Inflammatory Responses of RAW264.7 Macrophages When Treated with Ultralow Concentrations of Silver, Titanium Dioxide, and Zinc Oxide Nanoparticles.” Journal of Hazardous Materials 297: 146–152. doi:10.1016/j.jhazmat.2015.04.081.
- Goncalves, D. M., and D. Girard. 2014. “Zinc Oxide Nanoparticles Delay Human Neutrophil Apoptosis by a De Novo Protein Synthesis-Dependent and Reactive Oxygen Species-Independent Mechanism.” Toxicology In Vitro: An International Journal Published in Association with BIBRA 28 (5): 926–931. doi:10.1016/j.tiv.2014.03.002.
- Gonçalves, D. M., S. Chiasson, and D. Girard. 2010. “Activation of Human Neutrophils by Titanium Dioxide (TiO2) Nanoparticles.” Toxicology In Vitro: An International Journal Published in Association with BIBRA 24 (3): 1002–1008. doi:10.1016/j.tiv.2009.12.007.
- Gosens, I., A. Kermanizadeh, N. R. Jacobsen, A. -G. Lenz, B. Bokkers, W. H. de Jong, P. Krystek, et al. 2015. “Comparative Hazard Identification by a Single Dose Lung Exposure of Zinc Oxide and Silver Nanomaterials in Mice.” PloS One 10 (5): e0126934. doi:10.1371/journal.pone.0126934.
- Gosens, I., F. R. Cassee, M. Zanella, L. Manodori, A. Brunelli, A. L. Costa, B. G. H. Bokkers, et al. 2016. “Organ Burden and Pulmonary Toxicity of Nano-Sized Copper (II) Oxide Particles after Short-Term Inhalation Exposure.” Nanotoxicology 10 (8): 1084–1095. doi:10.3109/17435390.2016.1172678.
- Griendling, K. K., R. M. Touyz, J. L. Zweier, S. Dikalov, W. Chilian, Y. -R. Chen, D. G. Harrison, et al. 2016. “Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement from the American Heart Association.” Circulation Research 119 (5): e39–e75. doi:10.1161/RES.0000000000000110.
- Haslett, C., L. A. Guthrie, M. M. Kopaniak, R. B. Johnston, and P. M. Henson. 1985. “Modulation of Multiple Neutrophil Functions by Preparative Methods or Trace Concentrations of Bacterial Lipopolysaccharide.” The American Journal of Pathology 119 (1): 101–110.
- Hedenborg, M. 1988. “Titanium Dioxide Induced Chemiluminescence of Human Polymorphonuclear Leukocytes.” International Archives of Occupational and Environmental Health 61 (1-2): 1–6. doi:10.1007/BF00381600.
- Holmes, A. M., S. Creton, and K. Chapman. 2010. “Working in Partnership to Advance the 3Rs in Toxicity Testing.” Toxicology 267 (1-3): 14–19. doi:10.1016/j.tox.2009.11.006.
- Huang, C. -C., R. S. Aronstam, D. -R. Chen, and Y. -W. Huang. 2010. “Oxidative Stress, Calcium Homeostasis, and Altered Gene Expression in Human Lung Epithelial Cells Exposed to ZnO Nanoparticles.” Toxicology In Vitro: An International Journal Published in Association with BIBRA 24 (1): 45–55. doi:10.1016/j.tiv.2009.09.007.
- Jacobsen, N. R., G. Pojano, H. Wallin, and K. A. Jensen. 2010. Nanomaterial dispersion protocol for toxicological studies in ENPRA. Internal ENPRA Project Report.
- Johnston, H., D. M. Brown, N. Kanase, M. Euston, B. K. Gaiser, C. T. Robb, E. Dyrynda, et al. 2015. “Mechanism of Neutrophil Activation and Toxicity Elicited by Engineered Nanomaterials.” Toxicology In Vitro: An International Journal Published in Association with BIBRA 29 (5): 1172–1184. doi:10.1016/j.tiv.2015.04.021.
- Johnston, H. J., R. Verdon, S. Gillies, D. M. Brown, T. F. Fernandes, T. B. Henry, A. G. Rossi, et al. 2018. “Adoption of In Vitro Systems and Zebrafish Embryos as Alternative Models for Reducing Rodent Use in Assessments of Immunological and Oxidative Stress Responses to Nanomaterials.” Critical Reviews in Toxicology 48 (3): 252–271. doi:10.1080/10408444.2017.1404965.
