ABSTRACT
A significant proportion of the population is classified as having overweight or obesity. One framework which has attempted to explain biobehavioral mechanisms influencing the development of overweight and obesity is the energy balance model. According to this model, the body continually attempts to balance energy intake with energy expenditure. When energy intake and energy expenditure become imbalanced, there is an increase in homeostatic and allostatic pressure, generally to either increase energy intake or decrease energy expenditure, so as to restore energy homeostasis.Recent research has indicated that circadian aspects of energy intake and energy expenditure may influence energy balance. This paper provides a narrative review of existing evidence of the role of circadian timing on components of energy balance. Research on the timing of food intake, physical activity, and sleep indicates that unhealthy timing is likely to increase risk of weight gain. Public health guidelines focus on how much individuals eat and sleep, what foods are consumed, and the type and frequency of exercise, but the field of circadian science has begun to demonstrate that when these behaviors occur may also influence overweight and obesity prevention and treatment efforts.
Obesity is a significant public health concern:42.4% United States adultshave obesity (body mass index (BMI) ≥ 30.0 kg/m2) (Hales et al., Citation2020), and an additional 33% of adults are classified as having overweight (BMI of 25.0-29.9 kg/m2) (Flegal et al., Citation2016). Individuals who have overweight or obesity are at risk for numerous negative health consequences, including hypertension, type 2 diabetes, depression, and all-cause mortality (Flegal et al., Citation2013; Hruby & Hu, Citation2015). Given the array ofadverse health effects associated with overweight and obesity, decades of research have sought to elucidate the biobehavioral factors influencing overweight and obesity development, which can then inform effective overweight and obesity prevention and treatment strategies.
The energy balance model is onesuch theoretical framework which has been developed to understand physiological and behavioral factors which lead to obesity. This model posits that the body continually attempts to maintain its fat stores through a coordinated balance between energy intake and energy expenditure (Schwartz et al., Citation2003; Woods et al., Citation1998). While there is some evidence of a more dynamic process involved in energy balance (Manore et al., Citation2017), a balance between energy intake and energy expenditure is the foundation of the energy balance model at its most basic level. When the body detects that energy intake and energy expenditure are unbalanced, this sets off a coordinated network of physiological and behavioral changes to restore balance. These physiological and behavioral changes consist of both homeostatic and allostatic processes (McEwen & Wingfield, Citation2010). Homeostatic processes allow the body to maintain homeostasis, i.e., relatively constant levels of physiological factors necessary for sustaining life, such as body temperature and blood pH. Alternatively, allostatic processes involve allostasis, defined as stability through change. Allostatic processescan thus involve a larger magnitude of changes that help the body adapt to unpredictable changes, such as increased cortisol levels. providesan overview of the ways in which the body imparts homeostatic and allostatic pressure to either increase energy intake or decrease energy expenditureso as to restoreenergy balance, i.e., to ensure energy intake and energy expenditure are balanced (Schwartz et al., Citation2003).
Figure 1. Diagram of energy balance model depicting physiological, behavioral, and psychological changes aimed at balancing energy intake and energy expenditure.
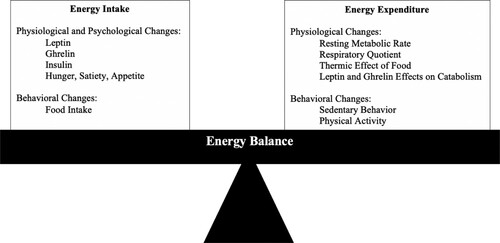
Various physiological, psychological, and behavioral factors are involved in maintaining energy balance through their role in energy intake. Physiological factors which contribute to energy intake include leptin, ghrelin, and insulin, and psychological factors include hunger, appetite, and satiety. Leptin is a hormone released by adipose tissue which signals the hypothalamus to stop energy intake. Ghrelin is a hormone primarily released by the stomach, which signals the hypothalamus to increase appetite, encouraging energy intake. Insulin is a pancreatic hormone that plays a vital role in maintaining blood glucose levels. The amount of glucose in blood rises after food intake. This increase in blood glucose triggers the release of insulin, which allows cells to either convert glucose into energy or store it for later use, thereby keeping blood glucose levels stable. A behavioral factor which contributes to energy intake is food intake. Major classes of nutrients which may be consumed during food intake are carbohydrates, fats, and proteins. Carbohydrates and fats provide the body with energy, and protein isimportant for the growth and repair of bodily tissues. Both physiological and behavioral changes may occur to increase or decrease energy intake (for reviews, see Hall et al., Citation2012; Hill et al., Citation2013; Keesey & Powley, Citation2008;Lenard & Berthoud, Citation2008; Schwartz et al., Citation2003). Physiological changes that may occur to increase energy intake include decreases inleptin and increases in ghrelin levels,decreases in insulin, increases in hungerand appetite, and decreases in satiety, and a behavioral change aimed at increasing energy intake is increased food intake (Schwartz et al., Citation2003). It should also be acknowledged that food choice, which is not under biological or homeostatic and allostatic control, also plays a role in determining food and macronutrient intake. When there is homeostatic and allostatic pressure to reduce energy intake, reciprocal biological and physiological processes typically occur (e.g., increases in leptin levels, decreased food intake).
Similarly, physiological and behavioral factors are involved in maintaining energy balance through their role in energy expenditure. Physiological components of energy expenditure include respiratory quotient, resting metabolic rate, and the thermic effect of food. The body’s respiratory quotient is the ratio of the volume of CO2 produced by the body to the volume of O2 used by the body. Resting metabolic rate is the largest proportion of energy expenditure; this term refers to the rate at which the body uses energy when it is at rest. The thermic effect of food refers to the energy required to ingest, digest, absorb, and store energy intake. Behavioral aspects of energy expenditure sedentary behavior and physical activity. Sedentary behavior is defined as any waking behavior that is characterized by low energy expenditure (i.e., ≤ 1.5 metabolic equivalents) performed while in a seated or lying posture (e.g., reading, watching television) (Tremblay et al., Citation2017). Physical activity, on the other hand, is any bodily movement that increases energy expenditure; it is commonly subcategorized into light, moderate, or vigorous physical activity based upon the perceived effort and energy expended. Thus, physiological and behavioral changes may also occur to either increase or decrease energy expenditure in response to homeostatic and allostatic pressure (for reviews, see Hall et al., Citation2012; Hill et al., Citation2013; Keesey & Powley, Citation2008; Lenard & Berthoud, Citation2008; Schwartz et al., Citation2003). Physiological adaptations to decrease energy expenditure may include an increased respiratory quotient, alowered resting metabolic rate, and decreased thermic effect of food, while behavioral changes aimed at decreasing energy expenditure consist of increased sedentary behavior and decreased time spent in moderate to vigorous physical activity. Opposite behavioral and biological changesgenerallyoccur in response to homeostatic and allostatic pressures toincrease energy expenditure (e.g., increased resting metabolic rate, decreased sedentary behavior).
Despite this sophisticated system of homeostatic and allostatic processes, energy imbalances do occur over time, and these imbalances alter body weight as well as health. If, over time, energy expenditure exceeds energy intake (i.e., negative energy balance), individuals lose weight. Alternatively, if energy intake chronically exceeds energy expenditure (i.e., positive energy balance), individuals gain weight. Likely because of the evolutionary advantage of engaging in behaviors that prevent starvation, this systemtends to favor weight gain in the modern obesogenic environment, in which individuals are eating, sleeping, working, and socializing throughout the 24-h day (Greenway, Citation2015; Schwartz et al., Citation2003). Thus, the advent of this 24-h society has indicated that when behaviors occur may also be important to consider in relation to energy balance, health, and obesity risk.
Table 1. Characteristics of original research studies cited in review.
Recent research seems to indicate that circadian aspects of energy intake and energy expenditure influence energy balance. To date, reviews have focused on links between genetic components of the circadian system and metabolism (e.g., Bass & Takahashi, Citation2010; Hurley et al., Citation2016; Kim et al., Citation2019; Lekkas & Paschos, Citation2019). Other reviews have examined links between metabolic processes, circadian processes, and individual behaviors related to energy balance (Chaput et al., Citation2020; Longo & Panda, Citation2016; Manoogian & Panda, Citation2017; Melkani & Panda, Citation2017; Serin & Acar Tek, Citation2019) while neglecting other behaviors which likelycontribute to energy balance. This paper willprovide a narrative review and critical analysis of existing evidence of the role ofcircadian timingof food intake, physical activity, and sleep behaviors on components of energy balance. In particular, this paper will examine homeostatic and allostatic processes that allow the body to adapt in an effortto restore balance. Thus, our paper builds upon existing reviews by incorporating relationships between the circadian timing of three key behaviors (i.e., food intake, physical activity, sleep) and energy balance, while also discussing evidence of allostatic and homeostatic pressuresthat promote energy balance.
A literature search was conduted in Google Scholar to identify relevant empirical articles using various combinations of search terms such as: chrononutrition, energy balance, circadian timing, food intake, sleep/wake timing, meal timing, physical activity timing, exercise timing, morning meals, breakfast eating, breakfast skipping, time-restricted feeding, time-restricted eating, evening meals, evening eating, night eating, body mass index, obesity, body weight, weight loss, energy intake, energy expenditure, weight gain, morning exercise, morning physical activity, evening exercise, evening physical activity, glucose, insulin, insulin resistance, social jetlag, shift work, morning shift, delayed bedtime, evening shift, overnight shift, fasting, eating window, metabolism, caloric intake, and metabolic hormones. References of retrieved articles and studies that were in the ‘Cited By’ list in Google Scholar were then screened to identify additional relevant studies. Included studies were those which examined at least one of the focal behaviors (i.e., timing of food intake, timing of physical activity, sleep/wake timing) and relationships between the behavior and components of energy balance (see for characteristics of original research studies cited in the present review). This search was limited to articles available in the English language.
The impact of circadian timing of food intake on energy balance
This section outlines literature on the circadian timing of food intake (i.e., chrononutrition) (Arble et al., Citation2009) and how it affects energy balance through physiological and behavioral changes that alter energy intake and energy expenditure. Key aspects of research are discussed, including late timing of food intake, early timing of food intake, and eating patterns across the 24-h day.
The timing of food intakeacross the 24-h day has been linked to components of energy balance in past research. A landmark study on chrononutrition was conducted by Arble et al. (Citation2009) in which nocturnal mice were fed a high-fat diet either during the typical inactive phase of the 24-h day (i.e., ‘wrong’ time of day), or during the typical active phase of the 24-h day (i.e., ‘right’ time of day). Extraordinarily, even though energy intake and energy expenditure did not differ between study conditions, the mice fed during the ‘wrong’ time of day gained significantly more weight than the mice fed during the ‘right’ time of day. Most notably, the rodent study conducted by Arble et al. (Citation2009) laid the foundation for the potential role of chrononutrition in energy balance. These findings have been replicated in mice by other researchers (Chaix et al., Citation2014; Hatori et al., Citation2012). This line of research has begun to be applied in human samples, though differences in study design somewhat preclude direct comparsions. In correlational studies of humans, eating the main meal late in the 24-h day (i.e., at the ‘wrong’ time of day), as opposed to eating an earlier main meal, was associated with less weight loss in individuals who had obesity, even when energy intake and energy expenditure were not different between study groups (Garaulet et al., Citation2013; Ruiz-Lozano et al., Citation2016). More recent animal research has shown that even if caloric intake is greater in a high-fat diet condition compared to a normal chow condition, the restriction of food intake to typical waking hours may still prevent weight gain and decrease adiposity; however, it may not protect against inflammation (Delahaye et al., Citation2018). This research has highlighted an important distinction between studies which hold caloric intake constant across conditions (e.g., Garaulet et al., Citation2013; Ruiz-Lozano et al., Citation2016) and those that do not hold caloric intake constant (Delahaye et al., Citation2018); due to this discrepancy, additional research is necessary to fully understand the relationships between caloric intake, timing of food intake, and energy balance. As few chrononutrition studies have reported data on energy expenditure, the existing evidence has focused on the quantity and quality of energy intake in relation to circadian timing.
Late timing of food intake
Overall, late timing of food intake in particular may be associated with increased energy intake. In their examination of Americans’ diurnal eating patterns, Gill and Panda (Citation2015) found that almost 40% of participants’ total daily energy intake was consumed after 6:00pm. This was deemed to be potentially problematic, as late meal intake was generally indicative of excessive total daily energy intake (i.e., contributing to a positive energy balance). Similar associations between eating later in the day anda greaterenergy intake have been reported by other researchers (Gluck et al., Citation2008; Reid et al., Citation2014), with men especially likely to engage in late-night eating (Spaeth et al., Citation2014). This increase in energy intake has also been linked to a positive energy balance, though the direction of this association remains unclear. While calorie consumption after 8:00pm has been linked togreater BMI (Baron et al., Citation2011), individuals who have obesitymay be more likely to engage in energy intake later in the day (Andersson & Rossner, Citation1996; Corbalan-Tutau et al., Citation2012; McHill et al., Citation2017; McHill et al., Citation2019; for exception, see Mills et al., Citation2011). Conversely, restricting nighttime energy intake (e.g., no energy intake from 7:00pm to 6:00am) may lead to a reduction in total daily energy intake (LeCheminant et al., Citation2013).
One factor which may be linked to increased homeostaticand allostatic pressure to engage in energy intake at night is a change in appetite for certain macronutrients throughout the 24-h day. Both humans and rats have an increased preference for high-fat foods at dinnertime compared to breakfast (Lax et al., Citation1998; Westerterp-Plantenga et al., Citation1996); preference for high-carbohydrate foods may also increase late at night (Gallant et al., Citation2014). Thisincrease in the homeostaticand allostatic pressure of appetite for certain nutrients may be problematic for energy balance, because foods consumed late at night typically are low in satiety (de Castro, Citation2004; de Castro, Citation2007; de Castro, Citation2009), may contain high amounts of sodium (Striegel-Moore et al., Citation2008) or carbohydrates (McHill et al., Citation2019), and may simply be less healthy (Barrington & Beresford, Citation2019); these so-called ‘empty calories’ may lead individuals to consume additional snacks or drinks in an attempt to be satiated. Based on experimental work in mice, an increased appetite for high-fat foods at night may exert homeostaticand allostatic pressure to increase the likelihood of weight gain: compared to mice who consumed a high-fat diet near the beginning of the active phase, consumption of a high-fat diet near the end of the active phase was associated with markers of poor metabolic health such as weight gain and glucose intolerance, though total daily energy intake was identical between groups (Bray et al., Citation2010).
