Abstract
Nanotechnology applications are fast-growing in many industrial fields. Consequently, health effects of engineered nanomaterials (ENMs) should be investigated. Within the EU-Life project NanoExplore, we developed a harmonized protocol of an international multicenter prospective cohort study of workers in ENM-producing companies. This article describes the development of the protocol, sample size calculation, data collection and management procedures and discusses its relevance with respect to research needs. Within this protocol, workers’ ENM exposure will be assessed over four consecutive working days during the initial recruitment campaign and the subsequent follow-up campaigns. Biomonitoring using noninvasive sampling of exhaled breath condensate (EBC), exhaled air, and urine will be collected before and after 4-day exposure monitoring. Both exposure and effect biomarkers, will be quantified along with pulmonary function tests and diagnosed diseases reported using a standardized epidemiological questionnaire available in four languages. Until now, this protocol was implemented at seven companies in Switzerland, Spain and Italy. The protocol is well standardized, though sufficiently flexible to include company-specific conditions and occupational hygiene measures. The recruitment, to date, of 140 participants and collection of all data and samples, enabled us launching the first international cohort of nanotechnology workers. All companies dealing with ENMs could join the NanoExplore Consortium, apply this harmonized protocol and enter in the cohort, concieved as an open cohort. Its protocol meets all requirements of a hypotheses-driven prospective study, which will assess and reassess effects of ENM exposure on workers’ health by updating the follow-up of the cohort. New hypothesis could be also considered.
Introduction
Background and rationale
Nanotechnology is an emerging field and fast growing in industry and science (Ellenbecker and Tsai Citation2015). Engineered nanomaterials (ENMs) are nanosize matter (approximately 1–100 nm) produced and used to develop novel material with exceptional physiochemical properties (European Commission Citation2011; NIOSH Citation2018; Kuempel et al., Citation2021). For these reasons, ENMs are used in many industry sectors including electronics, energy, healthcare, nutrition, and cosmetics (Chen et al. Citation2021, Schubauer-Berigan et al. Citation2020, Ursini et al. Citation2021, Wu et al. Citation2021). Although ENMs can be highly beneficial for several industrial fields, there are many unanswered questions about their potential environmental and health effects (Hodson, Geraci, and Schulte, Citation2019; Riediker et al. Citation2012).
Most of the current knowledge about ENMs’ toxicity comes from experimental in vitro and in vivo studies. These studies have shown that ENMs can induce harmful effects on cells, tissues, and organs, such as reduction ofimmune cell viability, increase in oxidative stress, inflammations, endothelial permeability, pro-thrombotic state, airways irritation, and can cause diseases such as cancer (Guseva Canu et al. Citation2018; Schulte et al. Citation2009; Mostovenko et al. Citation2021; Murphy et al. Citation2016; Müller et al. Citation2018; Dávila-Grana et al. Citation2018; Erdely et al. Citation2009; Li et al. Citation2007; Shvedova et al. Citation2005). Indeed, these effects can differ depending on the type of ENMs, but also within the same family of ENMs depending on some specific physicochemical properties (Drew et al. Citation2017; Stone et al. Citation2020). Such studies are necessary and important for identifying hazardous effects of nanoparticles, yet they are insufficient in assessing human exposure and health risks, developing recommendations and implementing occupational exposure limits to consistently manage ENMs’ risks. First, ENMs may react differently in simplified biological models (e.g. immune cells) compared to complex biological milieu such as specific human tissues, organs and systems (Cedervall et al. Citation2007; Monopoli et al. Citation2012). Second, some deleterious effects were observed only for extremely high doses that are not representative of working conditions (Krug Citation2014; Pauluhn Citation2011). Although no overt health effects in humans caused by ENMs have been reported yet, ensuring the safety of workers and consumers is mandatory for the responsible development and the long-term sustainability of nanotechnology-enabled industry (European Commission Citation2014).
Conducting epidemiological studies with a comprehensive exposure and health outcome assessment at short and long terms is, thus, the next necessary step in the anticipation of potential adverse health effects related to exposure to ENMs in humans, particularly for medium and longer-terms (Guseva Canu, Burstyn, and Richardson Citation2016b). This seems especially urgent, as by the end of 2020, the number of exposed workers has been estimated to be six million worldwide and this number is expected to grow (Ghafari, Moghadasi, and Shekaftik Citation2020; Roco Citation2011).
Epidemiological data from human populations specifically exposed to ENMs are currently very limited. A systematic review by Schulte et al. (Citation2019) identified 27 studies in humans exposed to ENMs published over the past 15 years. Eighteen of these 27 studies were cross-sectional, including four studies with no comparison (i.e. non-exposed) group, and a very small sample size (from two to 16 workers), i.e. characteristic of an exploratory, rather than epidemiological study design. Only four research teams conducted repeated measurements of exposure and outcomes, with variable follow-up duration (i.e. 5 months, 12 months, three and four years), usually by adopting a panel study design. Yet, this design is particularly sensitive to attrition (Lugtig and Smith Citation2019). In studies of ENM workers, the number of participants remaining at the subsequent follow-up drops dramatically and ranges between less than 30% (Ghosh et al. Citation2017; Kuijpers et al. Citation2018) and up to 60% (Afshari Citation2017; Pelclova et al. Citation2017) of the initial sample. The highest participation rate (85%) at one and two year follow-up was reported by Pelclova et al, (Pelclova et al. Citation2020), but the study consisted of only 20 exposed workers. Such settings preclude a causal inference analysis, therefore, the evidence on potential health effects of ENM exposure in humans remains limited (Schulte et al. Citation2019; Gulumian et al. Citation2016).
Launching epidemiological studies among nanotechnology workers is challenging (Guseva Canu et al. Citation2018). Besides methodological limitations pertaining to the epidemiological studies, numerous scientific issues hamper the realization of cohort studies of nanotechnology workers. They include a lack of standardized exposure assessment methods for quantifying ENM airborn concentrations as well as measuring relevant ENM exposure biomarkers; the large variety of existing ENMs and their properties; the lack of knowledge about ENMs toxicological modes of action, dose-response functions and target organs, as well as about specific biomarkers allowing to assess biological and health effects related to ENMs exposures; and the relatively small number of workers exposed to a given (subset of) ENMs for a large period of time (Guseva Canu et al. Citation2018; Riediker et al. Citation2012). In this regard, Riediker et al (Riediker et al. Citation2012) emphasized that, before launching large-scale studies on health monitoring, it is paramount to determine the feasibility of such studies, as well as usefulness and reliability of the results. They strongly suggested establishing well-defined frameworks that will properly identify study populations and select study designs, characterize exposures and define appropriate outcome measures. The usage of biomarkers is encouraged for both assessing and monitoring the exposure to ENMs, and the early biological and health effects of this exposure (Bergamaschi et al. Citation2015).
According to the World Health Organization (WHO), biomarkers are chemicals, their metabolites, or products of an interaction between a chemical and some target molecule that is measured in the human body compartments (WHO Citation2006). Exposure biomarkers reveal the concentration of a parent compound or its metabolites in human biological matrices (Nieuwenhuijsen, Paustenbach, and Duarte-Davidson Citation2006), such as urinary metal concentration after exposure to metal-containing ENMs. Effect biomarkers are measurable biochemical, physiological, and behavioral effects or other alterations within an organism that, depending on the magnitude, can be associated with an established or possible health impairment or disease (Zare Jeddi et al. Citation2021). They indicate changes in biological systems resulting from complex pathways of exposure. Since effect biomarkers often reflect subclinical changes before the onset of disease, they are a valuable tool for anticipating the potential adverse effects of ENMs and elucidating dose–effect relationships. However, in practice, the use of biomarkers for environmental and occupational exposure and health surveillance is limited (Viegas et al. Citation2020). Obstacles to the implementation of biomonitoring include the lack of standards and guidance values needed to interpret the results, as well as organizational and logistical aspects, which may be difficult to manage in some contexts (Crézé et al. Citation2021; Jones Citation2020). Biomarkers should ideally be measured in biological matrices collected noninvasively and enabling a routine screening and monitoring of workers (Crézé et al. Citation2021). In prospective studies, which need repeated measurements of biological matrices, the non-invasiveness of the procedure can increase the participation rate of the workers and of not exposed volunteers, thus allowing a more consistent and representative study sample. Exhaled breath condensate (EBC), exhaled air and urine could therefore be considered the three preferred biological matrices for non-invasive monitoring of ENM workers. Biomarkers measured inEBC can assess acute or long-term changes in lung biopathology, giving insights on oxidative stress and inflammatory response but also on tissue remodeling (e.g. lung fibrosis or COPD) (Pelclova et al. Citation2018; Graczyk et al. Citation2015), whereas urinary biomarkers can reflect systemic changes (Smolders et al. Citation2010; Manno et al. Citation2010; Bergamaschi et al. Citation2015; Schulte et al. Citation2018).
Objectives and research hypotheses
The NanoExplore project aimed at building an integrated network for studying biomarkers for identifying early effects on health related to ENM exposures in nanotechnology workers, in the European Union and Switzerland. In particular, it aims at assessing a representative panel of biomarkers known to reflect particle exposure and various short- and long-term biological endpoints such as inflammation and oxidative stress, immune system dysregulation, host defense impairment and activation of the pro-fibrotic cascade, both at local (pulmonary) and systemic levels. To address these research needs, we propose a harmonized protocol for a prospective epidemiological cohort, encompassing a comprehensive assessment of occupational and environmental exposure to ENMs combined with a feasible biological monitoring. Although we focus on ENMs, the protocol can be adapted to studies investigating exposures to nano-objects and their agglomerates and aggregates and non-engineered ultrafine particles.