- Kawakami, T., J. He, H. Morita, K. Yokoyama, H. Kaji, C. Tanaka, S. -I. Suemori, et al. 2014. “Rab27a Is Essential for the Formation of Neutrophil Extracellular Traps (NETs) in Neutrophil-like Differentiated HL60 Cells.” PLoS One 9 (1): e84704. doi:10.1371/journal.pone.0084704.
- Kelley, W. J., C. A. Fromen, G. Lopez-Cazares, and O. Eniola-Adefeso. 2018. “PEGylation of Model Drug Carriers Enhances Phagocytosis by Primary Human neutrophils.” Acta Biomaterialia 79: 283–293. 2018/09/06, doi:10.1016/j.actbio.2018.09.001.
- Kermanizadeh, A., G. Pojana, B. K. Gaiser, R. Birkedal, D. Bilanicˇová, H. Wallin, K. A. Jensen, et al. 2013. “In Vitro Assessment of Engineered Nanomaterials Using a Hepatocyte Cell Line: Cytotoxicity, Pro-Inflammatory Cytokines and Functional Markers.” Nanotoxicology 7 (3): 301–313. doi:10.3109/17435390.2011.653416.
- Keshavan, S., P. Calligari, L. Stella, L. Fusco, L. G. Delogu, and B. Fadeel. 2019. “Nano-Bio Interactions: A Neutrophil-Centric View.” Cell Death & Disease 10 (8): 569. doi:10.1038/s41419-019-1806-8.
- Kim, J. S., A. Adamcakova-Dodd, P. T. O'Shaughnessy, V. H. Grassian, and P. S. Thorne. 2011. “Effects of Copper Nanoparticle Exposure on Host Defense in a Murine Pulmonary Infection model.” Particle and Fibre Toxicology 8 (1): 29. doi:10.1186/1743-8977-8-29.
- Klein, C. L, S. Comero, B. Stahlmecke, J. Romazanov, T. A. J. Kuhlbusch, E. Van Doren, P. -J. De Temmerman, et al. 2011. NM-Series of Representative Manufactured Nanomaterials NM-300 Silver Characterisation, Stability, Homogeneity. doi:10.2788/23079.
- Kumar, V., N. Sharma, and S. S. Maitra. 2017. “In Vitro and In Vivo Toxicity Assessment of Nanoparticles.” International Nano Letters 7 (4): 243–256. doi:10.1007/s40089-017-0221-3.
- Lai, X., Y. Wei, H. Zhao, S. Chen, X. Bu, F. Lu, D. Qu, et al. 2015. “The Effect of Fe2O3 and ZnO Nanoparticles on Cytotoxicity and Glucose Metabolism in Lung Epithelial Cells.” Journal of Applied Toxicology: JAT 35 (6): 651–664. doi:10.1002/jat.3128.
- Ma-Hock, L., S. Burkhardt, V. Strauss, A. O. Gamer, K. Wiench, B. van Ravenzwaay, R. Landsiedel, et al. 2009. “Development of a Short-Term Inhalation Test in the Rat Using Nano-Titanium Dioxide as a Model Substance.” Inhalation Toxicology 21 (2): 102–118. doi:10.1080/08958370802361057.
- Monse, C., M. Raulf, O. Hagemeyer, V. van Kampen, B. Kendzia, V. Gering, E. -M. Marek, et al. 2019. “Airway Inflammation after Inhalation of Nano-Sized Zinc Oxide Particles in Human Volunteers.” BMC Pulmonary Medicine. England 19 (1): 266. doi:10.1186/s12890-019-1026-0.
- Morimoto, Y., H. Izumi, Y. Yoshiura, T. Tomonaga, T. Oyabu, T. Myojo, K. Kawai, et al. 2016. “Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation.” International Journal of Molecular Sciences 17 (8): 1241. doi:10.3390/ijms17081241.
- Nakayama, T., and H. Saitoh. 2015. “Tunicamycin-Induced Neutrophil Extracellular Trap (NET)-like Structures in Cultured Human Myeloid Cell Lines.” Cell Biology International 39 (3): 355–359. Blackwell Publishing Ltd, doi:10.1002/cbin.10396.