These effects of late-night eating on energy balance may also be due to melatonin, a hormone regulated by the central circadian clock that is released by the pineal gland at nighttime. Though melatonin may alter homeostatic and allostatic pressure in a variety of ways related to energy intake, melatonin seems to exert the greatest effects on insulin. During the day, i.e., when melatonin is low, insulin sensitivity is increased (Reutrakul & Van Cauter, Citation2014), whereas at night, i.e., when melatonin is high, insulin resistance is more likely (for reviews, see Cipolla-Neto et al., Citation2014; Owino et al., Citation2019). Additional research has shown that melatonin may suppress insulin release (Tuomi et al., Citation2016) and contribute to impaired glucose tolerance (Lopez-Minguez et al., Citation2018; Van Cauter et al., Citation1989), both of which have been related to obesity (Ferrannini & Camastra, Citation1998). Melatonin is considered a key regulator of leptin, as leptin also peaks late at night and decreases during the day (Saladin et al., Citation1995). Without melatonin, leptin signaling becomes impaired,potentially leading to leptin resistance over time (Buonfiglio et al., Citation2018). Thus, these physiological alterations in leptin, glucose, and ghrelin may increase homeostatic and allostatic pressure to engage in energy intake at night and could account for findings linking melatonin signaling to obesity (for review, see Cipolla-Neto et al., Citation2014). For example, energy intake close to melatonin onset has been associated with increased body fat and BMI (McHill et al., Citation2017). Taken together, individuals may be predisposed to engage in energy intake late at night due to increased preference for energy-dense foods, and melatonin may alter homeostatic and allostatic pressures such as insulin sensitivity and glucose tolerance when late-night energy intake occurs, contributing to a positive energy balance. Not all research to date has supported this relationship, however. Research in humans has highlighted some contrasting findings, such that higher concentrations of melatonin are linked to better glucose control (for review, see Garaulet et al., Citation2020). Garaulet et al. (Citation2020) hypothesize that the timing of the body’s melatonin cycle relative to the feeding/fasting cycle may account for these inconsistent findings; i.e., increased melatonin during the feeding cycle is linked to impaired glucose tolerance but increased melatonin during the fasting cycle may be beneficial for the body.
Early timing of food intake
Research has also examined how energy intake early in the day may affect energy balance. The first eating event of the day (i.e., breakfast) perhaps has the largest impact on the body’s metabolic processes for the rest of the day. This is likely because the first eating event occurring after the overnight fasting period (i.e., nocturnal sleep; suggested to be a minimum of 10-12 h (International Standards Organisation, Citation2010)) sets the phase of the body’s liver clock (Hirao et al., Citation2010). The liver clock refers to a set of genetic feedback loops within cells of the liver that function as as a self-sustained timekeeper to ensure vital metabolic processes (e.g., carbohydrate metabolism) occur at the ‘right’ time of day, and to help the liver and body maintain homeostasis through the varying physiological and behavioral time cues throughout the 24-hour day (for review, see Reinke & Asher, Citation2016). Thus, alterations to the timing of this first eating event may alter the timing of numerous metabolic processes, such as glucose homeostasis (Bolli et al., Citation1984; la Fleur et al., Citation2001; Lamia et al., Citation2008) and lipid metabolism (Adamovich et al., Citation2014; Masri et al., Citation2014) (for reviews, see Adamovich et al., Citation2015; Poggiogalle et al., Citation2018), while also altering the timing of other eating events.
Breakfast skipping may also alter homeostaticand allostatic pressures. Individuals who skip breakfast, for instance, may be more likely to engage in more mid-day snacking (Sajjad et al., Citation2014) or consume lunch earlier (Kant & Graubard, Citation2015) compared to those who eat breakfast. Though breakfast skipping – and, by extension, an earlier lunchtime – could hypothetically contribute to an earlier evening meal time, Nakajima (Citation2018) have suggested that breakfast skipping is actuallytied to a delayed eating schedule. Some evidence for this association has come from studies of night eating. Given that one criterion for night eating syndrome is lack of appetite in the morning following food intake late at night (Allison et al., Citation2010), nighttime eating may not only alter the phase of the body’s liver clock (Kuroda et al., Citation2012), but also decrease the homeostatic and allostatic pressure of appetite and delay the timing of individuals’ first eating event of the day, thus likely being linked to a delayed eating schedule throughout the 24-h day (Allison et al., Citation2005; Goel et al., Citation2009; O’Reardon et al., Citation2005). This association between late-night eating and skipping breakfast has also been observed in non-clinical samples (Karatzi et al., Citation2017; Kutsuma et al., Citation2014; Okada et al., Citation2019). In an effort to tease apart causal factors, experimental research has suggested that this relationship between breakfast skipping and late-night eating may be due in part to a daily endogenous peak in general hunger in the evening (i.e., 8:00pm) and a nadir in the morning (i.e., 8:00am) (Scheer et al., Citation2013), which would increase homeostaticand allostatic pressure to engage in late-night eating and decrease homeostatic and allostatic pressure to engage in energy intake in the morning. However, additional research is necessaryto determine directionality of the association between late-night eating and breakfast skipping.
In addition, research has examined the effect of consumption of certain nutrients at breakfast onhomeostatic and allostatic pressures tied to energy intake. For instance, a breakfast rich in protein has been associated with both initial and longer-term feelings of fullness and satiety, and reduced levels of the appetite-stimulating hormone ghrelin (de Castro, Citation2007; Leidy et al., Citation2007; Rains et al., Citation2015); a high-carbohydrate and protein breakfast may have similar effects on appetite and satiety (Jakubowicz et al., Citation2012). However, studies of the effect of breakfast skipping on body weight are inconsistent (for review, see St-Onge et al., Citation2017). For instance, a foundational clinical trial by Schlundt et al. (Citation1992) demonstrated that a change in breakfast consumption behaviors (i.e., habitual breakfast skippers beginning to eat breakfast and habitual breakfast eaters beginning to skip breakfast) led to greater weight loss compared to those who maintained breakfast consumption behaviors, rather than an individual’s breakfast behavioral pattern in and of itself. Other studies have reported no effects of breakfast skipping on body weight, compared to breakfast consumption in individuals with overweight and obesity (Chowdhury et al., Citation2016), and in individuals at a healthy weight (Betts et al., Citation2014). Other research has indicated greater weight loss due to breakfast skipping, although skipping breakfast did result in higher cholesterol levels in the blood (Geliebter et al., Citation2014). St-Onge (Citation2017) and colleagues suggest that minimal effects of breakfast consumption on weight loss efforts may be due to compensatory behaviors throughout the rest of the 24-hour day; further work is warranted to understand this complex relationship.
Evidence has also indicated that the consumption of certain nutrients can alter the timing of various metabolic processes and the phase of the liver clock (Hara et al., Citation2001; Stokkan et al., Citation2001; Vollmers et al., Citation2009). For instance, a high-fat diet can greatly distort daily rhythms of leptin and insulin (Kohsaka et al., Citation2007), leading to a high degree of variabilityin leptin and insulin levels during both the light and dark cycles. A high-fat diet may also blunt daily patterns of lipid metabolism (Eckel-Mahan et al., Citation2013; Kohsaka et al., Citation2007). A diet high in fat may also delay adiponectin signaling in the liver (Barnea et al., Citation2009), thereby altering the body’s glucose levels (Combs & Marliss, Citation2014; Gamberi et al., Citation2018). These physiological changes can increase homeostatic and allostatic pressure to engage in energy intake and lead to erratic eating behaviors that encourage a positive energy balance, such as increased energy intake during the ‘wrong’ time of day (Kohsaka et al., Citation2007). Conversely, a diet of carbohydrates and protein allows for stabilization of the phase of the liver clock (Hirao et al., Citation2009); therefore, these nutrients may enable synchronization of metabolic processes to optimize health and functioning. Insulin (Tahara et al., Citation2011), glucose and amino acids (Oike et al., Citation2011), and rapidly digested starches (Itokawa et al., Citation2013) may also be important for regulating the timing of the liver clock.
Proportion of energy intakeacross the 24-hday
Rather than focusing primarily on the timing of the first or last eating event of the day, other research has looked to identify the role of other eating patterns in energy balance. The largest meal (i.e., meal in which individuals consume the greatest proportion of energy across the 24-h day), relative to other meals, may be one relevant eating pattern. For instance, Rangaraj et al. (Citation2020) found that consuming a greater proportion of energy intake early in the day, as opposed to later in the day, was linked to increased insulin sensitivity. Astudy by Carlson et al. (Citation2007) examined the effects of a single meal consumed within a window from 5:00pm to 9:00pm as opposed to the traditional three meals per day, over a two-month period. Though fasting levels of metabolic hormones such as leptin and ghrelin did not differ between conditions, the single-meal condition resulted in increased glucose levels, a delayed insulin response, and lowered glucose tolerance in the morning (Carlson et al., Citation2007). The single-meal condition also resulted in decreased feelings of fullness and increased perceptions of hunger (Stote et al., Citation2007). As described by Stote et al. (Citation2007), this study protocol had a high rate of withdrawal (∼30%), perhaps due to the fact that this type of eating schedule is difficult to sustain for many adults. As such, results should be interpreted with caution. Overall, however, these results are generally consistent with findings that many others have demonstrated: consuming the majority of energy intake earlier in the day (i.e., at breakfast or lunch) may be more beneficial for energy balance and metabolic health than consuming most calories at a later mealtime (Bandín et al., Citation2015; Bo et al., Citation2014; Chaix et al., Citation2019; Dattilo et al., Citation2010; Garaulet et al., Citation2013; Jakubowicz et al., Citation2013a; Jakubowicz et al., Citation2013b; Jamshed et al., Citation2019; Kahleova et al., Citation2017; Kelly et al., Citation2020; Qin et al., Citation2003; for exceptions, see Kant et al. (Citation1997) and Versteeg et al. (Citation2018)); for reviews, see Allison and Goel (Citation2018) and Pellegrini et al. (Citation2020). Because of these findings demonstrating a link between energy balance and the timing of energy intake, other studies have attempted to uncover relationships between the timing of energy intake and other health outcomes related to energy balance.
Eating window within the 24-h day
Another relevant eating pattern may be an individual’s eating window (i.e., duration of time from first eating event of the day to last eating event of the day). As part of a larger study assessing diurnal eating patterns in American adults, Gill and Panda (Citation2015) conducted a pilot study in a subsample of individuals with overweight. These adults were asked to restrict their eating window from over 14 h per day to 10-11 h per day for three months, with the researchers providing no recommendations on managing nutrition or caloric intake. Participants in this pilot study lost weight, reported greater energy levels and greater sleep satisfaction, and, in contrast to the aforementioned studies of mice, daily energy intake decreased by approximately 20%. The innovative workconducted by Gill and Panda (Citation2015) showed that modifying the timing of energy intake may enable individuals to decrease their total daily energy intake, and this behavioral target may also serve as a novel avenue for weight loss and health improvement efforts; consistent findings have been reported by others (Moro et al., Citation2016).
Recent work by Wilkinson et al. (Citation2020) found that restricting one’s eating window may not only assist in weight loss efforts, but also in reducing waist circumference and improving blood pressure and cholesterol levels. Another study, conducted by Parr et al. (Citation2020), compared the effect of a restricted eating window (8 h per day) to a longer (15 h per day) eating window for 5days. Results indicated that participants’ nighttime glycemic control improved after adhering to the shorter eating window schedule, compared to the 15 h per day eating window schedule. Hutchison et al. (Citation2019) reported that following a 9 h per day restricted eating window schedulefor one week can improve glucose tolerance compared to baseline. An additional study suggested that, compared to an unrestricted eating schedule, an 8-h restricted eating window can decrease the frequency of eating events and reduce body weight andfat mass even when physical activity did not differ between conditions (Chow et al., Citation2020). Further work by Ravussin et al. (Citation2019) has indicated that restricting the eating window to early in the day (i.e., 8:00am to 2:00pm) can reduce appetite, compared to a 12 h eating window (i.e., 8:00am to 8:00pm); similar findings have been reported in other work (Przulj et al., Citation2021). Such restriction of one’s eating window has been shown to be a safe and feasible option for adults (Martens et al., Citation2020), and a recent meta-analysis suggests this is a promising strategy for improving metabolic health (Moon et al., Citation2020).
This area of research is developing rapidly, but many questions remain. One topic of inquiry relates to the effects of a nocturnal eating window, in which an individual fasts from sunrise to sunset (e.g., Ramadan fasting). Overall, evidence suggests that fasting during Ramadan is related to lower BMI and improved markers of metabolic health (for reviews, see Mindikoglu et al., Citation2017; Osman et al., Citation2020), thoughthese positive effects may be short-lived(Fernando et al., Citation2019; Sadeghirad et al., Citation2014). Studies to date tend to be limited by short follow-up periods and heterogeneous Ramadan fasting practices around the world. Further research is needed to explicate these relationships and to determine whether these effects are primarily due to restricting energy intake to a specific window, or whether these benefits may simply illustrate the effect of reduced caloric intake. A recent review suggests that restricting the eating window and restricting caloric intake may actually have equivalent effects on body weight (Rynders et al., Citation2019). Given that a reduction in caloric intake was shown to accompany a restricted eating window schedule in a preliminary study (Gill & Panda, Citation2015), perhaps restricting one’s eating window improves metabolic health primarily through a reduction in caloric intake and not through circadian mechanisms.
Taken together, this body of research indicates that the association between energy intake and energy expenditure may not simply be due to what and how much is eaten, and that improper timing of food intake may have a moderating effect on energy balance.
Impact of circadian timing of physical activity on energy balance
Research has also begun to examine the role of the timing of physical activity in energy balance. This section details the impact of the timing of physical activity on aspects of energy balance. It begins with a discussion of daily patterns of nonvolitional energy expenditure, then discussesthe timing of physical activityand componentsof energy balance.