As a first preparatory step, we identified and defined target ENMs, manufacturing processes, potentially exposed workers, biomarkers, and biomonitoring procedures relevant for exposure and health assessment by reviewing the published literature and technical reports (Bergamaschi et al. Citation2019; Domat Citation2019). The available toxicological findings suggested that the pulmonary exposure to some ENMs can cause cardiovascular and respiratory diseases, particularly the lung fibrosis, but also cancer (Schulte et al. Citation2018). However, these diseases usually appear after a long latency of several years or decades, while the majority of the current workforce in nanotechnology has not been exposed for a long enough time and/or at high enough level to develop such diseases at this time. Conversely, the early responses to ENM exposure are potentially appropriate outcomes to measure, even though most of them reflect short-term and sometimes reversible changes with uncertain clinical significance. In particular, ENMs are known to elicit the inflammatory responses that could be monitored in blood, serum, induced sputum, urine and EBC of exposed workers. Similarly, ENMs may activate oxidative stress responses that could reduce the concentration of biomarkers of antioxidant defense system at systemic level and increase the level of reactive oxygen species (ROS) (Schulte et al. Citation2019). We thus included the biomarkers of fibrosis, inflammation and oxidative stress that can be measure at pulmonary and systemic levels.
Second, we conducted an online survey of nanotechnology companies to assess the biomonitoring acceptance among managers and workers, as well as the practical aspects affecting its feasibility in the field (Crézé et al. Citation2021). We also assessed the propensity and barriers to participate in a longitudinal epidemiological study among these specific populations. In this article, we present the harmonized protocol with special emphasis on standardization of procedures, study sample representativeness and recruitment feasibility.
The protocol development integrated several underlying hypotheses. First, we hypothesized that the exposure and effect biomarkers selected for this study are associated with ENM exposure in a dose-response manner and that the fluctuation of ENM exposures would affect within working week the concentrations of the most sensitive biomarkers. Second, we hypothesized that the ENM exposure can also act on a longer-term basis (e.g. by affecting the biomarkers of chronic effect, such as a lung fibrosis or some clinically measured lung function parameters). Furthermore, we hypothesized that ENM exposure reduction recommendations can affect the biomarker profile and disease prevalence at the long term, e.g. between initial and subsequent follow-up campaigns, several years later. This set of hypotheses reflects the current overview of toxicological findings for ENMs, which in large majority suggest some exposure-related changes in early effect biomarkers, although varying depending on the type of ENMs handled. Human studies reporting changes corresponding to the pathological alterations, namely in pulmonary functional parameters, are still limited (Li et al. Citation2018; Schubauer-Berigan et al. Citation2018; Zhao et al. Citation2018; Cao et al. Citation2020). However, these long-term effects could be better evidenced with a longer follow-up of exposed workers in the context of chronic exposure (Guseva Canu et al. Citation2018; Mostovenko et al. Citation2021).
On the basis of the results of pilot studies, we discuss the most challenging aspects to be taken into account in cohort development and future studies.
Material and methods
Setting up the study protocol
There are four main prerequisites for the study protocol: i) to create a well-characterized registry of eligible participants (i.e. ENM worker registry) for prospective epidemiological studies by developing a solid, yet feasible recruitment strategy; ii) to harmonize and standardize all processes in deriving the exposure assessment strategy, in characterizing the population, in verifying pre-analytical requirements for the biomonitoring methods; iii) to select a panel of biomarkers for prospective epidemiological studies by assessing relationships between exposure and health effects at short-, medium- and longer-terms; iv) to evaluate associations between concentrations of airborne particle exposure and biomarkers of exposure and early effects in terms of causal inference after reducing, whenever possible, the extent of exposure (Schulte et al. 2016; Guseva Canu et al. Citation2013).
Study design
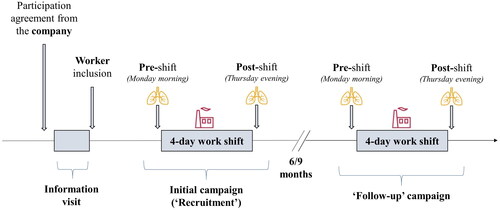
The proposed study design relies on an international multicenter open prospective cohort, with the first follow-up end-point planned at 6 to 9 months since the first recruitment (). The precise planning and frequency of the follow-up campaigns will be scheduled in close collaboration with companies, depending on their operational availability and budget.
Figure 1. Main steps of the NanoExplore cohort construction and follow-up. An information visit conducted beforehand will serve to collect company-related information, inform and enroll volunteer participants into the cohort. Participants’ data on exposure and health outcomes will be collected during the field campaigns. Both the initial (‘recruitment’) and 6 or 9-month follow-up campaigns will be conducted following similar procedures. Airborne exposure to ENMs (symbol: plant) will be monitored during a 4-day work shift. Biological matrices (i.e. EBC, exhaled air and urine; symbol: lungs) for biomarker quantification will be sampled in pre- and post-shift.
Study population and sample size
The target population encompasses workers handling ENMs and more generally nano-objects, their aggregates and agglomerates (European Commission Citation2014) during their occupational tasks. On the basis of the results of our preparatory survey (Crézé et al. Citation2021), we will focus on companies manufacturing and/or processing metal (Al, Ag, Cu), metal oxide (TiO2, ZnO, CeO2, SiO2 -both crystalline and amorphous-, FexOy, Al2O3, CaCO3), carbon-based ENMs (single- and multi-walled nanotubes, graphene) and nanocellulose. As 50% of responding companies reported to have less than 10 employees handling these ENMs, it is crucial to include a sufficient number of companies and workers to ensure an appropriate gradient of exposure and statistical power for causal inference analysis.
Two complementary approaches were used to determine the minimal sample size needed to attain sufficient statistical power. First, external exposure and post- vs. pre-shift variations of the exposure and effect biomarkers were estimated based on the study by Pelclova et al.(Pelclova et al. Citation2018). The corresponding within-group standard deviation of the individual within-subject variation was approximated based on results of the study by Hopf et al. (Hopf et al. Citation2019), where the same biomarkers of oxidative stress were quantified in urine and EBC. For all calculations, estimations were performed under the hypothesis of identical standard variation in both exposed and non-exposed workers. All estimations were calculated with a type I error (α) set at 5% and a statistical power (1 – β) set at 80%.
A second sample size calculation was performed for the exposure biomarkers, i.e. estimation of the difference that we expected to observe in biomarkers concentration between exposed and non-exposed workers. Between-subject internal exposure variations (i.e. particulate number concentration in EBC determined by Nanoparticle Tracking Analysis) were estimated from the study on airport worker populations exposed to ultrafine particles (Marie-Desvergne et al. Citation2016).
Based on these calculations, we determined that a minimal study sample size of 120 workers (60 exposed, 60 non-exposed) was needed to detect a significant difference of at least 25% between groups in the within-subject variation of biomarkers of effect. Taking into account a lost-to-follow-up ratio of 20% due to constraints related to the collection of biological samples and employee turnover (Liao et al. Citation2014), an optimal sample size would include 80 exposed workers and 80 non-exposed workers. For more details of sample size calculation, see Web Appendix A.
It is noteworthy that in many companies the so-called ‘non-exposed’ workers (whose occupational tasks are not related to ENMs per se, e.g. administrative tasks) may be non-intentionally exposed to ENMs, because of non-effective confinement between production and administration areas (Ellenbecker and Tsai Citation2015). Therefore, we will split the non-exposed group in two subgroups. A subgroup with negligible or low ENM exposure will consist of administrative office workers from the same companies where workers for the ‘Exposed’ group will be recruited. A second non-exposed subgroup will consist of workers with confirmed absence of exposure to ENMs in their occupational settings (i.e. workers from companies whose activities are not related with ENMs).
Recruitment strategy
Participants will be recruited in a two-step procedure. First, members of the NanoExplore Consortium will recruit eligible companies in their respective countries to get a representative sample of companies. For this, Consortium members will make use of professional contacts with companies established during the regular activity of or with occupational physicians, occupational hygienists or researchers during NanoExplore and previously conducted surveys (Schmid, Danuser, and Riediker Citation2008; Crézé et al. Citation2021), and with the help of an announcement posted on the NanoExplore website. For this recruitment step, priority will be given to companies with confirmed ENM exposure and with more than five ENM exposed workers).
In a second step, an onsite information visit will be organized in companies that previously gave their agreement to participate in the study (). During this visit, a questionnaire will be administrated to managers or health and safety specialists to collect standardized information on company activities, processes at risk for ENM exposure, as well as equipment and infrastructure already available for employee protection (further referred to as the company questionnaire and available on Unisanté data repository https://doi.org/10.16909/dataset/31). Next, eligible workers will be recruited. A member of the NanoExplore Consortium will explain study objectives, procedure and potential risks and benefits related to study participation. Workers willing to participate will then be provided with detailed information about data collection procedures. A written informed consent will be obtained from each participant before her/his inclusion. For practical and logistical reasons, the non-exposed group will consist of workers from NanoExploreConsortium institutions.
Data collection
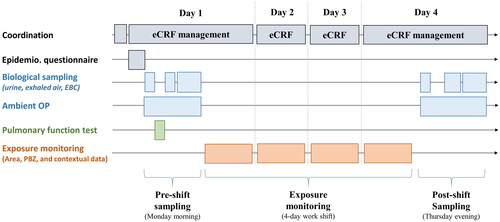
Individual data on ENM exposure, health outcomes and potentially confounding factors will be collected during the field campaigns, as illustrate in .
Figure 2. Structure and timeline of the NanoExplore campaign in the field. The field campaign lasts four days. The electronic epidemiological questionnaire is filled once, in the beginning of the campaign. Biological sampling takes place twice, i.e. in pre-shift on the 1st day and post-shift on the last day of the campaign. Pulmonary function test completes the pre-shift sampling. Exposure monitoring is conducted over a 4-day 8-hour work shift. The overall coordination is ensured using workers’ individual electronic Case Report Forms (eCRFs). EBC: exhaled air condensate, OP: oxidative potential
Main outcomes
In this study, we will assess several types of outcomes. As primary outcomes, we will consider the effect biomarkers reflecting biological changes at pulmonary and systemic levels. describes the effect biomarkers and their corresponding analytical methods. We selected both ‘traditional’ biomarkers of inflammation, immune dysfunction, and oxidative stress and ‘new’ biomarkers, namely High-Sensitivity C-Reactive Protein, Surfactant protein-D (SP-D), and Krebs von den Lungen glycoprotein 6 (KL-6), that will be measured in EBC for the first time. While most biomarkers reflect the early or short-term effects of the exposure, the SP-D and KL-6 are regarded as biomarkers resulting from long-term effects (Bergamaschi et al. Citation2022). The biomarkers will be measured in the initial and follow-up field campaigns in three types of biological matrix: urine, EBC and exhaled air. During each campaign, biological matrix sampling for biomarker analysis will be conducted twice, i.e. before and after a 4-day monitoring of external exposure (further referred to as pre- and post-shift, respectively). Web Appendix B summarizes the standardized biological sampling procedures.
