The publishers would like to apologise for an error that occurred in the publication of Nanotoxicology, December 2008; 2(4): 252–274.
Genotoxicity of engineered nanomaterials: A critical review LAETITIA GONZALEZ1, DOMINIQUE LISON2, & MICHELINE KIRSCH-VOLDERS1
Tables and contained some errors and should have appeared as below:
Figure 2. Summary of the available studies conducted with the comet assay (A) or the micronucleus test (B). Results are categorized on the basis of chemical nature of the ENMs and their mean primary particle size. Statistically significant genotoxic effects or absence of genotoxic effects are represented as ▪ or □, respectively. Comet assay data on specific lesions detected by enzymatic treatment with formamidopyrimidine DNA-glycosylase (fpg) and endonuclease III (EndoIII) are included. The numbers in brackets refer to the individual studies reviewed in .
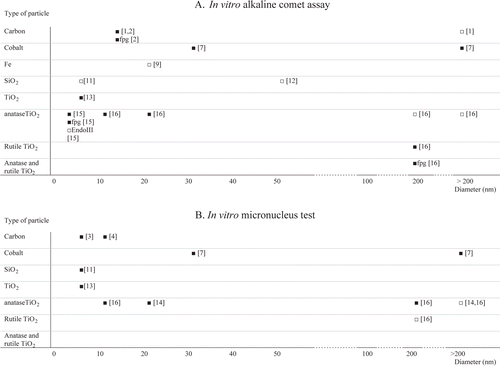
Table I. An overview of the genotoxicity endpoints that can be assessed in function of the cellular targets or functionality potentially affected by ENMs.
Table II. Summary of available in vitro and in vivo genotoxicity studies with ENMs (see text for selection procedure).
Table III. Evaluation of the available literature on in vitro and in vivo genotoxicity of ENMs based on the proposed minimal criteria for nanotoxicology and genotoxicology.