Daily patterns of nonvolitional energy expenditure
Early research on energy expenditure has examined daily patterns of the physiological components of energy expenditure (for review, see Shaw et al., Citation2019). Studies have demonstrated an endogenous circadian rhythm in resting metabolic rate (Spengler et al., Citation2000; Zitting et al., Citation2018) with resting metabolic rate increasing into the afternoon (Haugen et al., Citation2003) and peaking in the late evening (van Moorsel et al., Citation2016; Zitting et al., Citation2018). Romon and colleagues first reported a daily pattern of the thermic effect of food (Romon et al., Citation1993) while further research by Morris et al. (Citation2015) demonstrated the contribution of the circadian timing system in the thermic effect of food. Research has also suggested the presence of a daily rhythm in respiratory quotient. Kelly et al. (Citation2020) utilized whole-room calorimetry to discover a diurnal pattern in respiratory quotient. Zitting et al. (Citation2018) conducted a rigorous study which provided evidence for an unmasked circadian rhythm of respiratory quotient, though their study was limited by indirect calorimetry. Not all researchers have not found evidence of this daily pattern, however (Kräuchi & Wirz-Justice, Citation1994). Interestingly, Keim et al. (Citation1997) have suggested that food intake patterns throughout the day may affect components of nonvolitional energy expenditure, such as respiratory quotient. Daily variations of other biological and behavioral factors associated with energy expenditure (e.g., physical activity) have also been demonstrated.
Timing of Physical activity and components of energy balance
In addition to physiological rhythms in energy expenditure processes, rhythms in volitional energy-expending behavior (i.e., physical activity) have also been shown to relate to energy homeostatic and allostatic processes, though findings have been somewhat mixed (Blankenship et al., Citation2021). Maraki et al. (Citation2005) examined effects of a single exercise session, performed either in the morning or the evening, on appetite and energy intake. Exercise resulted in increased appetite and decreased satiety and fullness, without altering daily energy intake, with no differences between the morning and evening (Maraki et al., Citation2005). Another study found that a single session of exercise performed in the morning increased satiety more than an afternoon exercise session, but overall energy intake was not impacted (Alizadeh et al., Citation2015). Research by Larsen et al. (Citation2019), however, indicated that the time of day (morning, afternoon, or evening) in which individuals engage in exercise may not have an effect on appetite and energy intake. The discrepancy in findings between these studies may be due in part to methodological differences in the time frame in which appetite was assessed. Maraki et al. (Citation2005) measured appetite 45 min after participants completed aerobic exercise, after a cool down period, whereas Alizadeh et al. (Citation2015) evaluated appetite immediately following exercise; further, Larsen et al. (Citation2019) measured appetite up to 48 h after exercise was completed. Based on these findings, perhaps changes in appetite and satiety happen immediately following morning exercise, but these effects are less affected byexercise timing in the short-term, and then dissipate as time passes. A difference in the type of exercise and the intensity of exercise performed could also account for such a difference in results. Participants in the studies conducted by Maraki et al. (Citation2005) and Alizadeh et al. (Citation2015) engaged in aerobic exercise, but participants in Larsen et al.’s (Citation2019) study completed high-intensity interval exercise. This is supported by a recent review by Manore et al. (Citation2017) which concluded that higher-intensity exercise has a greater satiety effect and that specific exercises such as running may have greater effects on satiety. Additional work is necessary to determine impacts of various types of exercise on these homeostatic and allostatic processes, and to evaluate whether men and women differ in homeostatic and allostatic pressures in response to exercise.
Other studies involving long-term exercise have found mixed results in relation to weight outcomes as well. In a study of mice fed a high-fat diet, exercise late in the active part of the day protected against weight gain better than exercise performed early in the active part of the day (Dalbram et al., Citation2019). Surprisingly, the amount of energy intake was similar between study conditions, and the timing of exercise did not affect other homeostatic and allostatic pressures related to energy intake, such as insulin action or glucose homeostasis (Dalbram et al., Citation2019). The researchers posited that exercise late in the active phase may have protected against weight gain due to alterations in the timing of food intake (Dalbram et al., Citation2019), though the timing of food intake was not reported in this study. Additional research in human samples has provided conflicting findings. In a study of postmenopausal women, those who walked in the evening (i.e., before dinner, between 6:00 and 8:00pm) experienced a greater reduction in fat mass than women who walked in the morning (i.e., after breakfast, between 7:00 and 9:00am), as the evening walkers significantly increased their energy intake in the morning compared to the morning walkers (Di Blasio et al., Citation2010). Conceivably these effects are due to the repeated acute impact of exercise on satiety levels among indviduals who exercised prior to meals. These results contrast those from Alizadeh et al. (Citation2017). In their study, women who completed 6 weeks of aerobic exercise training in the morning (i.e., between 8:00 and 10:00am) lost significantly more weight than women who exercised in the afternoon (i.e., between 2:00 and 4:00pm), due at least in part to a significant reduction in caloric intake over six weeks in the morning exercise group (Alizadeh et al., Citation2017). Similarly, in a 10-month supervised exercise intervention aimed at inducing weight loss, young adults who chose to perform the majority of their exercise sessions in the morning (i.e., between 7:00 and 11:59am) lost more weight than those who completed the majority of their exercise sessions in the afternoon/evening (i.e., between 3:00 and 7:00pm) (Willis et al., Citation2020).
It should be noted that participants in the study by Di Blasio et al. (Citation2010) were instructed to engage in exercise either after breakfast or before dinner. Thus, those individuals who exercised in the morningate a meal and ended their nighttime fast prior to exercising, whereas those who exercised in the evening ate a meal after exercising. This difference in the timing of food intake patterns in relation to exercise could therefore have had a differential impact on the results of this exercise regimen. Alternatively, Alizadeh et al. (Citation2017) and Willis et al. (Citation2020) measured participants’ natural food intake patterns but did not provide participants instructions on when to eat throughout the day in relation to exercise. Therefore, participants in those studies could have self-selected their own chrononutrition patterns, such as whether to exercisebefore or after an eating event. Willis et al. (Citation2020) did report that the exercise groups did not differ in their proportion of energy intake consumed in the morning, afternoon, or evening, but Alizadeh et al. (Citation2017) did not report patterns of energy intake across the day. These studies also may have been confounded by differences in caloric intake. Mice in Dalbram et al.’s (Citation2019) and humans in Willis et al.’s (Citation2020) various study conditions did not significantly differ in energy intake. However, morning walkers reduced their caloric intake in research by Alizadeh et al. (Citation2017) and evening walkers in Di Blasio et al.’s (Citation2010) study increased their caloric intake in the morning. Taken together, participants in each of these exercise intervention studies seemed to display different patterns of chrononutrition and caloric intake. These exercise patterns and study designs likely influenced and shifted participants’ chrononutrition (e.g., eating window, first and last eating events of the day), and it is unclear whether the reported results are due to exercise timing or the timing and quantity of food intake. A recent randomized controlled trial assigned sedentary male participants with overweight or obesity to consume a high-fat diet for five days, with instructions to consume meals at scheduled times (7:30am, 1:00pm, and 7:30pm) (Moholdt et al., Citation2021). After the five days, participants continued consuming the high-fat diet but were also randomly assigned to an exercise condition (morning; evening; no exercise) for 5 days. Results indicated that five days of a high-fat diet led to changes in lipid and amino acid metabolism. Further, the evening exercise condition resulted in some reversal of these changes, such as decreases in fasting blood glucose, insulin, and LDL-cholesterol and triacylglycerol concentrations; these beneficial effects were not seen in the morning exercise and no exercise conditions (Moholdt et al., Citation2021). For this study, morning exercise occurred at 6:30am and evening exercise occurred at 6:30pm; many of the participants in the morning exercise condition had to alter their sleep timing to adhere to the exercise regimen. In addition, the morning exercise condition occurred after the overnight fast, while the evening exercise condition occurred after a 5.5 h fast. Perhaps these findings are due in part to these changes in sleep timing and differences in fasting duration. Because of these discrepancies, additional research should build off of work to date and continue to provide more consistent chrononutrition timing parameters to allow for a clearer evaluation of the isolated impact of exercise timing on body composition and weight loss.
Cross-sectional studies that have examined the association between physical activity patterns and body composition have, likewise, produced mixed results. In a sample of older women, those who performed a lower proportion of the day’s total physical activity in the morning had greater odds for obesity compared to those who performed a greater proportion of their daily activity in the morning (Chomistek et al., Citation2016). However, a separate study reported that the timing of physical activity was not related to BMI (Marinac et al., Citation2019). Findings from Marinac et al. (Citation2019) may be attributable to the fact that participants slept for approximately seven hours per night on average; this sufficient sleep duration may therefore have protected participants from such negative health effects of unhealthy timing of other behaviors. It is unclear whether sleep duration played a role in the effect seen by Chomistek et al. (Citation2016), as the researchers did not evaluate participants’ sleep duration. In sum, the available research has provided some conflicting evidence of the role of physical activity timing on energy homeostatic processes and body weight, but additional work is needed to better understand this relationship.
An integrated approach to the study of energy balance
Whereas most of the work described above studied individual circadian influences on components of energy balance in isolation, newer research has begun to take a more integrated approach by examining effects of both timing of food intake and timing of physical activity, rather than focusing on just one of these circadian-related behaviors. For instance, work that previously demonstrated the independent beneficial qualities of food intake early in the day and restricting food intake to a distinct window has recently been examined as an integrated intervention. In a randomized crossover study by Sutton et al. (Citation2018), men with prediabetes were assigned to either a restricted eating window condition with an early last meal (6-h eating window with dinner before 3:00PM) or a control condition (12-h eating window) for five weeks. Participants then switched conditions for another five weeks. Energy intake was equivalent across conditions, and no weight loss occurred; however, the early restricted eating window condition decreased homeostatic and allostatic pressures to engage in energy intake, such as improved insulin sensitivity and insulin levels and decreased appetite in the evening (Sutton et al., Citation2018). Otherexperimental work also showed an early time-restricted eating window resulted in reductions in appetite, without impacts on energy expenditure, compared to a control eating schedule (Ravussin et al., Citation2019).
An additional area of research has looked to incorporate promising results from chrononutrition with existing knowledge of physical activity. In mice fed an identical high-fat diet, eating a high-fat diet early in the active phase of the dayand then exercising later resulted in increased energy expenditure, decreased respiratory quotient, a smaller increase in body fat, and less weight gain overall, compared to early exercise followed by later eating (Sasaki et al., Citation2014). Research has also examined the combined effects of fasting and exercise training on homeostatic and allostatic pressures. In one study, individuals who fasted prior to undergoing morning exercise training had improved glucose tolerance and insulin sensitivity compared to those who consumed energy before and during the exercise training (Van Proeyen et al., Citation2010). Tinsley et al. (Citation2017) examined the effects of a restricted window of energy intake (4 h/day, 4 days/week) combined with resistance training three days per week. Compared to individuals who only completed the resistance training program, participants who completed both the resistance training and the restricted energy intake window displayed a greater degree of muscle endurance, and energy intake was reduced (Tinsley et al., Citation2017). This work indicates that a restricted window of energy intake, coupled with increased energy expenditure, has the potential to improve strength and enhance energy balance. Further work incorporating additional circadian factors will continue to improve our understanding of the complex relationships between energy balance-related homeostatic and allostatic pressures and weight outcomes.
Onesuch circadian factor that has garnered significant attentionas a topic of research is the sleep/wake cycle. Thus far,most of the evidence has focused on the impact of short sleep duration (i.e., sleep duration of less than 6 h/night)on energy balance. As a result of this reearch, a theoretical model has been proposed to explain the relationship between short sleep duration and energy balance.
In an updated iteration of the energy balance model, Penev (Citation2012) postulated that insufficient sleep results in physiological changes that disrupt energy balance. Examples of physiological changes related to insufficient sleep include altered leptin and ghrelin levels (Spiegel et al., Citation2004; Taheri et al., Citation2004), reduced insulin sensitivity (Wong et al., Citation2015), increased activation in brain areas associated with emotional responses, motivation, and reward (Greer et al., Citation2013; St-Onge et al., Citation2012), and decreased resting metabolic rate (Spaeth et al., Citation2015). According to Penev (Citation2012), these physiological changes then lead to behavioral changes aimed at conserving energy or providing the body with energy. Behavioral adaptations can involve changes in energy expenditure, e.g., increased sedentary behavior and decreased time spent engaging in moderate-vigorous physical activity (Booth et al., Citation2012; Schmid et al., Citation2009). Behavioral adaptations may also involve changes in energy intake, e.g., increased caloric intake (Calvin et al., Citation2013); increased preference for high-fat, sugary, and salty foods (Kant & Graubard, Citation2014; Kim et al., Citation2011; Spiegel et al., Citation2004); increased hunger and appetite (Nedeltcheva et al., Citation2010; Spiegel et al., Citation2004); and increased snacking (Kim et al., Citation2011). Collectively, this body of research indicates that while short sleepers may have increased energy expenditure due to extended wakefulness (Markwald et al., Citation2013), these individuals may be more likely to overcompensate with energy intake beyond what is necessary to sustain this extended wakefulness (Al Khatib et al., Citation2017; for review, see St-Onge, Citation2013). Thus, chronic short sleepers may have difficulty maintaining a healthy energy balance (i.e., maintain weight) or losing weight through a negative energy balance; these homeostatic and allostatic pressures tend to favor a positive energy balance.
Epidemiological studies and meta-analyses have indeed supported a link between sleep duration and energy balance,as short sleep duration is associated with increased likelihood of obesity in children and adults (Cappuccio et al., Citation2008; Chen et al., Citation2008; Ogilvie & Patel, Citation2017). While sleep duration is likely quite meaningful for health and energy balance, recent evidence has indicated that sleep/wake timing may also be important. For instance, studies focused on shift work have shown that its practice (i.e., workingduring the night and sleeping during day) has not only been linked to increased appetite for unhealthy foods (Souza et al., Citation2019) and decreased energy expenditure (McHill et al., Citation2014), but also to increased risk of obesity (Sun et al., Citation2017). While Penev’s (Citation2012) extension of the energy balance model has broadened our consideration of biobehavioral factors throughout the 24-h day, the current model fails to consider the specific time of the 24-h day in which sleep occurs.
Impact of circadian timing of sleep on energy balance
This section focuses on the effects of sleep timing and how it impacts aspects of energy balance. Key behaviors related to sleep timing are discussed, including sleep timing in non-shift workers,social jetlag, andshift work.
Sleep timing in non-shift workers
Abnormal sleeping patterns among non-shift workers may influence energy balance. For instance, in a study of children and adolescents, individuals with a delayed sleep/wake schedule (i.e., later bedtime and wake time) had higher BMI and were more likely to have an unhealthy diet compared to those who went to bed and woke up earlier (Golley et al., Citation2013). In a sample of adults, Baron et al. (Citation2011) reported that late sleepers, those individuals with a sleep midpoint (i.e., the time at the middle of the sleep interval)after 5:30am were more likely to have a delayed eating schedule, such that they tended to consume a later breakfast and more energy after dinner and after 8:00pm, compared to those with a sleep midpoint before 5:30am. Late sleeping was also linked to consumption ofa less healthy diet compared to normal sleepeing (Baron et al., Citation2011). Further, a delayed bedtime has been associated with increased intake of energy-dense foods (Golley et al., Citation2013) and greater caloric intake (Spaeth et al., Citation2013), perhaps because the energetic costs of this extended wakefulness may increase homeostatic and allostatic pressure for individuals to provide the body with energy.