Table 1. Effect biomarkers measured in the NanoExplore cohort.
Furthermore, pulmonary function parameters, including the Forced Expiratory Volume in 1 second (FEV1), the Forced Vital Capacity (FVC) and the Forced Expiratory Fluxes at 25% and 75% of FVC (FEF25–75%) will be considered as secondary outcome. These outcomes could reflect the early and/or delayed effects of ENM exposure on the respiratory system. The pulmonary function tests will be performed by an occupational physician of the NanoExplore Consortium (for details, see Web Appendix B).
Finally, some selected diseases diagnosed by participants’ physicians and self-declared by the study participants in the epidemiological questionnaire (https://doi.org/10.16909/dataset/31) could be considered as protracted effects of the ENM exposure and analyzed as tertiary health outcomes. These will include cardiovascular, respiratory, allergic, and cancer diseases selected based on the evidence from experimental and human studies on the effects of ultrafine and fine particle exposure.
Exposure assessment
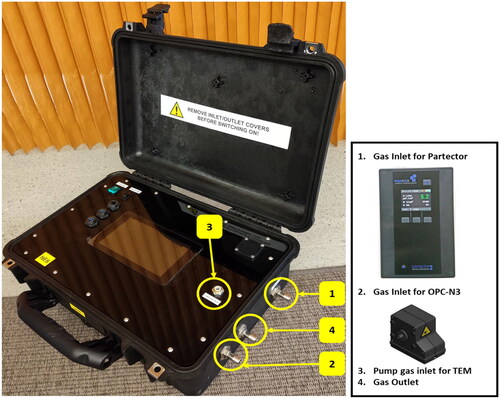
We will follow the Nanoparticle Emission Assessment Technique (NEAT) during the field campaigns. NEAT was established for the identification and measurement of potential inhalation exposure to ENM (Methner, Hodson, and Geraci Citation2010). Airborne particle number concentration will be measured over the full or a part of work shift for four consecutive days, i.e. between pre- and post-shift biological sampling sessions detailed in paragraph 2.5.1. Stationary and personal air monitoring will be conducted using NanoExplore kit of devices whose combined use offers an adequate multi-metric strategy of ENMs exposure assessment (), and consists of three built-in measuring devices (Methner, Hodson, and Geraci Citation2010):
Figure 3. The NanoExplore kit for exposure monitoring. The NanoExplore kit includes three built-in modules: the Optical Particle Counting module (OPC), the nanoparticle sensor (Partector 2), and the transmission electronic microscopy (TEM) particle head sampler.
The Optical Particle Counting (OPC) module measures mass concentrations for particulate matter (PM) with size lower than 1, 2.5 and 10 μm, respectively (PM1, PM2.5 and PM10 respectively), using real-time light scattering-based diameter measurements.
The nanoparticle sensor (Partector 2) measures the electrical diffusion charging behavior of airborne particles – submicronic particles passing through the impactor are electrically charged at the instrument inlet – in order to calculate the particle number concentration and average diameter, and consequently derive the Lung Deposited Surface Area (LDSA) parameter in real-time, with a time-resolution of one second. The mass concentration is thus obtained through a series of approximations on the measured particles (Asbach et al., Citation2017).
The transmission electronic microscopy (TEM) particle sampler consists of a holder of an organic polymer film hosting a metal grid) connected to a built-in pump (Rotary Vane Pump G 6/02-4 EB from THOMAS), operating at a maximum flow of 2.6 l/min, a maximum pressure of 230 mbar, and a maximum vacuum of 23%. This pump enables collecting airborne particles during ENM-releasing activities. The particles trapped on the filter will be analyzed following standardized laboratory procedures to evaluate particles’ chemical profile, average size distribution and shape (see Web Appendix C for details).
The ambient oxidative potential will be measured in order to evaluate the aerosol ability to modify the redox homeostasis in the lungs (Bates et al. Citation2019). This metric will be used to standardize the oxidative potential measured in workers’ exhaled air (OPEA, described in the ) (Goekce et al. Citation2022). The original approach developed by Unisanté consists of a sampling train made of a Teflon filter inserted in an IOM cassette followed by XAD-2 sorbent tube operating at a pump rate of 2 L/min. OPEA and ambient oxidative potential will be measured just before or just after the urine and EBC sample collection. Upon the end of the sampling period (typically 6–8 hours), both filters and XAD-2 tubes will be stored at 4 °C with the help of a cooler. The oxidative potential analysis will be performed following the procedure described in Sauvain et al (Sauvain et al. Citation2021). For details on exposure measurement methodology, see Web Appendix D.
Ambient temperature and relative humidity will be continuously monitored using a multifunction measuring instrument TESTO 435 (Strategies Citation2017). This instrument will be placed near the worker’s personal breathing zone to characterize the environmental conditions of the process and the value measured will be taken before the start of the shift or activities with ENMs.
This exposure assessment will be completed with biological exposure monitoring. Exposure biomarkers will be measured in urine and EBC as described in . While the metal concentration measured in urine is a widespread method of exposure monitoring, the quantification of number concentration of nanoparticles in the EBC samples is still rarely used (Guseva Canu et al. Citation2021b). This metric can be measured using the nanotracking analysis (NTA), which also determines the hydrodynamic size distribution with a diameter of approximately 40–1000 nm (nm) in liquid suspension (Sauvain et al. Citation2017).
Table 2. Exposure biomarkers measured in the NanoExplore cohort.
As effect biomarkers, exposure biomarkers will be measured twice at each field campaign according to the standardized procedures described in Web Appendix B.
It worth to mention that after the each field campaign, companies will receive the coded company-specific results of exposure measurement, and when appropriate, the advices and recommendations of a occupational hygienists from the NanoExplore Consortium to support companies in the implementation of remediation strategies for reducing exposure. The exposure measurements conducted in consecutive field campaigns will allow to evaluate, whether companies follow these recommendations and whether the implement exposure control measures are effective in lowering ENM exposure.
Individual risk factors and co-exposures
In order to reduce the likelihood of biased result interpretation when associating ENM exposure with individuals’ biomarker concentrations, individual, contextual and potentially confounding factors will be explored using an epidemiological questionnaire. This questionnaire will be administered to all workers enrolled in the study at baseline and will address determinants of occupational, environmental and domestic exposures (both past and present) to fine and ultrafine particles. Moreover, information on the smoking status, life habits related to inflammatory status (e.g. sleep, food and physical activity habits, paraclinical parameters) and health- and allergy-related parameters, with a specific focus on respiratory parameters, onco-hematological diseases, immuno-deficiency syndromes, auto-immune diseases and cardiovascular events (both past and present) will be collected. The baseline epidemiological questionnaireis is available at the Unisanté data repository (https://doi.org/10.16909/dataset/31).
Standardized coordination for field campaigns
The standardized procedure illustrated in and should ensure reproducibility, comparability and precision of data collected during different field campaigns. Procedures for the initial and the follow-up campaigns are nearly identical, except for the epidemiological questionnaire, which is significantly shorter at follow-up and only aims to update the information collected atthe initial campaign.
Once potentially eligible workers are identified during the information visit to the company (see Recruitment strategy), they are pre-allocated to the ‘Exposed’ or the ‘Non-exposed’ group according to the information about their exposures provided by the company’s occupational safety and health specialist. This pre-allocation aims at facilitating participant recruitment, to meet the sample size requirements, while the final classification of participants with respect to ENM exposure will be based on individual exposure monitoring data. Next, study participant IDs (coded) are created by the study coordinator at Unisanté using the REDCap software (Harris et al. Citation2009). Links to the questionnaire will be sent to participants by the partner institutions. Recruited participants will then complete the epidemiological questionnaire using either their mobile phone, tablet or personal computer upon a personalized invitation link managed via REDCap. All participants have at least one of these electronic devices available at work and will fill in the questionnaire during their working-time. Throughout biological sampling procedures, individual forms will also be completed in REDCap, thus ensuring standardized collection of information pertaining to biological samplings. The overall collection of all forms and questionnaires pertaining to one study participant constitutes his/her electronic Case Report Form (eCRF). Use of electronic questionnaire and forms was preferred as this prevents data collection from human errors related to entering data from paper questionnaires into the REDCap system. Moreover, REDCap allows the language choice for questionnaire completion between Spanish, Italian, French, German, and English. Electronic data collection and storage will be organized in accordance with the ethical guidelines. Only coded (de-identified) data will be centralized at the study coordinating center (Unisanté)(Tajani and Edtstadler Citation2018). In order to avoid analytical variability, the collected samples will beanalyzed in the same laboratory at the University of Torino and the coded results will be centralized at Unisanté secured server, curated and analyzed statistically.
A local project coordinator will be available on site to answer questions from workers or company representatives regarding study objectives and procedures during the sampling campaigns. Exposure assessment results will be communicated to companies in the form of a confidential report and explained by an occupational hygienist from the NanoExplore Consortium. Advice regarding exposure reduction measures will also be formulated. Whenever possible, biomarker concentrations will be compared with available reference intervals for European adult population and the meta-estimates of background values in healthy and non-exposed population reported in literature (Graille et al. Citation2020a; Graille et al. Citation2020b; Hemmendinger et al. Citation2020; Shoman et al. Citation2020; Toto et al. Citation2022; Turcu et al. Citation2022; Guseva Canu et al. Citation2022).
Code of ethics and participation consent
This study follows the principles of the Declaration of Helsinki. The study protocol was approved by the NanoExplore Consortium (https://www.lifenanoexplore.eu/about/consortium) and the EU monitor in charge of the NanoExplore project. Moreover, approvals have been obtained from the local ethics regulation organs: the Swissethics in Switzerland (approval 2020–01098); the Bio-ethical Committee of the University of Torino in Italy (approval 336577 8.08.2020); and the Health and Safety Board of the Catalan Institute of Nanoscience and Nanotechnology, in Spain (approvalI CN2-22-03-2022). All participants have given an individual written informed consent to the inclusion of material pertaining to themselves and acknowledged that they cannot be identified via the paper; and that we have fully de-identified their data.