- Ong, K. J., T. J. MacCormack, R. J. Clark, J. D. Ede, V. A. Ortega, L. C. Felix, M. K. M. Dang, et al. 2014. “Widespread Nanoparticle-Assay Interference: Implications for Nanotoxicity Testing.” PloS One 9 (3): e90650. doi:10.1371/journal.pone.0090650.
- Parkos, C. A. 2016. “Neutrophil-Epithelial Interactions a Double-Edged Sword.” The American Journal of Pathology 186 (6): 1404–1416. doi:10.1016/j.ajpath.2016.02.001.
- Pelletier, M., A. Savoie, and D. Girard. 2000. “Activation of Human Neutrophils by the Air Pollutant Sodium Sulfite (Na(2)SO(3)): comparison with immature promyelocytic HL-60 and DMSO-differentiated HL-60 cells reveals that Na(2)SO(3) is a neutrophil but not a HL-60 cell agonist .” Clinical Immunology (Orlando, Fla.) 96 (2): 131–139. Academic Press Inc., doi:10.1006/clim.2000.4883.
- Pettibone, J. M., A. Adamcakova-Dodd, P. S. Thorne, P. T. O'Shaughnessy, J. A. Weydert, and V. H. Grassian. 2008. “Inflammatory Response of Mice following Inhalation Exposure to Iron and Copper Nanoparticles.” Nanotoxicology 2 (4): 189–204. doi:10.1080/17435390802398291.
- Roda, E., M. G. Bottone, M. Biggiogera, G. Milanesi, and T. Coccini. 2019. “Pulmonary and Hepatic Effects after Low Dose Exposure to Nanosilver: Early and Long-Lasting Histological and Ultrastructural Alterations in Rat.” Toxicology Reports 6: 1047–1060. doi:10.1016/j.toxrep.2019.09.008.
- Rossi, A. G., C. Ward, and I. Dransfield. 2004. “Getting to Grips with the Granulocyte: Manipulation of Granulocyte Behaviour and Apoptosis by Protein Transduction Methods.” Biochemical Society Transactions 32 (3): 452–455. doi:10.1042/BST0320452.
- Russell, W. M. S., and R. L. Burch. 2005. The Principles of Humane Experimental Technique. Baltimore, MD: Johns Hopkins Center for Alternatives to Animal Testing. http://altweb.jhsph.edu
- Sager, T. M., C. Kommineni, and V. Castranova. 2008. “Pulmonary Response to Intratracheal Instillation of Ultrafine Versus Fine Titanium Dioxide: Role of Particle Surface Area.” Particle and Fibre Toxicology 5: 17 doi:10.1186/1743-8977-5-17.
- Sayes, C. M., K. L. Reed, and D. B. Warheit. 2007. “Assessing Toxicity of Fine and Nanoparticles: Comparing In Vitro Measurements to In Vivo Pulmonary Toxicity profiles.” Toxicological Sciences: An Official Journal of the Society of Toxicology 97 (1): 163–180. doi:10.1093/toxsci/kfm018.
- Seaton, A., D. Godden, W. MacNee, and K. Donaldson. 1995. “Particulate Air Pollution and Acute Health Effects.” The Lancet 345 (8943): 176–178. doi:10.1016/S0140-6736(95)90173-6.
- Silva, L. R., and D. Girard. 2016. “Human Eosinophils Are Direct Targets to Nanoparticles: Zinc Oxide Nanoparticles (ZnO) Delay Apoptosis and Increase the Production of the Pro-Inflammatory Cytokines IL-1β and IL-8.” Toxicology Letters 259 (8): 11–20. doi:10.1016/J.TOXLET.2016.07.020.
- Singh, S. 2019. “Zinc Oxide Nanoparticles Impacts: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity.” Toxicology Mechanisms and Methods 29 (4): 300–311. doi:10.1080/15376516.2018.1553221.
- Singh, C., S. Friedrichs, M. Levin, R. Birkedal, K. A. Jensen, G. Pojana, W. Wohlleben, et al. 2011. NM-Series of Representative Manufactured Nanomaterials – Zinc Oxide NM-110, NM-111, NM-112, NM-113: Characterisation and Test Item Preparation. Accessed 26 May 2020. https://publications.jrc.ec.europa.eu/repository/handle/JRC64075
- Skocaj, M., M. Filipic, J. Petkovic, and S. Novak. 2011. “Titanium Dioxide in Our Everyday Life; Is It Safe?” Radiology and Oncology 45 (4): 227–247. doi:10.2478/v10019-011-0037-0.