The sleep/wake cycle is known to significantly influence the body’s insulin and glucose levels throughout the 24-h day (Van Cauter & Copinschi, Citation2006). Thus, it seems plausible that delayed sleep timing may also exert physiological changes associated with increased energy intake. However, it has been difficult to distinguish the effects of delayed sleep timing on metabolism from those due to insufficient sleep, as bedtime delay or wake time advancements are often accompanied by insufficient sleep (e.g., Chaput et al., Citation2007; Markwald et al., Citation2013; Spaeth et al., Citation2013). In an effort to test this more thoroughly, results from a pilot study by Pizinger et al. (Citation2018) indicated that these negative effects of altered sleep timing on insulin sensitivity and glucose tolerance are seen only when accompanied by insufficient sleep. More research is therefore needed to determine the independent effects of altered sleep timing on physiological components of energy intake.
Later timing of sleep can also alter behavioral changes related to energy expenditure. For instance, Schechter and St-Onge (Citation2014) reported that delayed sleep timing was associated with less time spent engaging in moderate to vigorous physical activity and more time spent in sedentary behavior. In addition, compared to adolescents with an early bedtime and rise time, adolescents with a late bedtime and rise time engaged in almost 30 min/day less moderate to vigorous physical activity (Olds et al., Citation2011). Similar findings were also discovered in a large multinational study of children: a later bedtime was positively associated with sedentary behavior and negatively associated with time spent in moderate to vigorous physical activity (Chaput et al., Citation2015). Overall, this indicates that among both children and adults, individuals may experience increased homeostatic and allostatic pressure to decrease energy expenditure in order to compensate for extended wakefulness.
Evidence to date has failed to examine the independent effects of sleep timing on physiological components of energy expenditure. Instead, laboratory research has shown that insufficient sleep, by way of a delayed bedtime and an earlier wake time, is associated with increased energy expenditure over the 24-h period (Markwald et al., Citation2013; Schechter et al., Citation2013); however, respiratory quotient may not be affected (Schechter et al., Citation2013). Though altered sleep timing and short sleep duration may require a greater amount of energy to allow one to sustain wakefulness, this body of research has consistently shown that opposing homeostatic and allostatic pressures are enacted which aim to provide the body with energy, and the amount of energy intake which occurs is beyond that which is required to maintain energy balance.
Another area of sleep timing which research is beginning to examine as a potential contributor to energy balance is the variability in one’s sleep/wake schedule. Intra-individual variability in sleep/wake timing has been associated with components of energy balance, such as an unhealthy diet, more time spent engaging in sedentary behavior, and insufficient sleep (Duncan et al., Citation2016), as well as insulin resistance and BMI (Taylor et al., Citation2016; for review, see Bei et al., Citation2016). In addition, a recent longitudinal study by Kline et al. (Citation2021) found that later sleep timing and more variable wake times were associated with less weight loss, among individuals enrolled in a 12-month behavioral weight loss intervention. Sleep/wake timing variability is a relatively novel area of research which should be explored in future work to further identify physiological and behavioral mechanisms which may influence metabolic outcomes.
Social jetlag
An additional component of sleep timing which has been examined in relation to energy balance is social jetlag, i.e., misalignment between social and biological time (Wittmann et al., Citation2006). Because the timing of school and work constraints often hinder individuals’ natural sleep/wake timing behaviors, people tend to fall asleep and wake up later on free days than they do on work or school days; this discrepancy between sleep/wake timing on work or school days and free days has been coined social jetlag (Wittmann et al., Citation2006). Social jetlag has been related to markers of metabolic dysfunction and obesity (Parsons et al., Citation2015; Roenneberg et al., Citation2012); however, older adults may not be as strongly affected, potentially due to fewer work obligations as individuals reach retirement (Koopman et al., Citation2017). Subsequent research has attempted to uncover pathways underlying this relationship.
One way in which social jetlag may influence energy balance is through its effects on behavioral factors aimed at increasing energy intake. Social jetlag has been associated with increased total daily energy intake (Mota et al., Citation2019) and an increased likelihood of consuming an unhealthy diet (Mota et al., Citation2019; Silva et al., Citation2016; Zerón-Rugerio et al., Citation2019).
Research has also indicated that social jetlag can impart physiological changes that alter energy intake. In individuals with a metabolic-related chronic illness, social jetlag has been related to elevated fasting glucose levels (Mota et al., Citation2017). A recent study has suggested that the relationship between social jetlag and appetite may depend on the magnitude of social jetlag. Though energy intake was equivalent between comparison groups, Polugrudov et al. (Citation2017) reported that normal-weight individuals with > 2 h of difference in sleep timing between work days and free days were hungrier after breakfast compared to those with ≤ 1 h of social jetlag; participants with > 2 h of social jetlag were also less satiated 2 h after consuming breakfast. Taken together, this work indicates that social jetlag may increase homeostatic and allostatic pressures to engage in energy intake.
Social jetlag may also influence energy balance through its effects on energy expenditure, though research on this topic is scarce. One observational study found that individuals with a ≥2 h difference in sleep timing between work or school days and free days were less physically active compared to those with less severe social jetlag (i.e., ≤60 min) (Rutters et al., Citation2014). Interestingly, social jetlag may occur in shift workers, and this combination has been shown to relate to less time spent engaging in moderate to vigorous physical activity and less energy expended through physical activity (Alves et al., Citation2017). However, given the limited number of studies examining this association, additional research is needed to fully understand how social jetlag may relate to energy expenditure.
Shift work
The study of shift work, which examines individuals who work, eat, and are activeduring the typical inactive portion of the 24-h day and sleep during the active portion of the day, may provide further insight into the relationships between sleep timing andenergy balance. Shift work is increasingly common in the 24-h lifestyle of modern society; this work schedule has been associated with the development of numerous negative health consequences (e.g., Antunes et al., Citation2010; Van Drongelen et al., Citation2011), as well as weight gain and obesity (Sun et al., Citation2017). Due to these relationships, research has begun to investigate potential mechanisms underlying this association. One pathway through which shift work may be linked to energy balance and health is through its relationships with homeostatic and allostatic pressure to engage in energy intake.
Studies have indicated that shift work may increase homeostatic and allostatic pressure to provide the body with energy through a couple of avenues. Shift work may increase appetite for energy-dense foods, i.e., high-fat and high-calorie foods (Cain et al., Citation2015; Souza et al., Citation2019) and also be tied to consumption of an unhealthy diet, such as one high in fat and sugar and low in fruit and vegetable intake (Knutson et al., Citation1990;Lowden et al., Citation2010; Nakamura et al., Citation2018; Pepłonska et al., Citation2019; for exceptions, see Beebe et al., Citation2017; Hulsegge et al., Citation2016) or increased snacking during the work shift (de Assis et al., Citation2003; Lennernäs et al., Citation1995; Reinberg et al., Citation1979). Such findings may be due to shift workers’ increased homeostatic and allostatic pressure to engage in increased energy intake tostay awake and function during work. This pattern of eating during the night shift (i.e., typical inactive period of the 24-h day) has been linked to physiological changes which can impair metabolic health, such as a weakened glucose response (Centofanti et al., Citation2018) and reduced glucose metabolism (Grant et al., Citation2017).
Somecross-sectional research suggested that the negative health effects of shift work may be the result of greater total dailyenergy intake in shift workers (Hulsegge et al., Citation2016; Nakamura et al., Citation2018), but additional evidence does not support this explanation. In a recent systematic review, Cayanan et al. (Citation2019) compared energy intake for night shift workers and non-night shift workers. Interestingly, researchers discovered that total energy intake and macronutrient intake were equivalent between shift workers and non-shift workers (Cayanan et al., Citation2019). Similar findings regarding energy intake were also reported in other studies and systematic reviews (Bonham et al., Citation2016;de Assis et al., Citation2003;Lennernäs et al., Citation1995; Souza et al., Citation2019). Though the relationship between shift work and energy balance is quite complex, overall research suggests that this abnormal sleep timing within the 24-h day may differentially affect energy balance by altering patterns of energy intake, perhaps by affecting how energy intake is metabolized by the body.
Research on energy expenditure and shift work has been sparse, with mixed findings reported. In an acute laboratory study of simulated shift work, McHill et al. (Citation2014) demonstrated that just a few nights of shift work can lead to decreased total daily energy expenditure and a decreased thermic effect of food after a late dinner. Other research has shown contrasting effects of shift work on components of energy expenditure. For instance, in a study with objective measurements of physical activity via accelerometer, shift work wasnot associated with altered physical activity, compared to non-shift work (Roskoden et al., Citation2017). Additional research using objective assessments of physical activity showed no difference in total energy expenditure (Wakui et al., Citation2002) between shift workers and non-shift workers. However, Esquirol et al. (Citation2009) used a questionnaire to estimate physical activity and discovered that shift workers displayed greater physical activity during work compared to non-shift workers. These findings may be due to the type of occupation typically utilized in shift work, as shift work is often more physically demanding than non-shift work positions. More time spent in occupational physical activity has been related to less time spent in leisure-time physical activity (Nooijen et al., Citation2018); this would likely contribute to the lack of difference in overall energy expenditure seen between shift workers and non-shift workers. Contradictory findings reported in these studies may also be a consequence of the method used to measure physical activity: researchers that utilized objective assessment methods (Roskoden et al., Citation2017; Wakui et al., Citation2002) found no difference in components of energy expenditure, but researchers that utilized self-report methods did find a difference between shift workers’ and non-shift workers’ physical activity. Further, Roskoden et al. (Citation2017) and Wakui et al. (Citation2002) were limited by small sample sizes. Additional analyses by Roskoden et al. (Citation2017) compared shift-working nurses, non-shift-working nurses, and non-shift-working office workers. The researchersfound that non-shift working nurses had a lower resting metabolic rate compared to shift-working nurses and non-shift-working office staff members. Roskoden et al. (Citation2017) clarified that this result may be due to age effects on metabolism, as non-shift working nurse participants were older than the other two participant groups. Given the various energy expenditure assessment methods in these studies, additional research is needed to clarify patterns of physical activity and other components of energy expenditure among shift workers and to determine whether these relationships differ by the nature of the work (e.g., healthcare, call center, manufacturing plant).
In sum, research on sleep timing has indicated that abnormal sleep timing may increase homeostatic and allostatic pressure for individuals to increase energy intake, by altering appetitive hormones, appetite, and eating behaviors. A paucity of evidence exists regarding relationships between physical activity and sleep timing, but it seems plausible that individuals may face homeostatic and allostatic pressure to decrease energy expenditure in altered sleep timing conditions to conserve energy.
Conclusions and future directions
In conclusion, current evidence has indicated that the existing energy balance modeldoes not consider a crucial contributor to energy balance: the timing of behaviors in the 24-h day. The timing of food intake, physical activity, and sleep may each influence energy balance by exerting changes in physiology and behavior, thereby altering homeostatic and allostatic pressure to either provide the body with energy or conserve energy. Thus, expansion of the existing energy balance model may provide a more comprehensive approach to factors which impact energy balance and health.
The present review has identified relevant literature regarding the timing of behaviors in the 24-h day, but additional research is needed to fully understand how these behaviors affect components of energy expenditure and energy intake, and how these behaviors may affect the more dynamic and complex components of the energy balance model. Well-controlled studies which allow for assessment of the directionality of these relationships would be quite valuable. For example, research should attempt to identify whether late-night energy intake leads to an increased likelihood of breakfast skipping, or whether breakfast skipping leads to a delayed eating schedule.
In addition, it is important to recognize that a true circadian rhythm continues under constant conditions, i.e., when no environmental time cues are provided and when various ‘masking’ factors (e.g., physical activity, energy intake) are controlled. It is not always feasible to conduct studies under these very highly-controlled conditions, especially with research involving human subjects. However, more of this type of research is needed, as these studies allow for distinguishing between circadian rhythms and diurnal rhythms, and ensuring rhythms are independent of masking effects by behavioral and/or environmental cues.
The effectiveness of actually modifying the timing of food intake, physical activity, and sleep for weight loss should be further examined. While initial research has shown promising effects of restricting food intake to a certain time frame within the 24-h day, supplemental research is needed to determine whether this may serve as an effective tool for long-term weight loss or weight management. Studies could also be conducted to ascertain whether exercising in the morning, as opposed to the evening, may lead to increased weight loss, and to determine whether altering sleep timing can result in better weight loss or weight management. Perhaps research should also consider individuals’ preferences for engaging in these behaviors as well, as this could strongly influence adherence to certain eating, exercise, or sleep schedules.
Finally, research is needed to determine whether the timing of food intake, physical activity, and sleep are variable or stable over time. Knowledge of longitudinal patterns is essential for future research and weight management efforts because this would inform the most relevant targets for intervention and to identify trait and state mechanisms related to chrononutrition. If the timing of eating, physical activity, and sleep are stable over time, this would confirm the approach presently being taken in the chrononutrition literature with interventions targeting the timing of food intake, but interventions may benefit further by also targeting the timing of physical activity and sleep. If the circadian timing of these behaviors isfound to be variable over time,such findings might also suggest that circadian behavior-based interventions may be challenged by limited periods of effectiveness, and more intensive follow-up strategies could be important for maintaining energy balance and health.
As evidence continues to accumulate, this updated ‘circadian’ version of the energy balance model may be of use in clinical settings. Clinicians and researchers may consider healthy chrononutrition in the development of weight loss interventions; for example, individuals could simply be advised to stop energy intake after a certain time of day (e.g., 8:00pm), or to consume the majority of their calories early in the day. While the importance of adequate sleep duration in health and functioning has begun to be recognized by health care professionals, physicians and nurses should also consider sleep timing, especially if patients are involved in shift work or demonstrate variability in their sleep timing behaviors. After further research, health care professionals may also recommend engaging in exercise at a certain time of day. Future work might utilize this model to develop and test a range of weight management interventions to supplement and extend existing treatment options.