Statistical analysis
First, the data of the initial campaign will be analyzed as a cross-sectional sample, where non-exposed workers, workers with low exposure and exposed workers will be compared using linear models to ensure comparability in terms of demographic and socio-economic variables.
In a second step, short-term effects from occupational ENM exposures on the within work-week differences in the selected biomarkers will be investigated using multiple linear mixed models. The change in each biomarker concentration over 4-days will be modeled as function of individual ENM exposure measurements accounting for potential confounders or effect modifiers explored using the variables collected by epidemiological questionnaire. Such analyses will be performed on a within-campaign basis, to associate external and internal exposure patterns and biomarker concentrations. The effect of the different types of ENMs identified in the company and epidemiological questionnaires can be assessed and compared in stratified analysis comparing each of the most frequent types of ENMs with the non-exposed participants. Adequate control for multiplicity of tests will be performed using standard procedures. As a third step focusing on external exposure, the time-dependent minute-by-minute variations in particle number concentration will be investigated with respect to exposure determinants. These time series will be visually inspected for qualitative assessment, then quantitatively described using Bayesian spline models (Pétremand et al. Citation2021, Pétremand et al. Citation2022).
Finally, an overall analysis will consist in modeling, again using linear mixed models, the changes in biomarkers occurring along with the implementation of the exposure control measures over the 6/9-month follow-up by companies. These analyses will allow us to identify a set of best-fitting biomarkers to be used in future studies. Differences in exposure patterns (features of the released ENM and concentration) between initial and follow-up field campaigns will be investigated using linear models accounting for company-specific factors, to evaluate the validity of emission reduction strategies, when applicable.
Statistical analyses will be performed using a type I error of 5% (α = 0.05; two-sided tests) with the STATA software, version 17.
Discussion
As far as we know, this is the first harmonized protocol for an international multicenter prospective cohort study of ENM workers in the world. It includes harmonization and standardization in ENM exposure assessments and pulmonary functional tests coupled with biological monitoring of early effects from ENM exposures on human health. It has been approved by different research teams, companies and countries. The prospective design of this cohort with regular follow-up campaigns enables nesting intervention studies focused on ENM exposure controls in companies with ENM exposures and monitoring health effects over medium- and long terms. These features are paramount when assessing causality, particularly when the expected diseases are chronic non-communicable diseases with a long latency time (e.g. 2 to 30 years) before their clinical manifestation, such as cancer or cardiovascular diseases (Checkoway et al. Citation1990; Rose Citation2005). The originality and strength of this protocol consist of its highly standardized, though adaptive nature, with electronic multilingual procedures, facilitated by the use of RedCAP software. These procedures and tools for their implementation have been developed based on the previous exploratory studies conducted by the NanoExplore Consortium members (Bencsik, Lestaevel, and Guseva Canu Citation2018; Bergamaschi et al. Citation2021; Bergamaschi et al. Citation2015; Crézé et al. Citation2021; Graczyk et al. Citation2015; Guseva Canu et al. Citation2016a; Guseva Canu et al. Citation2020; Guseva Canu et al. Citation2021a; Guseva Canu et al. Citation2021b; Guseva Canu et al. Citation2017; Guseva Canu et al. Citation2016c; Guseva Canu et al. Citation2018; Hopf et al. Citation2019; Iavicoli et al. Citation2019; Sauvain et al. Citation2022; Bergamaschi et al. Citation2022), in a highly interdisciplinary framework. In fact, this Consortium includes experts in occupational hygiene, toxicology, biomonitoring, medicine, epidemiology, and biostatistics, air quality monitoring and assessment as well as in communication from six countries. Moreover, most partners are specialized in ENM exposure assessment and/ornanotoxicology, owing to their long-standing activity in EU- and internationally funded research projects and current technical/professional consultancy activity for companies. The protocol was based on the best evidence available as to the health outcomes to be measured and ENM methods of exposure and outcome assessments, in an integrated and practical approach.
This protocol is currently being implemented in a three-country pilot study (Switzerland, Spain and Italy). At the time of manuscript drafting, 140 eligible workers have been included at seven different companies. The same team will conduct the biological sampling and exposure assessments across the facilities, which would improve the consistency of the data. It is noteworthy that the protocol has been tested just at the beginning of the Covid-19 pandemic with complete or partial lockdowns and sanitary restrictions differing between participating countries. During the biological sample collection period, safety measures such as mandatory face mask wearing and disinfection of the equipment can require additional time, financial and human resources. Fear of potential contamination could decrease participation rate among workers. Additionally, exposure to ENMs could differ between the usual situation and pandemic period because of a decreased production activity/volume and an increased use of personal protective equipment. However, the study protocol appeared sufficiently flexible to include additional safety measures, being applicable even in extremely unusual conditions.
The ongoing pilot study confirmed the importance of a harmonized protocol and standardized procedures which should lead to an effective management of geographical and cultural differences in participating countries and companies. A good level of integration of local study coordinators within the NanoExplore Consortium also appeared paramount for organizational and logistical reasons, especially pertaining to biological sample storage and transportation/shipment (e.g. preserving biological specimens by freezing). Such an integrated approach is crucial for the investigation of ENM effects on human health as it allows standardizing not only the exposure assessment and recruitment process but also the procedures of biomarkers’ collection, storage and analyses. The use of EBC as a non-invasive method for the assessment of samples from the airways has become increasingly popular. The analysis of EBC allows for the measurement of mediators of local inflammation synthesized in airways of healthy and ill people as well as for the assessment of systemic inflammation through the determination of mediators coming from the bloodstream. It should be stressed that although promising, biomarkers measured in EBC are not yet validated clinically, in contrast with biomarkers of systemic inflammatory response measured in serum and changes in blood cell counts, considered more robust. Moreover, for some effect biomarkers, such as MDA, 8-isoprostane and 8-OHdG in EBC, the analytical methods still need validation and inter-laboratory comparisons (Hemmendinger et al. Citation2021). The latter could be facilitated through such an international collaboration. As this study encompasses a broad range of biomarkers, it will give insights on the most sensitive and relevant biomarkers among the selected subset. Their relevance with respect to clinical outcomes can be further assessedusing the Key characteristics framework (Smith et al. Citation2016; Lind et al. Citation2021). Non-invasive biological sampling implies minimal risks and burden for participants and investigators. Such procedures are easily accepted and their use is positively associated with participation rates (Crézé et al. Citation2021). Finally, this protocol meets all requirements of a hypotheses-driven longitudinal study, which will assess and reassess effects of ENM exposure on workers’ health by updating the follow-up of the cohort. A priori defined research questions and hypothesis testing render this study methodologically more robust and therefore more conclusive in comparison with exploratory studies conducted until now (Schulte et al. Citation2009). Therefore, this study will lead to an increasing knowledge of occupational exposures to ENMs across research fields and countries, and creating awareness of ENM exposures among workers, companies, as well as researchers.
It is worth mentioning that this protocol was developed for the real occupational settings and exposure scenarios. It showed its relevance in a company handling powders of micrometric range in which we found a non-negligible fraction of metal nanoparticles. In fact, several conventional materials (e.g. titanium dioxide or carbon black) considered simply as bulk powders, consist in reality of nanoparticles in aggregated or agglomerated forms (Bergamaschi et al. Citation2022; Fonseca et al. Citation2021), which may fall under the EU definition of nanomaterials. Moreover, several studies revealed that the release of ultrafine particles originating from the handling of conventional micrometer-sized materials may be substantial (Viitanen et al. Citation2017). As a result, workers are often exposed to a heterogeneous mixtures of different particles, which can make the quantitative exposure characterization and risk assessment very complex. Hence, the measurement of particle number concentration, size distribution and LDSA in workers’ PBZ represents a pragmatic approach to assess external exposure to nanosized particles considering all potential sources using relatively standardized exposure metrics common for the entire cohort. Several authors found that the surface area concentration of particles may have stronger correlation with the negative health effects than e.g. number or mass concentration and considered LDSA as a relevant metric when it comes to understanding the deposition of particles into the human lungs (Hennig et al. Citation2018; Patel et al. Citation2018; Oberdörster, Oberdörster, and Oberdörster Citation2005; Brown et al. Citation2001; Lepistö et al. Citation2022). Nevertheless, none of these metrics is specific with respect to ENM type. That is why, whenever possible, it is recommended to complement them with additional measurements targeting more specifically certain types of ENMs or some of their physicochemical properties. For instance, for carbon-based ENMs, such as CNTs, the measurement of elemental carbon mass concentration and quantification of CNT agglomerates and/or CNT contained fibers in PBZ could be implemented (Dahm, Bertke, and Schubauer-Berigan Citation2019), although concerns raised with respect to the standardization of these measures and metrics (Guseva Canu et al. Citation2020). For a metal-based ENM, the metal mass fraction in airborne particles can be measured, ideally along with assessment of bioavailability and bioaccessibility of this metal (CitationFigueroa-Lara et al. 2019; Guseva Canu et al. Citation2021a). However, this methodalsolacks consensus (Kastury, Smith, and Juhasz Citation2017). Although more specific to certain types of ENMs, these additional exposure measures are considered optional in this protocol, unless their cost, in field implementation challenges and lack of standardization can be reduced sufficiently to allow their generalization. It is also worth to stress, that handling only one type of ENM is an unrealistic exposure scenario in industry. A complex exposure assessment protocol with multiple exposure metrics justified in the research setting is hardly acceptable in some industrial settings. Moreover, for a mixed exposure to different ENMs at a workplace, distinguishing the effects of different particles and sizes will be also unrealistic. It would be also misleading from a toxicological point of view (Bergamaschi et al. Citation2015; Iavicoli et al. Citation2019; Iavicoli, Leso, and Schulte Citation2016). In this situation, biomonitoring as the only tool to assess the effects of such mixed exposures (Schulte et al. Citation2018; Schulte et al. Citation2016; Schulte et al. Citation2019; Zare Jeddi et al. Citation2021).