- Smulders, S., K. Luyts, G. Brabants, K. V. Landuyt, C. Kirschhock, E. Smolders, L. Golanski, et al. 2014. “Toxicity of Nanoparticles Embedded in Paints Compared with Pristine Nanoparticles in Mice.” Toxicological Sciences: An Official Journal of the Society of Toxicology 141 (1): 132–140. doi:10.1093/toxsci/kfu112.
- Stensberg, M. C., Q. Wei, E. S. McLamore, D. M. Porterfield, A. Wei, and M. S. Sepúlveda. 2011. “Toxicological Studies on Silver Nanoparticles: Challenges and Opportunities in Assessment, Monitoring and Imaging.” Nanomedicine 6 (5): 879–898. doi:10.2217/nnm.11.78.
- Stoiber, W., A. Obermayer, P. Steinbacher, and W. -D. Krautgartner. 2015. “The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans.” Biomolecules 5 (2): 702–723. doi:10.3390/biom5020702.
- Tamassia, N., F. Bianchetto-Aguilera, F. Arruda-Silva, E. Gardiman, S. Gasperini, F. Calzetti, M. A. Cassatella, et al. 2018. “Cytokine Production by Human Neutrophils: Revisiting the “Dark Side of the Moon”.” European Journal of Clinical Investigation 48 Suppl 2: e12952. doi:10.1111/eci.12952.
- Taylor, E. L., A. G. Rossi, I. Dransfield, and S. P. Hart. 2007. “Analysis of Neutrophil Apoptosis.” Methods in Molecular Biology (Clifton, N.J.) 412: 177–200. doi:10.1007/978-1-59745-467-4_12.
- Tecchio, C., A. Micheletti, and M. A. Cassatella. 2014. “Neutrophil-Derived Cytokines: Facts Beyond Expression.” Frontiers in Immunology 5: 508. doi:10.3389/fimmu.2014.00508.
- Ude, V. C., D. M. Brown, L. Viale, N. Kanase, V. Stone, and H. J. Johnston. 2017. “Impact of Copper Oxide Nanomaterials on Differentiated and Undifferentiated Caco-2 Intestinal Epithelial Cells; Assessment of Cytotoxicity, Barrier Integrity, Cytokine Production and Nanomaterial Penetration.” Particle and Fibre Toxicology 14 (1): 31. doi:10.1186/s12989-017-0211-7.
- Wilhelmi, V., U. Fischer, H. Weighardt, K. Schulze-Osthoff, C. Nickel, B. Stahlmecke, T. A. J. Kuhlbusch, et al. 2013. “Zinc Oxide Nanoparticles Induce Necrosis and Apoptosis in Macrophages in a p47phox- and Nrf2-independent manner.” PLoS One 8 (6): e65704. doi:10.1371/journal.pone.0065704.
- Xu, L., A. Shao, Y. Zhao, Z. Wang, C. Zhang, Y. Sun, J. Deng, et al. 2015. “Neurotoxicity of Silver Nanoparticles in Rat Brain after Intragastric Exposure.” Journal of Nanoscience and Nanotechnology 15 (6): 4215–4223. doi:10.1166/jnn.2015.9612.
- Yan, J., X. Meng, L. M. Wancket, K. Lintner, L. D. Nelin, B. Chen, K. P. Francis, et al. 2012. “Glutathione Reductase Facilitates Host Defense by Sustaining Phagocytic Oxidative Burst and Promoting the Development of Neutrophil Extracellular Traps.” Journal of Immunology (Baltimore, Md.: 1950) 188 (5): 2316–2327. doi:10.4049/jimmunol.1102683.
- Yang, H., T. N. Marion, Y. Liu, L. Zhang, X. Cao, H. Hu, Y. Zhao, et al. 2019. “Nanomaterial Exposure Induced Neutrophil Extracellular Traps: A New Target in Inflammation and Innate Immunity.” Journal of Immunology Research 2019: 3560180. doi:10.1155/2019/3560180.
- Yaseen, R., S. Blodkamp, P. Lüthje, F. Reuner, L. Völlger, H. Y. Naim, M. von Köckritz-Blickwede, et al. 2017. “Antimicrobial Activity of HL-60 Cells Compared to Primary Blood-Derived Neutrophils Against Staphylococcus aureus.” Journal of Negative Results in Biomedicine 16 (1): 2. doi:10.1186/s12952-017-0067-2.