In sum, this area of research is continually evolving, with many new opportunities for further research. Existing evidence has indicated that the circadian timing of food intake, physical activity, and sleep may have substantial effects on energy intake and energy expenditure, but timing of behaviors in the 24-h day is largely ignored. Much of the current recommendations focus on how much individuals eat and sleep, what foods are consumed, and the type and frequency of exercise, but emerging research suggests that the timing of these behaviors should also be prioritized in interventions. Targeting the timing of these behaviors in interventions may serve as a novel avenue to optimize obesity prevention and treatment efforts.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- International Standards Organisation. (2010). Food products – determination of the glycemic index (GI) and recommendation for food classification; ISO 26642-1010. International Standards Organization.
- Adamovich, Y., Aviram, R., & Asher, G. (2015). The emerging roles of lipids in circadian control. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids Acta, 1851(8), 1017–1025. https://doi.org/https://doi.org/10.1016/j.bbalip.2014.11.013
- Adamovich, Y., Rousso-Noori, R., Zwighaft, Z., Neufeld-Cohen, A., Golik, M., Kraut-Cohen, J., Wang, M., Han, X., & Asher, G. (2014). Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metabolism, 19(2), 319–330. https://doi.org/https://doi.org/10.1016/j.cmet.2013.12.016
- Alizadeh, Z., Mostafaee, M., Mazaheri, R., & Younespour, S. (2015). Acute effect of morning and afternoon aerobic exercise on appetite of overweight women. Asian Journal of Sports Medicine, 6(2), e24222. https://doi.org/https://doi.org/10.5812/asjsm.6(2)20156.24222
- Alizadeh, Z., Younespour, S., Rajabian Tabesh, M., & Haghraven, S. (2017). Comparison between the effect of 6 weeks of morning or evening aerobic exercise on appetite and anthropometric indices: A randomized controlled trial. Clinical Obesity, 7(3), 157–165. https://doi.org/https://doi.org/10.1111/cob.12187
- Al Khatib, H. K., Harding, S. V., Darzi, J., & Pot, G. K. (2017). The effects of partial sleep deprivation on energy balance: A systematic review and meta-analysis. European Journal of Clinical Nutrition, 71(5), 614–624. https://doi.org/https://doi.org/10.1038/ejcn.2016.201
- Allison, K. C., Ahima, R. S., O’Reardon, J. P., Dinges, D. F., Sharma, V., Cummings, D. E., Heo, M., Martino, N. S., & Stunkard, A. J. (2005). Neuroendocrine profiles associated with energy intake, sleep, and stress in the night eating syndrome. The Journal of Clinical Endocrinology & Metabolism, 90(11), 6214–6217. https://doi.org/https://doi.org/10.1210/jc.2005-1018
- Allison, K. C., & Goel, N. (2018). Timing of eating in adults across the weight spectrum: Metabolic factors and potential circadian mechanisms. Physiology& Behavior, 192, 158–166. https://doi.org/https://doi.org/10.1016/j.physbeh.2018.02.047
- Allison, K. C., Lundgren, J. D., O’Reardon, J. P., Geliebter, A., Gluck, M. E., Vinai, P., Mitchell, J. E., Schenck, C. H., Howell, M. J., Crow, S. J., Engel, S., Latzer, Y., Tzischinsky, O., Mahowald, M. W., & Stunkard, A. J. (2010). Proposed diagnostic criteria for night eating syndrome. International Journal of Eating Disorders, 43(3), 241–247. https://doi.org/https://doi.org/10.1002/eat.20693
- Alves, M. S., Andrade, R. Z., Silva, G. C., Mota, M. C., Resende, S. G., Teixeira, K. R., Gonçalves, B. F., & Crispim, C. A. (2017). Social jetlag among night workers is negatively associated with the frequency of moderate or vigorous physical activity and with energy expenditure related to physical activity. Journal of Biological Rhythms, 32(1), 83–93. https://doi.org/https://doi.org/10.1177/0748730416682110
- Andersson, I., & Rossner, S. (1996). Meal patterns in obese and normal weight men: The ‘gustaf’ study. European Journal of Clinical Nutrition, 50, 639–646.
- Antunes, L. C., Levandovski, R., Dantas, G., Caumo, W., & Hidalgo, M. P. (2010). Obesity and shift work: Chronobiological aspects. Nutrition Research Reviews, 23(1), 155–168. https://doi.org/https://doi.org/10.1017/S0954422410000016
- Arble, D. M., Bass, J., Laposky, A. D., Vitaterna, M. H., & Turek, F. W. (2009). Circadian timing of food intake contributes to weight gain. Obesity, 17(11), 2100–2102. https://doi.org/https://doi.org/10.1038/oby.2009.264
- Bandín, C., Scheer, F. A., Luque, A. J., Ávila-Gandía, V., Zamora, S., Madrid, J. A., Gómez-Abellán, P., & Garaulet, M. (2015). Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. International Journal of Obesity (London), 39(5), 828–833. https://doi.org/https://doi.org/10.1038/ijo.2014.182
- Barnea, M., Madar, Z., & Froy, O. (2009). High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology, 150(1), 161–168. https://doi.org/https://doi.org/10.1210/en.2008-0944
- Baron, K. G., Reid, K. J., Kern, A. S., & Zee, P. C. (2011). Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring), 19(7), 1374–1381. https://doi.org/https://doi.org/10.1038/oby.2011.100
- Barrington, W. E., & Beresford, S. A. A. (2019). Eating occasions, obesity and related behaviors in working adults: Does it matter when you snack? Nutrients, 11(10), 2320. https://doi.org/https://doi.org/10.3390/nu11102320
- Bass, J., & Takahashi, J. S. (2010). Circadian integration of metabolism and energetics. Science, 330(6009), 1349–1354. https://doi.org/https://doi.org/10.1126/science.1195027
- Beebe, D., Chang, J. J., Kress, K., & Mattfeldt-Beman, M. (2017). Diet quality and sleep quality among day and night shift nurses. Journal of Nursing Management, 25(7), 549–557. https://doi.org/https://doi.org/10.1111/jonm.12492
- Bei, B., Wiley, J. F., Trinder, J., & Manber, R. (2016). Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews, 28, 108–124. https://doi.org/https://doi.org/10.1016/j.smrv.2015.06.003
- Betts, J. A., Richardson, J. D., Chowdhury, E. A., Holman, G. D., Tsintzas, K., & Thompson, D. (2014). The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. American Journal of Clinical Nutrition, 100(2), 539–547. https://doi.org/https://doi.org/10.3945/ajcn.114.083402
- Blankenship, J. M., Rosenberg, R. C., Rynders, C. A., Melanson, E. L., Catenacci, V. A., & Creasy, S. A. (2021). Examining the role of exercise timing in weight management: A review. International Journal of Sports Medicine. Advance online publication, https://doi.org/https://doi.org/10.1055/a-1485-1293
- Bo, S., Musso, G., Beccuti, G., Fadda, M., Fedele, D., Gambino, R., Gentile, L., Durazzo, M., Ghigo, E., Cassader, M., & Cassader, M. (2014). Consuming more of daily caloric intake at dinner predisposes to obesity. A 6-year population based prospective cohort study. PLoS One, 9(9), e108467. https://doi.org/https://doi.org/10.1371/journal.pone.0108467
- Bolli, G. B., De Feo, P., De Cosmo, S., Perriello, G., Ventura, M. M., Calcinaro, F., Lolli, C., Campbell, P., Brunetti, P., & Gerich, J. E. (1984). Demonstration of a Dawn phenomenon in normal human volunteers. Diabetes, 33(12), 1150–1153. https://doi.org/https://doi.org/10.2337/diab.33.12.1150
- Bonham, M. P., Bonnell, E. K., & Huggins, C. E. (2016). Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiology International, 33(8), 1086–1100. https://doi.org/https://doi.org/10.1080/07420528.2016.1192188
- Booth, J. N., Bromley, L. E., Darukhanavala, A. P., Whitmore, H. R., Imperial, J. G., & Penev, P. D. (2012). Reduced physical activity in adults at risk for type 2 diabetes who curtail their sleep. Obesity (Silver Spring, 20(2), 278–284. https://doi.org/https://doi.org/10.1038/oby.2011.306
- Bray, M. S., Tsai, J.-Y., Villegas-Montoa, C., Boland, B. B., Blasier, Z., Egbejimi, O., Kueht, M., & Young, M. E. (2010). Time-of-day dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. International Journal of Obesity (London), 34(11), 1589–1598. https://doi.org/https://doi.org/10.1038/ijo.2010.63
- Buonfiglio, D., Parthmios, R., Dantas, R., Silva, R. C., Gomes, G., Andrade-Silva, J., Ramos-Lobo, A., Amaral, F. G., Matos, R., Sinésio, J., Motta-Teixeira, L. C., Donato, J., Reiter, R. J., & Cipolla-Neto, J. (2018). Melatonin absence leads to long-term leptin resistance and overweight in rats. Frontiers in Endocrinology, 9, 122. https://doi.org/https://doi.org/10.3389/fendo.2018.00122
- Cain, S. W., Filtness, A. J., Phillips, C. L., & Anderson, C. (2015). Enhanced preference for high-fat foods following a simulated night shift. Scandinavian Journal of Work, Environment & Health, 41(3), 288–293. https://doi.org/https://doi.org/10.5271/sjweh.3486
- Calvin, A. D., Carter, R. E., Adachi, T., Macedo, P. G., Albuquerque, F. N., van der Walt, C., & Somers, V. K. (2013). Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest, 144(1), 79–86. https://doi.org/https://doi.org/10.1378/chest.12-2829
- Cappuccio, F. P., Taggart, F. M., Kandala, N. B., Currie, A., Peile, E., Stranges, S., & Miller, M. A. (2008). Meta-analysis of short sleep duration and obesity in children and adults. Sleep, 31(5), 619–626. https://doi.org/https://doi.org/10.1093/sleep/31.5.619
- Carlson, O., Martin, B., Stote, K. S., Golden, E., Maudsley, S., Najjar, S. S., Ferrucci, L., Ingram, D. K., Longo, D. L., Rumpler, W. V., Baer, D. J., Egan, J., & Mattson, M. P. (2007). Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal weight middle-aged men and women. Metabolism, 56(12), 1729–1734. https://doi.org/https://doi.org/10.1016/j.metabol.2007.07.018
- Cayanan, E. A., Eyre, N. A. B., Lao, V., Comas, M., Hoyos, C. M., Marshall, N. S., Phillips, C. L., Shiao, J. S. C., Guo, Y.-L. L., & Gordon, C. J. (2019). Is 24-hour energy intake greater during night shift compared to non-night shift patterns? A systematic review. Chronobiology International, 36(12), 1599–1612. https://doi.org/https://doi.org/10.1080/07420528.2019.1666865
- Centofanti, S., Dorrian, J., Hilditch, C., Grant, C., Coates, A., & Banks, S. (2018). Eating on nightshift: A big vs small snack impairs glucose response to breakfast. Neurobiology of Sleep and Circadian Rhythms, 4, 44–48. https://doi.org/https://doi.org/10.1016/j.nbscr.2017.12.001
- Chaix, A., Manoogian, E. N. C., Melkani, G. C., & Panda, S. (2019). Time-restricted eating to prevent and manage chronic metabolic diseases. Annual Review of Nutrition, 39(1), 291–315. https://doi.org/https://doi.org/10.1146/annurev-nutr-082018-124320
- Chaix, A., Zarrinpar, A., Miu, P., & Panda, S. (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metabolism, 20(6), 991–1005. https://doi.org/https://doi.org/10.1016/j.cmet.2014.11.001
- Chaput, J.-P., Dutil, C., Featherstone, R., Ross, R., Giangregorio, L., Saunders, T. J., Janssen, I., Poitras, V. J., Kho, M. E., Ross-White, A., Zankar, S., & Carrier, J. (2020). Sleep timing, sleep consistency, and health in adults: A systematic review. Applied Physiology, Nutrition, and Metabolism, 45(10), S232–S247. https://doi.org/https://doi.org/10.1139/apnm-2020-0032
- Chaput, J.-P., Katzmarzyk, P. T., LeBlanc, A. G., Tremblay, M. S., Barreira, T. V., Broyles, S. T., Fogelholm, M., Hu, G., Kuriyan, R., Kurpad, A., Lambert, E. V., Rae, D. E., Maher, C., Maia, J., Matsudo, V., Onywera, V., Sarmiento, O. L., Standage, M., Tudor-Locke, C., … Olds, T. (2015). Associations between sleep patterns and lifestyle behaviors in children: An international comparison. International Journal of Obesity Supplements, 5(S2), S59–S65. https://doi.org/https://doi.org/10.1038/ijosup.2015.21
- Chaput, J. P., Després, J. P., Bouchard, C., & Tremblay, A. (2007). Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring), 15(1), 253–261. https://doi.org/https://doi.org/10.1038/oby.2007.512
- Chen, X., Beydoun, M. A., & Wang, Y. (2008). Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity, 16(2), 265–274. https://doi.org/https://doi.org/10.1038/oby.2007.63
- Chomistek, A. K., Shiroma, E. J., & Lee, I. M. (2016). The relationship between time of day of physical activity and obesity in older women. Journal of Physical Activity & Health, 13(4), 416–418. https://doi.org/https://doi.org/10.1123/jpah.2015-0152
- Chow, L. S., Manoogian, E. N. C., Alvear, A., Fleischer, J. G., Thor, H., Dietsche, K., Wang, Q., Hodges, J. S., Esch, N., Malaeb, S., Harindhanavudhi, T., Nair, K. S., Panda, S., & Mashek, D. G. (2020). Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity, 28(5), 860–869. https://doi.org/https://doi.org/10.1002/oby.22756
- Chowdhury, E. A., Richardson, J. D., Holma, G. D., Tzintkas, K., Thompson, D., & Betts, J. A. (2016). The causal role of breakfast in energy balance and health: A randomized controlled trial in obese adults. American Journal of Clinical Nutrition, 103(3), 747–756. https://doi.org/https://doi.org/10.3945/ajcn.115.122044
- Cipolla-Neto, J., Amaral, F. G., Afeche, S. C., Tan, D. X., & Reiter, R. J. (2014). Melatonin, energy metabolism, and obesity: A review. Journal of Pineal Research, 56(4), 371–381. https://doi.org/https://doi.org/10.1111/jpi.12137
- Combs, T. P., & Marliss, E. B. (2014). Adiponectin signaling in the liver. Reviews in Endocrine and Metabolic Disorders, 15(2), 137–137. https://doi.org/https://doi.org/10.1007/s11154-013-9280-6
- Corbalan-Tutau, M. D., Madrid, J. A., & Garaulet, M. (2012). Timing and duration of sleep and meals in obese and normal weight women. Appetite, 59(1), 9–16. https://doi.org/https://doi.org/10.1016/j.appet.2012.03.015
- Dalbram, E., Basse, A. L., Zierath, J. R., & Treebak, J. T. (2019). Voluntary wheel running in the late dark phase ameliorates diet-induced obesity in mice without altering insulin action. Journal of Applied Physiology, 126(4), 993–1005. https://doi.org/https://doi.org/10.1152/japplphysiol.00737.2018
- Dattilo, M., Crispim, C. A., Zimberg, I. Z., Tufik, S., & de Mello, M. T. (2010). Meal distribution across the day and its relationship with body composition. Biological Rhythm Research, 42(2), 119–129. https://doi.org/https://doi.org/10.1080/09291011003729270
- de Assis, M. A. A., Kupekb, E., & Nahasc, M. V. (2003). Food intake and circadian rhythms in shift workers with a high workload. Appetite, 40(2), 175–183. https://doi.org/https://doi.org/10.1016/S0195-6663(02)00133-2
- de Castro, J. M. (2004). The time of day of food intake influences overall intake in humans. Journal of Nutrition, 134(1), 104–111. https://doi.org/https://doi.org/10.1093/jn/134.1.104
- de Castro, J. M. (2007). The time of day and the proportions of macronutrients are related to total daily food intake. British Journal of Nutrition, 98(5), 1077–1083. https://doi.org/https://doi.org/10.1017/S0007114507754296
- de Castro, J. M. (2009). When, how much and what foods are eaten are related to total daily food intake. British Journal of Nutrition, 102(8), 1228–1237. https://doi.org/https://doi.org/10.1017/S0007114509371640
- Delahaye, L. B., Bloomer, R. J., Butawan, M. B., Wyman, J. M., Hill, J. L., Lee, H. W., Liu, A. C., McAllan, L., Han, J. C., & van der Merwe, M. (2018). Time-restricted feeding of a high-fat diet in male C57Bl/6 mice reduces adiposity but does not protect against increased systemic inflammation. Applied Physiology, Nutrition, and Metabolism, 43(10), 1033–1042. https://doi.org/https://doi.org/10.1139/apnm-2017-0706
- Di Blasio, A., Di Donato, F., Mastrodicasa, M., Fabrizio, N., Di Renzo, D., Napolitano, G., Petrella, V., Gallina, S., & Ripari, P. (2010). Effects of the time of day of walking on dietary behavior, body composition and aerobic fitness in post-menopausal women. The Journal of Sports Medicine and Physical Fitness, 50(2), 196–201.