Importantly, the apparently small minimum sample size of this cohort with 120–160 workers actually corresponds to one of the largest studies of workers exposed to ENMs in the world. For instance, the unique cohort study of ENM workers (the EpiNano cohort) launched in 2012 in France, has included 130 workers so far (Guseva Canu et al. Citation2016c) while the future US National Institute for Occupational Safety and Health (US-NIOSH) cohort of carbon nanotube and nanofiber workers had 108 participants at baseline (Beard et al. Citation2018). The Taiwanese national panel study currently includes 206 exposed and 108 unexposed workers recruited at 14 different ENMs producing plants (Wu et al. Citation2019). It is noteworthy that in the US-NIOSH study, a personal exposure monitoring has been conducted while in the French cohort, the exposure is assessed only qualitatively since the (semi)quantitative exposure assessment has been discontinued (Renaudie et al. Citation2018). In the Taiwanese study, the exposure is assessed using control banding despite its high bias potential (Guseva Canu, Burstyn, and Richardson Citation2016b). The assessment of airborne exposure to ENMs based on aerosol sampling analysis should be considered as the minimal requirement pondering between the study feasibility in different occupational settings and the scientific value of its results. Whenever possible, it should be completed with a more thorough individual exposure assessment.
The relatively small numbers of participants as compared to the estimated nanotechnology workforce consistently reflect the difficulties in endorsement and recruitment of companies for epidemiological studies, and particularly the low response rate of ENM manufacturers. For example, the response rate in the NanoExplore survey was 2.4% (Crézé et al. Citation2021), in the International Commission on Occupational Health (ICOH) survey 2.6% (Iavicoli et al. Citation2019), in a Canadian survey 8.4% (Endo, Ostiguy, and Emond Citation2014), and in the French EpiNano program 16.0% (Guseva Canu et al. Citation2016c). The two rare examples of high company’ participation rates are surveys carried out in Switzerland (58.3%) (Schmid, Danuser, and Riediker Citation2010) and in the US (80%) (Schubauer-Berigan et al. Citation2018) in collaboration with the Swiss Ministry of Economy and Industry and the Swiss national work accident insurance, and the US-NIOSH, respectively. The difficulties experienced in research projects regarding company recruitment reflect the lack of awareness and regulation on emerging risks. It is noteworthy that once companies gave their agreement, workers generally have a favorable attitude to such studies and their participation rate can reach 75% (Beard et al. Citation2018) or even 99% (Guseva Canu et al. Citation2016c).
A successful implementation of a harmonized protocol in occupational settings concerned with ENM exposures in the ongoing study should demonstrate the feasibility of similar research projects in the future, facilitate further epidemiological studies and health surveillance programs, and inform stakeholders of regulatory aspects targeting occupational exposure to engineered and incidental nanoparticles. This protocol enables the launching of an international cohort of nanotechnology workers and, more generally, of workers exposed to a broad range of nanomaterials. As an open cohort, it could grow by including additional workers from new nanotechnology companies from various countries, which could join the NanoExplore Consortium and apply this harmonized protocol.
FUNDING
This work was supported by the European Commission LIFE program [Grant LIFE17 ENV/GR/000285, "NanoExplore" project]
Supplemental Material
Download Zip (346.6 KB)Acknowledgments
The authors acknowledge all members of NanoExplore consortium, and particularly Ms Judith Friesl, from Yordas GmbH (Germany), Ms Veronica Vela Vela from ITENE (Spain); Dr. Aikaterini -Maria Zarogianni, from NECCA (Greece);Dr Victor Dorribo and Dr Veronica Turcu from Unisanté (Switzerland); Dr. Valeria Bellisario, Dr. Federica Ghelli, Dr. Martina Buglisi, Prof. Roberto Bono from the Department of Public Health and Pediatrics at UNITO (Italy).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- 2021. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee: Second Regulatory Review on Nanomaterials. Brussels: European Commission.
- Afshari, R. 2017. “Reported Association of Air Pollution and Suicide Rate Could be Confounded.” Environmental Health : a Global Access Science Source 16 (1): 16. doi:10.1186/s12940-017-0219-3.
- Asbach, C., V. Neumann, C. Monz, D. Dahmann, M. VAN Tongeren, C. Alexander, L. Maccalman, and A. M. J. E. S. N. Todea. 2017. “On the Effect of Wearing Personal Nanoparticle Monitors on the Comparability of Personal Exposure Measurements.” Environmental Science: Nano 4 (1): 233–243. doi:10.1039/C6EN00362A.
- Audignon-Durand, S., C. Gramond, S. Ducamp, G. Manangama, A. Garrigou, F. Delva, P. Brochard, and A. Lacourt. 2021. “Development of a Job-Exposure Matrix for Ultrafine Particle Exposure: The MatPUF JEM.” Annals of Work Exposures and Health 65 (5): 516–527. doi:10.1093/annweh/wxaa126.
- Bates, J. T., T. Fang, V. Verma, L. H. Zeng, R. J. Weber, P. E. Tolbert, J. Y. Abrams, et al. 2019. “Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects.” Environmental Science & Technology 53 (8): 4003–4019. doi:10.1021/acs.est.8b03430.
- Beard, John D., Aaron Erdely, Matthew M. Dahm, Marie A. de Perio, M. Eileen Birch, Douglas E. Evans, Joseph E. Fernback, et al. 2018. “Carbon Nanotube and Nanofiber Exposure and Sputum and Blood Biomarkers of Early Effect among US Workers.” Environment International 116: 214–228. doi:10.1016/j.envint.2018.04.004.
- Bencsik, A., P. Lestaevel, and I. Guseva Canu. 2018. “Nano- and Neurotoxicology: An Emerging Discipline.” Progress in Neurobiology 160: 45–63. doi:10.1016/j.pneurobio.2017.10.003.
- Bergamaschi, E., V. Bellisario, M. Macrì, M. Buglisi, G. Garzaro, G. Squillacioti, F. Ghelli, et al. 2022. “A Biomonitoring Pilot Study in Workers from a Paints Production Plant Exposed to Pigment-Grade Titanium Dioxide (TiO2).” Toxics 10 (4): 171. doi:10.3390/toxics10040171.
- Bergamaschi, E., G. Garzaro, V. Bellisario, and F. Donato. 2019. DA3. Report on Critical Evaluation of Current Data on Biological Effects and Existing Biomarkers of Nanomaterial Exposure. Torino: NanoExplore Consortium.
- Bergamaschi, E., G. Garzaro, G. Wilson Jones, M. Buglisi, M. Caniglia, A. Godono, D. Bosio, I. Fenoglio, and I. Guseva Canu. 2021. “Occupational Exposure to Carbon Nanotubes and Carbon Nanofibres: More than a Cobweb.” Nanomaterials (Basel) 11 (3): 745. doi:10.3390/nano11030745.
- Bergamaschi, E., C. Poland, I. G. Canu, and A. Prina-Mello. 2015. “The Role of Biological Monitoring in Nano-Safety.” Nano Today. 10 (3): 274–277. doi:10.1016/j.nantod.2015.02.001.
- Brown, D. M., M. R. Wilson, W. Macnee, V. Stone, and K. Donaldson. 2001. “Size-Dependent Proinflammatory Effects of Ultrafine Polystyrene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines.” Toxicology and Applied Pharmacology 175 (3): 191–199. doi:10.1006/taap.2001.9240.
- Cao, X., L. Lin, A. Sood, Q. Ma, X. Zhang, Y. Liu, H. Liu, et al. 2020. “Small Airway Wall Thickening Assessed by Computerized Tomography is Associated with Low Lung Function in Chinese Carbon Black Packers.” Toxicological Sciences : An Official Journal of the Society of Toxicology 178 (1): 26–35. doi:10.1093/toxsci/kfaa134.
- Cedervall, T., I. Lynch, S. Lindman, T. Berggård, E. Thulin, H. Nilsson, K. A. Dawson, and S. Linse. 2007. “Understanding the Nanoparticle–Protein Corona Using Methods to Quantify Exchange Rates and Affinities of Proteins for Nanoparticles.” Proceedings of the National Academy of Sciences of the United States of America 104 (7): 2050–2055. doi:10.1073/pnas.0608582104.
- Checkoway, H., N. Pearce, J. L. Hickey, and J. M. Dement. 1990. “Latency Analysis in Occupational Epidemiology.” Archives of Environmental Health 45 (2): 95–100. doi:10.1080/00039896.1990.9935932.
- Chen, Z., S. Han, J. Zhang, P. Zheng, X. Liu, Y. Zhang, and G. Jia. 2021. “Metabolomics Screening of Serum Biomarkers for Occupational Exposure of Titanium Dioxide Nanoparticles.” Nanotoxicology 15 (6): 832–849. doi:10.1080/17435390.2021.1921872. [AQ3]
- Crézé, Camille, Marjorie François, Nancy B. Hopf, Victor Dorribo, Jean-Jacques Sauvain, Enrico Bergamaschi, Giacomo Garzaro, et al. 2021. “Producers of Engineered Nanomaterials—What Motivates Company and Worker Participation in Biomonitoring Programs?” International Journal of Environmental Research and Public Health 18 (8): 3851. doi:10.3390/ijerph18083851.
- Dahm, M. M., S. Bertke, and M. K. Schubauer-Berigan. 2019. “Predicting Occupational Exposures to Carbon Nanotubes and Nanofibers Based on Workplace Determinants Modeling.” Annals of Work Exposures and Health 63 (2): 158–172. doi:10.1093/annweh/wxy102.
- Dávila-Grana, Á., L. Diego-González, Á. González-Fernández, and R. Simón-Vázquez. 2018. “Synergistic Effect of Metal Oxide Nanoparticles on Cell Viability and Activation of MAP Kinases and NFκB.” International Journal of Molecular Sciences 19 (1): 246. doi:10.3390/ijms19010246.
- Domat, M. 2019. DA.1 Report on Selected ENMs and Critical Processes. Paterna, Spain: ITENE.
- Drew, N. M., E. D. Kuempel, Y. Pei, and F. Yang. 2017. “A Quantitative Framework to Group Nanoscale and Microscale Particles by Hazard Potency to Derive Occupational Exposure Limits: Proof of Concept Evaluation.” Regulatory Toxicology and Pharmacology : RTP 89: 253–267. doi:10.1016/j.yrtph.2017.08.003.
- Ellenbecker, M. J., and C. S.-J. Tsai. 2015. Exposure Assessment and Safety Considerations for Working with Engineered Nanoparticles. Hoboken, New Jersey, John Wiley & Sons.
- Endo, C.-A., C. Ostiguy, and C. Emond. 2014. Portrait de la nanotechnologie au Québec dans les milieux industriels et de la recherche universitaire et publique Institut de recherche Robert-Sauvé en santé et en sécurité du travail.
- Erdely, Aaron, Tracy Hulderman, Rebecca Salmen, Angie Liston, Patti C. Zeidler-Erdely, Diane Schwegler-Berry, Vincent Castranova, et al. 2009. “Cross-Talk between Lung and Systemic Circulation during Carbon Nanotube Respiratory Exposure. Potential Biomarkers.” Nano Letters 9 (1): 36–43. doi:10.1021/nl801828z.
- European Commission 2011. “Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial Text with EEA Relevance.” Official Journal of the European Union L275: 38–40.
- European Commission 2014. Guidance on the Protection of the Health and Safety of Workers from the Potential Risks Related to Nanomaterials at Work. Brussels: European Commission.
- Figueroa-Lara, J. J., J. M. Murcia-González, R. García-Martínez, M. Romero-Romo, M. Torres Rodríguez, and V. Mugica-Álvarez. 2019. “Effect of Platform Subway Depth on the Presence of Airborne PM2.5, Metals, and Toxic Organic Species.” Journal of Hazardous Materials 377: 427–436. doi:10.1016/j.jhazmat.2019.05.091.
- Fonseca, A. S., A.-K. Viitanen, T. Kanerva, A. Säämänen, O. Aguerre-Chariol, S. Fable, A. Dermigny, et al. 2021. “Occupational Exposure and Environmental Release: The Case Study of Pouring TiO2 and Filler Materials for Paint Production.” International Journal of Environmental Research and Public Health 18 (2): 418. doi:10.3390/ijerph18020418.
- Ghafari, J., N. Moghadasi, and S. O. Shekaftik. 2020. “Oxidative Stress Induced by Occupational Exposure to Nanomaterials: A Systematic Review.” Industrial Health 58 (6): 492–502. doi:10.2486/indhealth.2020-0073.
- Ghosh, M., D. Öner, K. Poels, A. M. Tabish, J. Vlaanderen, A. Pronk, E. Kuijpers, et al. 2017. “Changes in DNA Methylation Induced by Multi-Walled Carbon Nanotube Exposure in the Workplace.” Nanotoxicology 11 (9-10): 1195–1210. doi:10.1080/17435390.2017.1406169.
- Goekce, S., N. Concha-Lozano, J.-J. Sauvain, M. Hemmendinger, A. Portela, E. Sergent, P. Andujar, J.-C. Pairon, P. Wild, and G. J. M. I. N. T. Suárez, 2022. "Multiscattering-enhanced Absorbance to Enable the Sensitive Analysis of Extremely Diluted Biological Samples: Determination of Oxidative Potential in Exhaled Air". Medicine in Novel Technology and Devices 14, 100120.
- Graczyk, H., N. Lewinski, J. Zhao, J.-J. Sauvain, G. Suarez, P. Wild, B. Danuser, and M. Riediker. 2015. “Increase in Oxidative Stress Levels following Welding Fume Inhalation: A Controlled Human Exposure Study.” Particle and Fibre Toxicology 13 (1): 1–14. doi:10.1186/s12989-016-0143-7.
- Graille, M., P. Wild, J. J. Sauvain, M. Hemmendinger, I. Guseva Canu, and N. B. Hopf. 2020b. “Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis.” International Journal of Molecular Sciences. 21 (11): 3743.
- Graille, M., P. Wild, J.-J. Sauvain, M. Hemmendinger, I. Guseva Canu, and N. B. Hopf. 2020a. “Urinary 8-Isoprostane as a Biomarker for Oxidative Stress. A Systematic Review and Meta-Analysis.” Toxicology Letters 328: 19–27. doi:10.1016/j.toxlet.2020.04.006.
- Gulumian, M., J. Verbeek, C. Andraos, N. Sanabria, and P. DE Jager. 2016. “Systematic Review of Screening and Surveillance Programs to Protect Workers from Nanomaterials.” PloS One 11 (11): e0166071. doi:10.1371/journal.pone.0166071.
- Guseva Canu, I., O. Boutou-Kempf, L. Delabre, S. Ducamp, Y. Iwatsubo, J.-L. Marchand, and E. Imbernon. 2013. “French Registry of Workers Handling Engineered Nanomaterials as an Instrument of Integrated System for Surveillance and Research.” Journal of Physics: Conference Series 429: 012066. IOP Publishing. doi:10.1088/1742-6596/429/1/012066.
- Guseva Canu, I., T. F. Bateson, V. Bouvard, M. Debia, C. Dion, K. Savolainen, and I. J. Yu. 2016a. “Human Exposure to Carbon-Based Fibrous Nanomaterials: A Review.” International Journal of Hygiene and Environmental Health. 219 (2): 166–175. doi:10.1016/j.ijheh.2015.12.005.
- Guseva Canu, I., K. Batsungnoen, A. Maynard, and N. B. Hopf. 2020. “State of Knowledge on the Occupational Exposure to Carbon Nanotubes.” International Journal of Hygiene and Environmental Health 225: 113472. doi:10.1016/j.ijheh.2020.113472.
- Guseva Canu, I., I. Burstyn, and D. B. Richardson. 2016b. “Commentary: The Order of Things: Control Bands for Risk Managers and Scientists.” Epidemiology 27 (5): 765–768. doi:10.1097/EDE.0000000000000491.
- Guseva Canu, I., C. Crézé, M. Hemmendinger, T. Ben Rayana, S. Besançon, V. Jouannique, A. Debatisse, et al. 2021a. “Particle and Metal Exposure in Parisian Subway: Relationship between Exposure Biomarkers in Air, Exhaled Breath Condensate, and Urine.” International Journal of Hygiene and Environmental Health. 237: 113837. doi:10.1016/j.ijheh.2021.113837.
- Guseva Canu, I., M. Hemmendinger, J. J. Sauvain, G. Suarez, N. B. Hopf, J. A. Pralong, T. Ben Rayana, et al. 2021b. “Respiratory Disease Occupational Biomonitoring Collaborative Project (ROBoCoP): a Longitudinal Pilot Study and Implementation Research in the Parisian Transport Company.” Journal of Occupational Medicine and Toxicology 16 (1): 22. doi:10.1186/s12995-021-00312-4.
- Guseva Canu, I., M. Hemmendinger, A. Toto, P. Wild, C. Veys-Takeuchi, M. Bochud, G. Suárez. 2022. “Oxidative Potential in Exhaled Air (OP EA) as a Tool for Predicting Certain Respiratory Disorders in the General Adult Population: Cross-Sectional Analysis Nested in the Swiss Health Study”. Antioxidants, 11(10):2079. https://doi.org/10.3390/antiox11102079
- Guseva Canu, I., D. Jezewski-Serra, L. Delabre, S. Ducamp, Y. Iwatsubo, S. Audignon-Durand, C. Ducros, et al. 2017. “Qualitative and Semiquantitative Assessment of Exposure to Engineered Nanomaterials within the French EpiNano Program: inter-and Intramethod Reliability Study.” Annals of Work Exposures and Health 61 (1): 87–97. doi:10.1093/annweh/wxw008.
- Guseva Canu, I., D. Jezewski-Serra, D. Laurene, S. Ducamp, A. Lemaitre, K. Chami, I. Jaegle, and Y. Iwatsubo. 2016c. "O33-2 Epinano, the French Program for Epidemiological Surveillance of Workers Potentially Exposed to Engineered Nanomaterials: The State of Play after First 18 Months of Operation." Occupational and Environmental Medicine 73 (Suppl 1): A61.2–A61.
- Guseva Canu, I., P. A. Schulte, M. Riediker, L. Fatkhutdinova, and E. Bergamaschi. 2018. “Methodological, Political and Legal Issues in the Assessment of the Effects of Nanotechnology on Human Health.” Journal of Epidemiology and Community Health 72 (2): 148–153. doi:10.1136/jech-2016-208668.
- Harris, P. A., R. Taylor, R. Thielke, J. Payne, N. Gonzalez, and J. G. Conde. 2009. “Research Electronic Data Capture (REDCap) - a Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support.” Journal of Biomedical Informatics 42 (2): 377–381. doi:10.1016/j.jbi.2008.08.010.
- Hemmendinger, M., J. J. Sauvain, N. B. Hopf, P. Wild, G. Suárez, and I. Guseva Canu. 2021. “Method Validation and Characterization of the Associated Uncertainty for Malondialdehyde Quantification in Exhaled Breath Condensate.” Antioxidants (Basel) 10 (11): 1661.
- Hemmendinger, M., P. Wild, Y. Shoman, M. Graille, E. Bergamaschi, N. Hopf, and I. Guseva Canu. 2020. “Reference Ranges of Oxidative Stress Biomarkers Selected for Non-Invasive Biological Surveillance of Nanotechnology Workers: Study Protocol and Meta-Analysis Results for 8-OHdG in Exhaled Breath Condensate.” Toxicology Letters 327: 41–47.
- Hennig, F., U. Quass, B. Hellack, M. Küpper, T. A. J. Kuhlbusch, M. Stafoggia, and B. Hoffmann. 2018. “Ultrafine and Fine Particle Number and Surface Area Concentrations and Daily Cause-Specific Mortality in the Ruhr Area, Germany, 2009–2014.” Environmental Health Perspectives 126 (2): 027008. doi:10.1289/EHP2054.
- Hodson L, C. Geraci, and P. Schulte 2019. NIOSH nanotechnology research plan for 2018–2025. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH). doi:10.26616/NIOSHPUB2019116.
- Hopf, N. B., E. Bourgkard, V. Demange, S. Hulo, J. J. Sauvain, R. Levilly, F. Jeandel, et al. 2019. “Early Effect Markers and Exposure Determinants of Metalworking Fluids among Metal Industry Workers: Protocol for a Field Study.” JMIR Research Protocols 8 (8): e13744. doi:10.2196/13744.
- Iavicoli, I., V. Leso, M. Piacci, D. L. Cioffi, I. Guseva Canu, and P. A. Schulte. 2019. “An Exploratory Assessment of Applying Risk Management Practices to Engineered Nanomaterials.” International Journal of Environmental Research and Public Health 16 (18): 3290. doi:10.3390/ijerph16183290.