- Duncan, M. J., Kline, C. E., Rebar, A. L., Vandelanotte, C., & Short, C. E. (2016). Greater bed- and wake-time variability is associated with less healthy lifestyle behaviors: A cross-sectional study. Journal of Public Health, 24(1), 31–40. https://doi.org/https://doi.org/10.1007/s10389-015-0693-4
- Eckel-Mahan, K. L., Patel, V. R., de Mateo, S., Orozco-Solis, R., Ceglia, N. J., Sahar, S., Dilag-Penilla, S., Dyar, K., Baldi, P., & Sassone-Corsi, P. (2013). Reprogramming of the circadian clock by nutritional challenge. Cell, 155(7), 1464–1478. https://doi.org/https://doi.org/10.1016/j.cell.2013.11.034
- Esquirol, Y., Bongard, V., Mabile, L., Jonnier, B., Soulat, J.-M., & Perret, B. (2009). Shift work and metabolic syndrome: Respective impacts of job strain, physical activity, and dietary rhythms. Chronobiology International, 26(3), 544–559. https://doi.org/https://doi.org/10.1080/07420520902821176
- Fernando, H. A., Zibellini, J., Harris, R. A., Seimon, R. V., & Sainsbury, A. (2019). Effect of Ramadan fasting on weight and body composition in healthy non-athlete adults: A systematic review and meta-analysis. Nutrients, 11(2), 478. https://doi.org/https://doi.org/10.3390/nu11020478
- Ferrannini, E., & Camastra, S. (1998). Relationship between impaired glucose tolerance, non-insulin-dependent diabetes mellitus and obesity. European Journal of Clinical Investigation, 28(S2), 3–7. https://doi.org/https://doi.org/10.1046/j.1365-2362.1998.0280s2003.x
- Flegal, K. M., Kit, B. K., Orpana, H., & Graubard, B. I. (2013). Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. Journal of the American Medical Association, 309(1), 71–82. https://doi.org/https://doi.org/10.1001/jama.2012.113905
- Flegal, K. M., Kruszon-Moran, D., Carroll, M. D., Fryar, C. D., & Ogden, C. L. (2016). Trends in obesity among adults in the United States, 2005 to 2014. Journal of the American Medical Association, 315(21), 2284–2291. https://doi.org/https://doi.org/10.1001/jama.2016.6458
- Gallant, A., Lundgren, J., & Drapeau, V. (2014). Nutrition aspects of late eating and night eating. Current Obesity Reports, 3(1), 101–107. https://doi.org/https://doi.org/10.1007/s13679-013-0081-8
- Gamberi, T., Magherini, F., Modesti, A., & Fiaschi, T. (2018). Adiponectin signaling pathways in liver diseases. Biomedicines, 6(2), 52. https://doi.org/https://doi.org/10.3390/biomedicines6020052
- Garaulet, M., Gómez-Abellán, P., Alburquerque-Béjar, J. J., Lee, Y.-C., Ordovás, J. M., & Scheer, F. A. J. L. (2013). Timing of food intake predicts weight loss effectiveness. International Journal of Obesity, 37(4), 604–611. https://doi.org/https://doi.org/10.1038/ijo.2012.229
- Garaulet, M., Qian, J., Florez, J. C., Arendt, J., Saxena, R., & Scheer, F. A. J. L. (2020). Melatonin effects on glucose metabolism: Time to unlock the controversy. Trends in Endocrinology and Metabolism, 31(3), 192–204. https://doi.org/https://doi.org/10.1016/j.tem.2019.11.011
- Geliebter, A., Astbury, N. M., Aviram-Friedman, R., Yahav, E., & Hashim, S. (2014). Skipping breakfast leads to weight loss but also elevated cholesterol compared with consuming daily breakfasts of oat porridge or frosted cornflakes in overweight individuals: A randomised controlled trial. Journal of Nutrition Science, 3, e56. https://doi.org/https://doi.org/10.1017/jns.2014.51
- Gill, S., & Panda, S. (2015). A smartphone app reveals erratic diurnal eating patterns in humans that can be modified for health benefits. Cell Metabolism, 22(5), 789–798. https://doi.org/https://doi.org/10.1016/j.cmet.2015.09.005
- Gluck, M. E., Venti, C. A., Salbe, A. D., & Krakoff, J. (2008). Nighttime eating: Commonly observed and related to weight gain in an inpatient food intake study. American Journal of Clinical Nutrition, 88(4), 900–905. https://doi.org/https://doi.org/10.1093/ajcn/88.4.900
- Goel, N., Stunkard, A. J., Rogers, N. L., Van Dongen, H. P. A., Allison, K. C., O’Reardon, J. P., Ahima, R. S., Cummings, D. E., Heo, M., & Dinges, D. F. (2009). Circadian rhythm profiles in women with night eating syndrome. Journal of Biological Rhythms, 24(1), 85–94. https://doi.org/https://doi.org/10.1177/0748730408328914
- Golley, R. K., Maher, C. A., Matricciani, L., & Olds, T. S. (2013). Sleep duration or bedtime? Exploring the association between sleep timing behavior, diet and BMI in children and adolescents. International Journal of Obesity (London), 37(4), 546–551. https://doi.org/https://doi.org/10.1038/ijo.2012.212
- Grant, C. L., Coates, A. M., Dorrian, J., Kennaway, D. J., Wittert, G. A., Heilbronn, L. K., Pajcin, M., Della Vedova, C., Gupta, C. C., & Banks, S. (2017). Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiology International, 34(8), 1003–1013. https://doi.org/https://doi.org/10.1080/07420528.2017.1335318
- Greenway, F. L. (2015). Physiological adaptations to weight loss and factors favouring weight regain. International Journal of Obesity, 39(8), 1188–1196. https://doi.org/https://doi.org/10.1038/ijo.2015.59
- Greer, S. M., Goldstein, A. N., & Walker, M. P. (2013). The impact of sleep deprivation on food desire in the human brain. Nature Communications, 4(1), 2259. https://doi.org/https://doi.org/10.1038/ncomms3259
- Hales, C. M., Carroll, M. D., Fryar, C. D., & Ogden, C. L. (2020). Prevalence of obesity and severe obesity among adults and youth: United States, 2017-2019 (No. 360). National Center for Health Statistics.
- Hall, K. D., Heymsfield, S. B., Kemnitz, J. W., Klein, S., Schoeller, D. A., & Speakman, J. R. (2012). Energy balance and its components: Implications for body weight regulation. American Journal of Clinical Nutrition, 95(4), 989–994. https://doi.org/https://doi.org/10.3945/ajcn.112.036350
- Hara, R., Wan, K., Wakamatsu, H., Aida, R., Moriya, T., Akiyama, M., & Shibata, S. (2001). Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes to Cells, 6(3), 269–278. https://doi.org/https://doi.org/10.1046/j.1365-2443.2001.00419.x
- Hatori, M., Vollmers, C., Zarrinpar, A., DiTacchio, L., Bushong, E. A., Gill, S., Leblanc, M., Chaix, A., Joens, M., Fitzpatrick, J. J., Ellisman, M., & Panda, S. (2012). Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet. Cell Metabolism, 15(6), 848–860. https://doi.org/https://doi.org/10.1016/j.cmet.2012.04.019
- Haugen, H. A., Melanson, E. L., Tran, Z. V., Kearney, J. T., & Hill, J. O. (2003). Variability of measured resting metabolic rate. American Journal of Clinical Nutrition, 78(6), 1141–1145. https://doi.org/https://doi.org/10.1093/ajcn/78.6.1141
- Hill, J. O., Wyatt, H. R., & Peters, J. C. (2013). The importance of energy balance. European Endocrinology, 9(2), 111–115. https://doi.org/https://doi.org/10.17925/EE.2013.09.02.111
- Hirao, A., Nagahama, H., Tsuboi, T., Hirao, M., Tahara, Y., & Shibata, S. (2010). Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. American Journal of Physiology -Gastrointestinal and Liver Physiology, 299(5), G1045–G1053. https://doi.org/https://doi.org/10.1152/ajpgi.00330.2010
- Hirao, A., Tahara, Y., Kimura, I., & Shibata, S. (2009). A balanced diet is necessary for proper entrainment signals of the mouse liver clock. PLoS One, 4(9), e6909. https://doi.org/https://doi.org/10.1371/journal.pone.0006909
- Hruby, A., & Hu, F. B. (2015). The epidemiology of obesity: A big picture. Pharmacoeconomics, 33(7), 673–689. https://doi.org/https://doi.org/10.1007/s40273-014-0243-x
- Hulsegge, G., Boer, J. M. A., van der Beek, A. J., Verschuren, W. M. M., Sluijs, I., Wermeulen, R., & Proper, K. I. (2016). Shift workers have a similar diet quality but higher energy intake than day workers. Scandinavian Journal of Work and Environmental Health, 42(6), 459–468. https://doi.org/https://doi.org/10.5271/sjweh.3593
- Hurley, J. M., Loros, J. J., & Dunlap, J. C. (2016). The circadian system as an organizer of metabolism. Fungal Genetics and Biology, 90, 39–43. https://doi.org/https://doi.org/10.1016/j.fgb.2015.10.002
- Hutchison, A. T., Regmi, P., Manoogian, E. N. C., Fleischer, J. G., Wittert, G. A., Panda, S., & Heilbronn, L. K. (2019). Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity (Silver Spring), 27(5), 724–732. https://doi.org/https://doi.org/10.1002/oby.22449
- Itokawa, M., Hirao, A., Nagahama, H., Otsuka, M., Ohstu, T., Furutani, N., Hirao, K., Hatta, T., & Shibata, S. (2013). Time-restricted feeding of rapidly digested starches causes stronger entrainment of the liver clock in PER2::LUCIFERASE knock-in mice. Nutrition Research, 33(2), 109–119. https://doi.org/https://doi.org/10.1016/j.nutres.2012.12.004
- Jakubowicz, D., Barnea, M., Wainstein, J., & Froy, O. (2013a). Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clinical Science (London), 125(9), 423–432. https://doi.org/https://doi.org/10.1042/CS20130071
- Jakubowicz, D., Barnea, M., Wainstein, J., & Froy, O. (2013b). High caloric intake at breakfast vs. Dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring), 21(12), 2504–2512. https://doi.org/https://doi.org/10.1002/oby.20460
- Jakubowicz, D., Froy, O., Wainstein, J., & Boaz, M. (2012). Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids, 77(4), 323–331. https://doi.org/https://doi.org/10.1016/j.steroids.2011.12.006
- Jamshed, H., Beyl, R. A., Della Manna, D. L., Yang, E. S., Ravussin, E., & Peterson, C. M. (2019). Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients, 11(6), E1234. https://doi.org/https://doi.org/10.3390/nu11061234
- Kahleova, H., Lloren, J. I., Mashchak, A., Hill, M., & Fraser, G. E. (2017). Meal frequency and timing are associated with changes in body mass index in Adventist health study 2. Journal of Nutrition, 147(9), 1722–1728. https://doi.org/https://doi.org/10.3945/jn.116.244749
- Kant, A. K., & Graubard, B. I. (2014). Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010. American Journal of Clinical Nutrition, 100(3), 938–947. https://doi.org/https://doi.org/10.3945/ajcn.114.085191
- Kant, A. K., & Graubard, B. I. (2015). 40-year trends in meal and snack eating behaviors of American adults. Journal of the Academy of Nutrition and Dietetics, 115(1), 50–63. https://doi.org/https://doi.org/10.1016/j.jand.2014.06.354
- Kant, A. K., Schatzkin, A., & Ballard-Barbash, R. (1997). Evening eating and subsequent long-term weight change in a national cohort. International Journal of Obesity and Related Metabolic Disorders, 21(5), 407–412. https://doi.org/https://doi.org/10.1038/sj.ijo.0800422
- Karatzi, K., Moschonis, G., Choupi, E., & Manios, Y. (2017). Late-night overeating is associated with smaller breakfast, breakfast skipping, and obesity in children: The healthy growth study. Nutrition, 33, 141–144. https://doi.org/https://doi.org/10.1016/j.nut.2016.05.010
- Keesey, R. E., & Powley, T. L. (2008). Body energy homeostasis. Appetite, 51(3), 442–445. https://doi.org/https://doi.org/10.1016/j.appet.2008.06.009
- Keim, N. L., Van Loan, M. D., Horn, W. F., Barbieri, T. F., & Mayclin, P. L. (1997). Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. The Journal of Nutrition, 127(1), 75–82. https://doi.org/https://doi.org/10.1093/jn/127.1.75
- Kelly, K. P., McGuinness, O. P., Buchowski, M., Hughey, J. J., Chen, H., Powers, J., Page, T., & Johnson, C. H. (2020). Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biology, 18(2), e3000622. https://doi.org/https://doi.org/10.1371/journal.pbio.3000622
- Kim, P., Oster, H., Lehnert, H., Schmid, S. M., Salamat, N., Barclay, J. L., Maronde, E., Inder, W., & Rawashdeh, O. (2019). Coupling the circadian clock to homeostasis: The role of period in timing physiology. Endocrine Reviews, 40(1), 66–95. https://doi.org/https://doi.org/10.1210/er.2018-00049
- Kim, S., DeRoo, L. A., & Sandler, D. P. (2011). Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutrition, 14(5), 889–895. https://doi.org/https://doi.org/10.1017/S136898001000296X
- Kline, C. E., Chasens, E. R., Bizhanova, Z., Sereika, S. M., Buysse, D. J., Imes, C. C., Kariuki, J. K., Mendez, D. D., Cajita, M. I., Rathbun, S. L., & Burke, L. E. (2021). The association between sleep health and weight change during a 12-month behavioral weight loss intervention. International Journal of Obesity, 45(3), 639–649. https://doi.org/https://doi.org/10.1038/s41366-020-00728-8