- Iavicoli, I., V. Leso, and P. A. Schulte. 2016. “Biomarkers of Susceptibility: State of the Art and Implications for Occupational Exposure to Engineered Nanomaterials.” Toxicology and Applied Pharmacology 299: 112–124. doi:10.1016/j.taap.2015.12.018.
- Jones, K. 2020. “Occupational Biological Monitoring-is Now the Time?” Industrial Health 58 (6): 489–491. doi:10.2486/indhealth.58_600.
- Kastury, F., E. Smith, and A. Juhasz. 2017. “A Critical Review of Approaches and Limitations of Inhalation Bioavailability and Bioaccessibility of Metal(Loid)s from Ambient Particulate Matter or Dust.” The Science of the Total Environment 574: 1054–1074. doi:10.1016/j.scitotenv.2016.09.056.
- Krug, H. F. 2014. “Nanosafety Research—Are we on the Right Track?” Angewandte Chemie (International ed. in English) 53 (46): 12304–12319. doi:10.1002/anie.201403367.
- Kuempel E, J. R. Roberts, G. Roth, K. L. Dunn, R. Zumwalde, N. Drew, A. Hubbs, D. Trout, and G. Holdsworth. 2021. Current Intelligence Bulletin 70: health effects of occupational exposure to silver nanomaterials. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH). doi:10.26616/NIOSHPUB2021112.
- Kuijpers, E., A. Pronk, R. Kleemann, J. Vlaanderen, Q. Lan, N. Rothman, D. Silverman, P. Hoet, L. Godderis, and R. Vermeulen. 2018. “Cardiovascular Effects among Workers Exposed to Multiwalled Carbon Nanotubes.” Occupational and Environmental Medicine 75 (5): 351–358. doi:10.1136/oemed-2017-104796.
- Lepistö, T., H. Kuuluvainen, H. Lintusaari, N. Kuittinen, L. Salo, A. Helin, J. V. Niemi, et al. 2022. “Connection between Lung Deposited Surface Area (LDSA) and Black Carbon (BC) Concentrations in Road Traffic and Harbour Environments.” Atmospheric Environment 272: 118931. doi:10.1016/j.atmosenv.2021.118931.
- Liao, Hui-Yi, Yu-Teh Chung, Ching-Huang Lai, Shu-Li Wang, Hung-Che Chiang, Lih-Ann Li, Tsui-Chun Tsou, et al. 2014. “Six-Month Follow-up Study of Health Markers of Nanomaterials among Workers Handling Engineered Nanomaterials.” Nanotoxicology 8 (sup1): 100–110. doi:10.3109/17435390.2013.858793.
- Li, Z., T. Hulderman, R. Salmen, R. Chapman, S. S. Leonard, S.-H. Young, A. Shvedova, M. I. Luster, and P. P. Simeonova. 2007. “Cardiovascular Effects of Pulmonary Exposure to Single-Wall Carbon Nanotubes.” Environmental Health Perspectives 115 (3): 377–382. doi:10.1289/ehp.9688.
- Li, G., L. Liang, J. Yang, L. Zeng, Z. Xie, Y. Zhong, X. Ruan, et al. 2018. “Pulmonary Hypofunction Due to Calcium Carbonate Nanomaterial Exposure in Occupational Workers: A Cross-Sectional Study.” Nanotoxicology 12 (6): 571–585. doi:10.1080/17435390.2018.1465606.
- Lind, L., J. A. Araujo, A. Barchowsky, S. Belcher, B. R. Berridge, N. Chiamvimonvat, W. A. Chiu, et al. 2021. “Key Characteristics of Cardiovascular Toxicants.” Environmental Health Perspectives 129 (9): 95001. doi:10.1289/EHP9321.
- Lugtig, P., and P. A. Smith. 2019. The Choice between a Panel and Cohort Study Design. Southampton, UK: University of Southampton. https://eprints.soton.ac.uk/435301/1/WP4_The_choice_between_a_panel_and_cohort_study_design.pdf
- Manno, M., C. Viau, J. Cocker, C. Colosio, L. Lowry, A. Mutti, M. Nordberg, and S. Wang, 2010. “Biomonitoring for Occupational Health Risk Assessment (BOHRA).” Toxicology Letters 192 (1): 3–16. doi:10.1016/j.toxlet.2009.05.001.
- Marie-Desvergne, C., M. Dubosson, L. Touri, E. Zimmermann, M. Gaude-Môme, L. Leclerc, C. Durand, et al. 2016. “Assessment of Nanoparticles and Metal Exposure of Airport Workers Using Exhaled Breath Condensate.” Journal of Breath Research 10 (3): 036006. doi:10.1088/1752-7155/10/3/036006.
- Methner, M., L. Hodson, and C. Geraci. 2010. “Nanoparticle Emission Assessment Technique (NEAT) for the Identification and Measurement of Potential Inhalation Exposure to Engineered Nanomaterials–Part A.” Journal of Occupational and Environmental Hygiene 7 (3): 127–132. doi:10.1080/15459620903476355.
- Monopoli, M. P., C. Åberg, A. Salvati, and K. A. Dawson. 2012. “Biomolecular Coronas Provide the Biological Identity of Nanosized Materials.” Nature Nanotechnology 7 (12): 779–786. doi:10.1038/nnano.2012.207.
- Mostovenko, E., M. M. Dahm, M. K. Schubauer-Berigan, T. Eye, A. Erdely, T. L. Young, M. J. Campen, and A. K. Ottens. 2021. “Serum Peptidome: diagnostic Window into Pathogenic Processes following Occupational Exposure to Carbon Nanomaterials.” Particle and Fibre Toxicology 18 (1): 39. doi:10.1186/s12989-021-00431-0.
- Müller, L., S. K. Steiner, L. Rodriguez-Lorenzo, A. Petri-Fink, B. Rothen-Rutishauser, and P. Latzin. 2018. “Exposure to Silver Nanoparticles Affects Viability and Function of Natural Killer Cells, Mostly via the Release of Ions.” Cell Biology and Toxicology 34 (3): 167–176. doi:10.1007/s10565-017-9403-z.
- Murphy, A., A. Casey, G. Byrne, G. Chambers, and O. Howe. 2016. “Silver Nanoparticles Induce Pro‐Inflammatory Gene Expression and Inflammasome Activation in Human Monocytes.” Journal of Applied Toxicology : JAT 36 (10): 1311–1320. doi:10.1002/jat.3315.
- Nieuwenhuijsen, M., D. Paustenbach, and R. Duarte-Davidson. 2006. “New Developments in Exposure Assessment: The Impact on the Practice of Health Risk Assessment and Epidemiological Studies.” Environment International 32 (8): 996–1009. doi:10.1016/j.envint.2006.06.015.
- NIOSH 2018. Workplace Design Solutions: Protecting Workers during the Handling of Nanomaterials.
- Oberdörster, G., E. Oberdörster, and J. Oberdörster. 2005. “Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles.” Environmental Health Perspectives 113 (7): 823–839. doi:10.1289/ehp.7339.
- Patel, S., A. Leavey, A. Sheshadri, P. Kumar, S. Kandikuppa, J. Tarsi, K. Mukhopadhyay, et al. 2018. “Associations between Household Air Pollution and Reduced Lung Function in Women and Children in Rural Southern India.” Journal of Applied Toxicology : JAT 38 (11): 1405–1415. doi:10.1002/jat.3659.
- Pauluhn, J. 2011. “Poorly Soluble Particulates: searching for a Unifying Denominator of Nanoparticles and Fine Particles for DNEL Estimation.” Toxicology 279 (1-3): 176–188. doi:10.1016/j.tox.2010.10.009.
- Pelclova, D., V. Zdimal, P. Kacer, M. Komarc, Z. Fenclova, S. Vlckova, N. Zikova, et al. 2017. “Markers of Lipid Oxidative Damage among Office Workers Exposed Intermittently to Air Pollutants Including nanoTiO(2) Particles.” Reviews on Environmental Health 32 (1-2): 193–200. doi:10.1515/reveh-2016-0030.
- Pelclova, D., V. Zdimal, M. Komarc, J. Schwarz, J. Ondracek, L. Ondrackova, M. Kostejn, et al. 2020. “Three-Year Study of Markers of Oxidative Stress in Exhaled Breath Condensate in Workers Producing Nanocomposites, Extended by Plasma and Urine Analysis in Last Two Years.” Nanomaterials (Basel) 10 (12): 2440.
- Pelclova, D., V. Zdimal, J. Schwarz, S. Dvorackova, M. Komarc, J. Ondracek, M. Kostejn, et al. 2018. “Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites.” Nanomaterials 8 (8): 611. doi:10.3390/nano8080611.
- Pétremand, R., P. Wild, C. Crézé, G. Suarez, S. Besançon, V. Jouannique, A. Debatisse, and I. Guseva Canu. 2021. “Application of the Bayesian Spline Method to Analyze Real-Time Measurements of Ultrafine Particle Concentration in the Parisian Subway.” Environment International 156: 106773. doi:10.1016/j.envint.2021.106773.
- Pétremand R., G. Suarez, S. Besançon, H. Dil, I. Guseva Canu. 2022. “A real-time comparison of four particulate matter size fractions in the personal breathing zone of Paris subway workers: A six-week prospective study.” Sustainability 14: 5999. https://doi.org/10.3390/su14105999
- Renaudie, N., V. Tassy, Y. Iwatsubo, B. Sobczak, I. Tordjman, P. Maladry, L. Delabre, et al. 2018. “EpiNano Fait Peau Neuv.” Archives Des Maladies Professionnelles Et De L'Environnement 79 (4): 574. doi:10.1016/j.admp.2018.05.031.
- Riediker, M., M. K. Schubauer-Berigan, D. H. Brouwer, I. Nelissen, G. Koppen, E. Frijns, K. A. Clark, et al. 2012. “A Road Map toward a Globally Harmonized Approach for Occupational Health Surveillance and Epidemiology in Nanomaterial Workers.” Journal of Occupational & Environmental Medicine 54 (10): 1214–1223. doi:10.1097/JOM.0b013e31826e27f1.