- Knutson, A., Andersson, H., & Berglund, U. (1990). Serum lipoproteins in day and shift workers: A prospective study. British Journal of Industrial Medicine, 47(2), 132–134. https://doi.org/https://doi.org/10.1136/oem.47.2.132
- Kohsaka, A., Laposky, A. D., Ramsey, K. M., Estrada, C., Joshu, C., Kobayashi, Y., Turek, F. W., & Bass, J. (2007). High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabolism, 6(5), 414–421. https://doi.org/https://doi.org/10.1016/j.cmet.2007.09.006
- Koopman, A. D. M., Rauh, S. P., van ‘t Riet, E., Groeneveld, L., van der Heijden, A. A., Elders, P. J., Dekker, J. M., Nijpels, G., Beulens, J. W., & Rutters, F. (2017). The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New hoorn study. Journal of Biological Rhythms, 32(4), 359–368. https://doi.org/https://doi.org/10.1177/0748730417713572
- Kräuchi, K., & Wirz-Justice, A. (1994). Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. American Journal of Physiology, 267(3 Pt 2), R819–R829. https://doi.org/https://doi.org/10.1152/ajpregu.1994.267.3.R819
- Kuroda, H., Tahara, Y., Saito, K., Ohnishi, N., Kubo, Y., Seo, Y., Otsuka, M., Fuse, Y., Ohura, Y., Hirao, A., & Shibata, S. (2012). Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Scientific Reports, 2(1), 711. https://doi.org/https://doi.org/10.1038/srep00711
- Kutsuma, A., Nakajima, K., & Suwa, K. (2014). Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica, 2014, 1. https://doi.org/https://doi.org/10.1155/2014/253581
- la Fleur, S. E., Kalsbeek, A., Wortel, J., Fekkes, M. L., & Buijs, R. M. (2001). A daily rhythm in glucose tolerance: A role for the suprachiasmatic nucleus. Diabetes, 50(6), 1237–1243. https://doi.org/https://doi.org/10.2337/diabetes.50.6.1237
- Lamia, K. A., Storch, K. F., & Weitz, C. J. (2008). Physiological significance of a peripheral tissue circadian clock. Proceedings of the National Academy of Sciences, 105(39), 15172–15177. https://doi.org/https://doi.org/10.1073/pnas.0806717105
- Larsen, P., Marino, F., Melehan, K., Guelfi, K. J., Duffield, R., & Skein, M. (2019). Evening high-intensity interval exercise does not disrupt sleep or energy intake despite changes in acylated ghrelin in middle-aged men. Experimental Physiology, 104(6), 826–836. https://doi.org/https://doi.org/10.1113/EP087455
- Lax, P., Larue-Achagiotis, C., Martel, P., Madrid, J. A., & Verge, P. (1998). Repeated short-fasting modifies the macronutrient self-selection pattern in rats. Physiology & Behavior, 65(1), 69–76. https://doi.org/https://doi.org/10.1016/S0031-9384(98)00123-1
- LeCheminant, J. D., Christenson, E., Bailey, B. W., & Tucker, L. A. (2013). Restricting night-time eating reduces daily energy intake in healthy young men: A short-term cross-over study. British Journal of Nutrition, 110(11), 2108–2113. https://doi.org/https://doi.org/10.1017/S0007114513001359
- Leidy, H. J., Mattes, R. D., & Campbell, W. W. (2007). Effects of acute and chronic protein intake on metabolism, appetite and ghrelin during weight loss. Obesity (Silver Spring), 15(5), 1215–1225. https://doi.org/https://doi.org/10.1038/oby.2007.143
- Lekkas, D., & Paschos, G. K. (2019). The circadian clock control of adipose tissue physiology and metabolism. Autonomic Neuroscience, 219, 66–70. https://doi.org/https://doi.org/10.1016/j.autneu.2019.05.001
- Lenard, N. R., & Berthoud, H.-R. (2008). Central and peripheral regulation of food intake and physical activity: Pathways and genes. Obesity (Silver Spring), 16(S3), S11–S22. https://doi.org/https://doi.org/10.1038/oby.2008.511
- Lennernäs, M., Hambraeus, L., & Åkerstedt, T. (1995). Shift related dietary intake in day and shift workers. Appetite, 25(3), 253–266. https://doi.org/https://doi.org/10.1006/appe.1995.0060
- Longo, V. D., & Panda, S. (2016). Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabolism, 23(6), 1048–1059. https://doi.org/https://doi.org/10.1016/j.cmet.2016.06.001
- Lopez-Minguez, J., Saxena, R., Bandín, C., Scheer, F. A., & Garaulet, M. (2018). Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clinical Nutrition, 37(4), 1133–1140. https://doi.org/https://doi.org/10.1016/j.clnu.2017.04.003
- Lowden, A., Moreno, C., Holmbäck, U., Lennernäs, M., & Tucker, P. (2010). Eating and shift work – effects on habits, metabolism and performance. Scandinavian Journal of Work, Environment, & Health, 36(2), 150–162. https://doi.org/https://doi.org/10.5271/sjweh.2898
- Manoogian, E. N. C., & Panda, S. (2017). Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Research Reviews, 39, 59–67. https://doi.org/https://doi.org/10.1016/j.arr.2016.12.006
- Manore, M. M., Larson-Meyer, D. E., Lindsay, A. R., Hongu, N., & Houtkooper, L. (2017). Dynamic energy balance: An integrated framework for discussing diet and physical activity in obesity prevention – is it more than eating less and exercising more? Nutrients, 9(8), 905. https://doi.org/https://doi.org/10.3390/nu9080905
- Maraki, M., Tsofliou, F., Pitsiladis, Y. P., Malkova, D., Mutrie, N., & Higgins, S. (2005). Acute effects of a single exercise class on appetite energy intake and mood. Is there a time of day effect? Appetite, 45(3), 272–278. https://doi.org/https://doi.org/10.1016/j.appet.2005.07.005
- Marinac, C. R., Quante, M., Mariani, S., Weng, J., Redline, S., Cespedes Feliciano, E. M., Hipp, J. A., Wang, D., Kaplan, E. R., James, P., & Mitchell, J. A. (2019). Associations between timing of meals, physical activity, light exposure, and sleep with body mass index in free-living adults. Journal of Physical Activity & Health, 16(3), 214–221. https://doi.org/https://doi.org/10.1123/jpah.2017-0389
- Markwald, R. R., Melanson, E. L., Smith, M. R., Higgins, J., Perreault, L., Eckel, R. H., & WrightJr.K. P. (2013). Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences, 110(14), 5695–5700. https://doi.org/https://doi.org/10.1073/pnas.1216951110
- Martens, C. R., Rossman, M. J., Mazzo, M. R., Jankowski, L. R., Nagy, E. E., Denman, B. A., Richey, J. J., Johnson, S. A., Ziemba, B. P., Wang, Y., Peterson, C. M., Chonchol, M., & Seals, D. R. (2020). Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience, 42(2), 667–686. https://doi.org/https://doi.org/10.1007/s11357-020-00156-6
- Masri, S., Rigor, P., Cervantes, M., Ceglia, N., Sebastian, C., Xiao, C., Roqueta-Rivera, M., Deng, C., Osborne, T., Mostoslavsky, R., Baldi, P., & Sassone-Corsi, P. (2014). Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell, 158(3), 659–672. https://doi.org/https://doi.org/10.1016/j.cell.2014.06.050
- McEwen, B. S., & Wingfield, J. C. (2010). What’s in a name? Integrating homeostasis, allostasis and stress. Hormones and Behavior, 57(2), 105. https://doi.org/https://doi.org/10.1016/j.yhbeh.2009.09.011
- McHill, A. W., Czeisler, C. A., Phillips, A. J. K., Keating, L., Barger, L. K., Garaulet, M., Scheer, F. A., & Klerman, E. B. (2019). Caloric and macronutrient intake differ with circadian phase and between lean and overweight young adults. Nutrients, 11(3), 587. https://doi.org/https://doi.org/10.3390/nu11030587
- McHill, A. W., Melanson, E. L., Higgins, J., Connick, E., Moehlman, T. M., Stothard, E. R., & Wright Jr. K. P. (2014). Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences, 111(48), 17302–17307. https://doi.org/https://doi.org/10.1073/pnas.1412021111
- McHill, A. W., Phillips, A. J. K., Czeisler, C. A., Keating, L., Yee, K., Barger, L. K., Garaulet, M., Scheer, F. A., & Klerman, E. B. (2017). Later circadian timing of food intake is associated with increased body fat. The American Journal of Clinical Nutrition, 106(5), 1213–1219. https://doi.org/https://doi.org/10.3945/ajcn.117.161588
- Melkani, G. C., & Panda, S. (2017). Time-restricted feeding for the prevention and treatment of cardiometabolic disorders. The Journal of Physiology, 595(12), 3691–3700. https://doi.org/https://doi.org/10.1113/JP273094
- Mills, J. P., Perry, C. D., & Reicks, M. (2011). Eating frequency is associated with energy intake but not obesity in midlife women. Obesity (Silver Spring), 19(3), 552–559. https://doi.org/https://doi.org/10.1038/oby.2010.265
- Mindikoglu, A. L., Opekun, A. R., Gagan, S. K., & Devaraj, S. (2017). Impact of time-restricted feeding and Dawn-to-sunset fasting on circadian rhythm, obesity, metabolic syndrome, and nonalcoholic fatty liver disease. Gastroenterology Research and Practice, 2017, 3932491. https://doi.org/https://doi.org/10.1155/2017/3932491
- Moholdt, T., Parr, E. B., Devlin, B. L., Debik, J., Giskeødegård, G., & Hawley, J. A. (2021). The effect of morning vs evening exercise training on glycemic control and serum metabolites in overweight/obese men: A randomized trial. Diabetologia. Advance online publication, https://doi.org/https://doi.org/10.1007/s00125-021-05477-5
- Moon, S., Kang, J., Kim, S. H., Chung, H. S., Kim, Y. J., Yu, J. M., Cho, S. T., Oh, C.-M., & Kim, T. (2020). Beneficial effects of time-restricted eating on metabolic diseases: A systematic review and meta-analysis. Nutrients, 12(5), 1267. https://doi.org/https://doi.org/10.3390/nu12051267
- Moro, T., Tinsley, G., Bianco, A., Marcolin, G., Pacelli, Q. F., Battaglia, G., Palma, A., Gentil, P., Neri, M., & Paoli, A. (2016). Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of Translational Medicine, 14(1), 290. https://doi.org/https://doi.org/10.1186/s12967-016-1044-0
- Morris, C. J., Garcia, J. I., Myers, S., Yang, J. N., Trienekens, N., & Scheer, F. A. J. L. (2015). The human circadian system has a dominating role in causing the morning/evening difference in early diet-induced thermogenesis. Obesity (Silver Spring), 23(10), 2053–2058. https://doi.org/https://doi.org/10.1002/oby.21189
- Mota, M. C., Silva, C. M., Balieiro, L. C. T., Fahmy, W. M., & Crispim, C. A. (2017). Social jetlag and metabolic control in non-communicable chronic diseases: A study addressing different obesity statuses. Scientific Reports, 7(1), 6358. https://doi.org/https://doi.org/10.1038/s41598-017-06723-w
- Mota, M. C., Silva, C. M., Balieiro, L. C. T., Gonçalves, B. F., Fahmy, W. M., & Crispim, C. A. (2019). Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS One, 14(2), e0212126. https://doi.org/https://doi.org/10.1038/s41598-017-06723-w
- Nakajima, K. (2018). Unhealthy eating habits around sleep and sleep duration: To eat or fast? World Journal of Diabetes, 9(11), 190–194. https://doi.org/https://doi.org/10.4239/wjd.v9.i11.190
- Nakamura, M., Miura, A., Nagahata, T., Toki, A., Shibata, Y., Okada, E., & Ojima, T. (2018). Dietary intake and dinner timing among shift workers in Japan. Journal of Occupational Health, 60(6), 467–474. https://doi.org/https://doi.org/10.1539/joh.2018-0070-OA
- Nedeltcheva, A. V., Kilkus, J. M., Imperial, J., Schoeller, D. A., & Penev, P. D. (2010). Insufficient sleep undermines dietary efforts to reduce adiposity. Annals of Internal Medicine, 153(7), 435–441. https://doi.org/https://doi.org/10.7326/0003-4819-153-7-201010050-00006
- Nooijen, C. F., Pozo-Cruz, B. D., Nyberg, G., Sanders, T., Galanti, M. R., & Forsell, Y. (2018). Are changes in occupational physical activity level compensated by changes in exercise behavior? European Journal of Public Health, 28(5), 940–943. https://doi.org/https://doi.org/10.1093/eurpub/cky007
- Ogilvie, R. P., & Patel, S. R. (2017). The epidemiology of sleep and obesity. Sleep Health, 3(5), 383–388. https://doi.org/https://doi.org/10.1016/j.sleh.2017.07.013
- Oike, H., Nagai, K., Fukushima, T., Ishida, N., & Kobori, M. (2011). Feeding cues and injected nutrients induce acute expression of multiple clock genes in the mouse liver. PLoS One, 6(8), e23709. https://doi.org/https://doi.org/10.1371/journal.pone.0023709
- Okada, C., Imano, H., Muraki, I., Yamada, K., & Iso, H. (2019). The association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. Journal of Obesity, 2019, 2439571. https://doi.org/https://doi.org/10.1155/2019/2439571
- Olds, T. S., Maher, C. A., & Matricciani, B. A. (2011). Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep, 34(10), 1299–1307. https://doi.org/https://doi.org/10.5665/SLEEP.1266
- O’Reardon, J. P., Peshek, A., & Allison, K. C. (2005). Night eating syndrome: Diagnosis, epidemiology and management. CNS Drugs, 19(12), 997–1008. https://doi.org/https://doi.org/10.2165/00023210-200519120-00003