- Roco, M. C. 2011. “The Long View of Nanotechnology Development: The National Nanotechnology Initiative at 10 Years.” Journal of Nanoparticle Research13: 427–445. doi:10.1007/s11051-010-0192-z.
- Rose, G. 2005. “Incubation Period of Coronary Heart Disease.” International Journal of Epidemiology 34 (2): 242–244. doi:10.1093/ije/dyh308.
- Sauvain, J. J., M. Hemmendinger, G. Suárez, C. Creze, N. B. Hopf, V. Jouannique, A. Debatisse, J. A. Pralong, P. Wild, and I. Guseva Canu. 2022. “Malondialdehyde and Anion Patterns in Exhaled Breath Condensate among Subway Workers.” Particle and Fibre Toxicology 19 (1): 16. doi:10.1186/s12989-022-00456-z.
- Sauvain, J. J., G. Suarez, J. L. Edmé, O. M. Bezerra, K. G. Silveira, L. S. Amaral, A. P. Carneiro, N. Chérot-Kornobis, A. Sobaszek, and S. Hulo. 2017. “Method Validation of Nanoparticle Tracking Analysis to Measure Pulmonary Nanoparticle Content: The Size Distribution in Exhaled Breath Condensate Depends on Occupational Exposure.” Journal of Breath Research 11 (1): 016010. doi:10.1088/1752-7163/aa56dd.
- Sauvain, J. J., G. Suarez, N. B. Hopf, K. Batsungnoen, N. Charriere, F. Andre, R. Levilly, and P. Wild. 2021. “Oxidative Potential of Aerosolized Metalworking Fluids in Occupational Settings.” International Journal of Hygiene and Environmental Health 235: 113775. doi:10.1016/j.ijheh.2021.113775.
- Schmid, K., B. Danuser, and M. Riediker. 2008. Swiss nano-inventory. An assessment of the usage in Swiss Industry, final report, Edition IST
- Schmid, K., B. Danuser, and M. Riediker. 2010. “Nanoparticle Usage and Protection Measures in the Manufacturing Industry–a Representative Survey.” Journal of Occupational and Environmental Hygiene 7 (4): 224–232. doi:10.1080/15459621003609127.
- Schubauer-Berigan, M. K., M. M. Dahm, A. Erdely, J. D. Beard, M. Eileen Birch, D. E. Evans, J. E. Fernback, et al. 2018. “Association of Pulmonary, Cardiovascular, and Hematologic Metrics with Carbon Nanotube and Nanofiber Exposure among U.S. workers: A Cross-Sectional Study.” Particle and Fibre Toxicology 15 (1): 22. doi:10.1186/s12989-018-0258-0.
- Schubauer-Berigan, M. K., M. M. Dahm, C. A. Toennis, D. L. Sammons, T. Eye, V. Kodali, P. C. Zeidler-Erdely, and A. Erdely. 2020. “Association of Occupational Exposures with ex Vivo Functional Immune Response in Workers Handling Carbon Nanotubes and Nanofibers.” Nanotoxicology 14 (3): 404–419. doi:10.1080/17435390.2020.1717007.
- Schulte, Paul A., Ivo Iavicoli, Jorma H. Rantanen, Dirk Dahmann, Sergio Iavicoli, Rüdiger Pipke, Irina Guseva Canu, et al. 2016. “Assessing the Protection of the Nanomaterial Workforce.” Nanotoxicology 10 (7): 1013–1019. doi:10.3109/17435390.2015.1132347.
- Schulte, P. A., V. Leso, M. Niang, and I. Iavicoli. 2019. “Current State of Knowledge on the Health Effects of Engineered Nanomaterials in Workers: A Systematic Review of Human Studies and Epidemiological Investigations.” Scandinavian Journal of Work, Environment & Health 45 (3): 217–238. doi:10.5271/sjweh.3800.
- Schulte, P., V. Leso, M. Niang, and I. Iavicoli. 2018. “Biological Monitoring of Workers Exposed to Engineered Nanomaterials.” Toxicology Letters 298: 112–124. doi:10.1016/j.toxlet.2018.06.003.
- Schulte, P. A., M. K. Schubauer-Berigan, C. Mayweather, C. L. Geraci, R. Zumwalde, and J. L. Mckernan. 2009. “Issues in the Development of Epidemiologic Studies of Workers Exposed to Engineered Nanoparticles.” Journal of Occupational and Environmental Medicine 51 (3): 323–335. doi:10.1097/JOM.0b013e3181990c2c.
- Shoman, Y., P. Wild, M. Hemmendinger, M. Graille, J. J. Sauvain, N. B. Hopf, and I. Guseva Canu. 2020. “Reference Ranges of 8-Isoprostane Concentrations in Exhaled Breath Condensate (EBC): a Systematic Review and Meta-Analysis.” International Journal of Molecular Sciences. 21 (11): 3822.
- Shvedova, A. A., E. R. Kisin, R. Mercer, A. R. Murray, V. J. Johnson, A. I. Potapovich, Y. Y. Tyurina, et al. 2005. “Unusual Inflammatory and Fibrogenic Pulmonary Responses to Single-Walled Carbon Nanotubes in Mice.” American Journal of Physiology. Lung Cellular and Molecular Physiology 289 (5): L698–L708. doi:10.1152/ajplung.00084.2005.
- Smith, M. T., K. Z. Guyton, C. F. Gibbons, J. M. Fritz, C. J. Portier, I. Rusyn, D. M. Demarini, et al. 2016. “Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis.” Environmental Health Perspectives 124 (6): 713–721. doi:10.1289/ehp.1509912.
- Smolders, R., A. Bartonova, P. Boogaard, M. Dusinska, G. Koppen, F. Merlo, R. Sram, P. Vineis, and G. Schoeters. 2010. “The Use of Biomarkers for Risk Assessment: Reporting from the INTARESE/ENVIRISK Workshop in Prague.” International Journal of Hygiene and Environmental Health 213 (5): 395–400. doi:10.1016/j.ijheh.2010.05.006.
- Stone, V., S. Gottardo, E. A. J. Bleeker, H. Braakhuis, S. Dekkers, T. Fernandes, A. Haase, et al. 2020. “A Framework for Grouping and Read-across of Nanomaterials- Supporting Innovation and Risk Assessment.” Nano Today. 35: 100941. doi:10.1016/j.nantod.2020.100941.
- Strategies, O. J. J. N. R. O. 2017. Techniques and sampling protocols for determining the concentrations of manufactured nanomaterials in air at the workplace. Series on the safety of manufactured nanomaterials organisation for economic co-operation and development. 82.
- Tajani, A., and K. Edtstadler. 2018. Regulation (EU) 2018/1725 of the European Parlament and of the Council on the protection of natural persons with regard to the processing of personal data by the Union institutions, bodies, offices and agencies and on the free movement of such data, and repealing Regulation (EC).
- Toto, A., P. Wild, M. Graille, V. Turcu, C. Crézé, M. Hemmendinger, J. J. Sauvain, E. Bergamaschi, I. Guseva Canu, and N. B. Hopf. 2022. "Urinary Malondialdehyde (MDA) Concentrations in the General Population-A Systematic Literature Review and Meta-Analysis.” Toxics 10 (4): 160.
- Turcu, V., P. Wild, M. Hemmendinger, J. J. Sauvain, E. Bergamaschi, N. B. Hopf, and I. Guseva Canu. 2022. “Towards Reference Values for Malondialdehyde on Exhaled Breath Condensate: A Systematic Literature Review and me-ta-Analysis.” Toxics 10 (5): 258. doi:10.3390/toxics10050258.
- Ursini, C. L., A. M. Fresegna, A. Ciervo, R. Maiello, V. DEL Frate, G. Folesani, M. Galetti, et al. 2021. “Occupational Exposure to Graphene and Silica Nanoparticles. Part II: pilot Study to Identify a Panel of Sensitive Biomarkers of Genotoxic, Oxidative and Inflammatory Effects on Suitable Biological Matrices.” Nanotoxicology 15 (2): 223–237. doi:10.1080/17435390.2020.1850903.
- Viegas, S., Zare Jeddi , M. B. Hopf, N. Bessems, J. Palmen, N. S. Galea, K. Jones, et al. 2020. “Biomonitoring as an Underused Exposure Assessment Tool in Occupational Safety and Health Context-Challenges and Way Forward.” International Journal of Environmental Research and Public Health 17 (16): 5884. doi:10.3390/ijerph17165884.
- Viitanen, A.-K., S. Uuksulainen, A. J. Koivisto, K. Hämeri, and T. Kauppinen. 2017. “Workplace Measurements of Ultrafine Particles—a Literature Review.” Annals of Work Exposures and Health 61 (7): 749–758. doi:10.1093/annweh/wxx049.
- WHO 2006. Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals. Geneva: World Health Organization.
- Wu, W.-T., W.-T. Jung, and H.-L. Lee. 2021. “Lipid Peroxidation Metabolites Associated with Biomarkers of Inflammation and Oxidation Stress in Workers Handling Carbon Nanotubes and Metal Oxide Nanoparticles.” Nanotoxicology 15 (5): 577–587. doi:10.1080/17435390.2021.1879303.
- Wu, W. T., L. A. Li, T. C. Tsou, S. L. Wang, H. L. Lee, T. S. Shih, and S. H. Liou. 2019. “Longitudinal Follow-up of Health Effects among Workers Handling Engineered Nanomaterials: A Panel Study.” Environmental Health : a Global Access Science Source 18 (1): 107. doi:10.1186/s12940-019-0542-y.
- Zare Jeddi, M., N. B. Hopf, S. Viegas, A. B. Price, A. Paini, C. VAN Thriel, E. Benfenati, et al. 2021. “Towards a Systematic Use of Effect Biomarkers in Population and Occupational Biomonitoring.” Environment International 146: 106257. doi:10.1016/j.envint.2020.106257.
- Zhao, Lin., Yifang Zhu, Zhangjian Chen, Huadong Xu, Jingwen Zhou, Shichuan Tang, Zhizhen Xu, et al. 2018. “Cardiopulmonary Effects Induced by Occupational Exposure to Titanium Dioxide Nanoparticles.” Nanotoxicology 12 (2): 169–184. doi:10.1080/17435390.2018.1425502.