- Osman, F., Haldar, S., & Henry, C. J. (2020). Effects of time-restricted feeding during Ramadan on dietary intake, body composition and metabolic outcomes. Nutrients, 12(8), 2478. https://doi.org/https://doi.org/10.3390/nu12082478
- Owino, S., Buonfiglio, D. D. C., Tchio, C., & Tosini, G. (2019). Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Frontiers in Endocrinology, 10, 488. https://doi.org/https://doi.org/10.3389/fendo.2019.00488
- Parr, E. B., Devlin, B. L., Radford, B. E., & Hawley, J. A. (2020). A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: A randomized controlled trial. Nutrients, 12(2), E505. https://doi.org/https://doi.org/10.3390/nu12020505
- Parsons, M. J., Moffitt, T. E., Gregory, A. M., Goldman-Mellor, S., Nolan, P. M., Poulton, R., & Caspi, A. (2015). Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. International Journal of Obesity (London), 39(5), 842–848. https://doi.org/https://doi.org/10.1038/ijo.2014.201
- Pellegrini, M., Cioffi, I., Evangelista, A., Ponzo, V., Goitre, I., Ciccone, G., Ghigo, E., & Bo, S. (2020). Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Reviews in Endocrine and Metabolic Disorders, 21(1), 17–33. https://doi.org/https://doi.org/10.1007/s11154-019-09524-w
- Penev, P. D. (2012). Update on energy homeostasis and insufficient sleep. Journal of Clinical Endocrinology and Metabolism, 97(6), 1792–1801. https://doi.org/https://doi.org/10.1210/jc.2012-1067
- Pepłonska, B., Nowak, P., & Trafalska, E. (2019). The association between night shift work and nutrition patterns among nurses. Medcyna Pracy, 70(3), 363–376. https://doi.org/https://doi.org/10.13075/mp.5893.00816
- Pizinger, T., Kovtun, K., RoyChodhury, A., Laferrère, B., Schechter, A., & St-Onge, M. P. (2018). Pilot study of sleep and meal timing effects, independent of sleep duration and food intake, on insulin sensitivity in healthy individuals. Sleep Health, 4(1), 33–39. https://doi.org/https://doi.org/10.1016/j.sleh.2017.10.005
- Poggiogalle, E., Jamshed, H., & Peterson, C. M. (2018). Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism, 84, 11–27. https://doi.org/https://doi.org/10.1016/j.metabol.2017.11.017
- Polugrudov, A., Popov, S., Smirnov, V., Panev, A., Ascheulova, E., Kuznetsova, E., Tserne, T., & Borisenkov, M. (2017). Association of social jetlag experienced by young northerners with their appetite after having breakfast. Biological Rhythm Research, 48(6), 917–929. https://doi.org/https://doi.org/10.1080/09291016.2017.1323391
- Przulj, D., Ladmore, D., Smith, K. M., Phillips-Waller, A., & Hajek, P. (2021). Time restricted eating as a weight loss intervention in adults with obesity. PLoS One, 16(1), e0246186. https://doi.org/https://doi.org/10.1371/journal.pone.0246186
- Qin, L.-Q., Li, J., Wang, Y., Wang, J., Xu, J.-Y., & Kaneko, T. (2003). The effects of nocturnal life on endocrine patterns in healthy adults. Life Sciences, 73(19), 2467–2475. https://doi.org/https://doi.org/10.1016/S0024-3205(03)00628-3
- Rains, T. M., Leidy, H. J., Sanoshy, K. D., Lawless, A. L., & Maki, K. C. (2015). A randomized, controlled, crossover trial to assess the acute appetitive and metabolic effects of sausage and egg-based convenience breakfast meals in overweight premenopausal women. Nutrition Journal, 14(1), 17. https://doi.org/https://doi.org/10.1186/s12937-015-0002-7
- Rangaraj, V. R., Siddula, A., Burgess, H. J., Pannain, S., & Knutson, K. L. (2020). Association between timing of energy intake and insulin sensitivity: A cross-sectional study. Nutrients, 12(2), 503. https://doi.org/https://doi.org/10.3390/nu12020503
- Ravussin, E., Beyl, R. A., Poggiogalle, E., Hsia, D. S., & Peterson, C. M. (2019). Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity, 27(8), 1244–1254. https://doi.org/https://doi.org/10.1002/oby.22518
- Reid, K. J., Baron, K. G., & Zee, P. C. (2014). Meal timing influences daily caloric intake in healthy adults. Nutrition Research, 34(11), 930–935. https://doi.org/https://doi.org/10.1016/j.nutres.2014.09.010
- Reinberg, A., Migraine, C., Apfelbaum, M., Brigant, L., Ghata, J., Vieux, N., Laporte, A., & Nicolai, H. (1979). Circadian and ultradian rhythms in the eating behaviour and nutrient intake of oil refinery operators with shift-work every 3-4 days. Diabete & Metabolisme, 5, 33–41.
- Reinke, H., & Asher, G. (2016). Circadian clock control of liver metabolic functions. Gastroenterology, 150(3), 574–580. https://doi.org/https://doi.org/10.1053/j.gastro.2015.11.043
- Reutrakul, S., & Van Cauter, E. (2014). Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Annals of the New York Academy of Sciences, 1311(1), 151–173. https://doi.org/https://doi.org/10.1111/nyas.12355
- Roenneberg, T., Allebrandt, K. V., Merrow, M., & Vetter, C. (2012). Social jetlag and obesity. Current Biology, 22(10), 939–943. https://doi.org/https://doi.org/10.1016/j.cub.2012.03.038
- Romon, M., Edme, J. L., Boulenguez, C., Lescroart, J. L., & Frimat, P. (1993). Circadian variation of diet-induced thermogenesis. American Journal of Clinical Nutrition, 57(4), 476–480. https://doi.org/https://doi.org/10.1093/ajcn/57.4.476
- Roskoden, F. C., Krüger, J., Vogt, L. J., Gärtner, S., Hannich, H. J., Steveling, A., Lerch, M. M., Aghdassi, A. A., & Aghdassi, A. A. (2017). Physical activity, energy expenditure, nutritional habits, quality of sleep and stress levels in shift-working health care personnel. PLoS One, 12(1), e0169983. https://doi.org/https://doi.org/10.1371/journal.pone.0169983
- Ruiz-Lozano, T., Vidal, J., de Hollanda, A., Scheer, F. A. J. L., Garaulet, M., & Izquierdo-Pulido, M. (2016). Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clinical Nutrition, 35(6), 1308–1314. https://doi.org/https://doi.org/10.1016/j.clnu.2016.02.007
- Rutters, F., Lemmens, S. G., Adam, T. C., Bremmer, M. A., Elders, P. J., Nijpels, G., & Dekker, J. M. (2014). Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? Journal of Biological Rhythms, 29(5), 377–383. https://doi.org/https://doi.org/10.1177/0748730414550199
- Rynders, C. A., Thomas, E. A., Zaman, A., Pan, Z., Catenacci, V. A., & Melanson, E. L. (2019). Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients, 11(10), 2442. https://doi.org/https://doi.org/10.3390/nu11102442
- Sadeghirad, B., Motaghipisheh, S., Kolahdooz, F., Zahedi, M. J., & Haghdoost, A. A. (2014). Islamic fasting and weight loss: A systematic review and meta-analysis. Public Health Nutrition, 17((02|2)), 396–406. https://doi.org/https://doi.org/10.1017/S1368980012005046
- Sajjad, A., Anwer, M. O., Anwer, S., Zaidi, S. A. A., & Hasan, A. (2014). Missing breakfast, sleep, and exercise: Are you skipping out years of life. Journal of Nutrition and Health Sciences, 1(3), 1–7. https://doi.org/https://doi.org/10.15744/2393-9060.1.308
- Saladin, R., De Vos, P., Guerre-Millo, M., Leturque, A., Girard, J., Staels, B., & Auwerx, J. (1995). Transient increase in obese gene expression after food intake or insulin administration. Nature, 377(6549), 527–528. https://doi.org/https://doi.org/10.1038/377527a0
- Sasaki, H., Ohtsu, T., Ikeda, Y., Tsubosaka, M., & Shibata, S. (2014). Combination of meal and exercise timing with a high-fat diet influences energy expenditure and obesity in mice. Chronobiology International, 31(9), 959–975. https://doi.org/https://doi.org/10.3109/07420528.2014.935785
- Schechter, A., Rising, R., Albu, J. B., & St-Onge, M.-P. (2013). Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. American Journal of Clinical Nutrition, 98(6), 1433–1439. https://doi.org/https://doi.org/10.3945/ajcn.113.069427
- Schechter, A., & St-Onge, M. P. (2014). Delayed sleep timing is associated with low levels of free-living physical activity in normal sleeping adults. Sleep Medicine, 15(12), 1586–1589. https://doi.org/https://doi.org/10.1016/j.sleep.2014.07.010
- Scheer, F. A. J. L., Morris, C. J., & Shea, S. A. (2013). The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity, 21(3), 421–423. https://doi.org/https://doi.org/10.1002/oby.20351
- Schlundt, D. G., Hill, J. O., Sbrocco, T., Pope-Cordle, J., & Sharp, T. (1992). The role of breakfast in the treatment of obesity: A randomized clinical trial. American Journal of Clinical Nutrition, 55(3), 645–651. https://doi.org/https://doi.org/10.1093/ajcn/55.3.645
- Schmid, S. M., Hallschmid, M., Jauch-Chara, K., Wilms, B., Benedict, C., Lehnhert, H., Born, J., & Schultes, B. (2009). Sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. American Journal of Clinical Nutrition, 90(6), 1476–1482. https://doi.org/https://doi.org/10.3945/ajcn.2009.27984
- Schwartz, M. W., Woods, S. C., Seeley, R. J., Barsh, G. S., Baskin, D. G., & Leibel, R. L. (2003). Is the energy homeostasis system inherently biased toward weight gain? Diabetes, 52(2), 232–238. https://doi.org/https://doi.org/10.2337/diabetes.52.2.232
- Serin, Y., & Acar Tek, N. (2019). Effect of circadian rhythm on metabolic processes and the regulation of energy balance. Annals of Nutrition and Metabolism, 74(4), 322–330. https://doi.org/https://doi.org/10.1159/000500071
- Shaw, E., Leung, G. K. W., Jong, J., Coates, A. M., Davis, R., Blair, M., Huggins, C. E., Dorrian, J., Banks, S., Kellow, N. J., & Bonham, M. P. (2019). The impact of time of day on energy expenditure: Implications for long-term energy balance. Nutrients, 11(10), E2383. https://doi.org/https://doi.org/10.3390/nu11102383
- Silva, C. M., Mota, M. C., Miranda, M. T., Paim, S. L., Waterhouse, J., & Crispim, C. A. (2016). Chronotype, social jetlag and sleep debt are associated with dietary intake among Brazilian undergraduate students. Chronobiology International, 33(6), 740–748. https://doi.org/https://doi.org/10.3109/07420528.2016.1167712
- Souza, R. V., Sarmento, R. A., de Almeida, J. C., & Canuto, R. (2019). The effect of shift work on eating habits: A systematic review. Scandinavian Journal of Work, Environment, & Health, 45(1), 7–21. https://doi.org/https://doi.org/10.5271/sjweh.3759
- Spaeth, A. M., Dinges, D. F., & Goel, N. (2013). Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep, 36(7), 981–990. https://doi.org/https://doi.org/10.5665/sleep.2792
- Spaeth, A. M., Dinges, D. F., & Goel, N. (2014). Sex and race differences in caloric intake during sleep restriction in healthy adults. The American Journal of Clinical Nutrition, 100(2), 559–566. https://doi.org/https://doi.org/10.3945/ajcn.114.086579
- Spaeth, A. M., Dinges, D. F., & Goel, N. (2015). Resting metabolic rate varies by race and by sleep duration. Obesity, 23(12), 2349–2356. https://doi.org/https://doi.org/10.1002/oby.21198
- Spengler, C. M., Czeisler, C. A., & Shea, S. A. (2000). An endogenous circadian rhythm of respiratory control in humans. The Journal of Physiology, 526(3), 683–694. https://doi.org/https://doi.org/10.1111/j.1469-7793.2000.00683.x
- Spiegel, K., Tasali, E., Penev, P., & Van Cauter, E. (2004). Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine, 141, 846–850. https://doi.org/https://doi.org/10.7326/0003-4819-141-11-200412070-00008
- St-Onge, M.-P. (2013). The role of sleep duration in the regulation of energy balance: Effects on energy intakes and expenditure. Journal of Clinical Sleep Medicine, 9(1), 73–80. https://doi.org/https://doi.org/10.5664/jcsm.2348
- St-Onge, M. P., Ard, J., Baskin, M. L., Chiuve, S. E., Johnson, H. M., Kris-Etherton, P., & Varady, K. (2017). Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the American heart association. Circulation, 136(14), e96–e121. https://doi.org/https://doi.org/10.1161/CIR.0000000000000476
- St-Onge, M. P., McReynolds, A., Triveldi, Z. B., Roberts, A. L., Sy, M., & Hirsch, J. (2012). Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. American Journal of Clinical Nutrition, 95(4), 818–824. https://doi.org/https://doi.org/10.3945/ajcn.111.027383
- Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y., & Menaker, M. (2001). Entrainment of the circadian clock in the liver by feeding. Science, 291(5503), 490–493. https://doi.org/https://doi.org/10.1126/science.291.5503.490
- Stote, K. S., Baer, D. J., Spears, K., Paul, D. R., Harris, G. K., Rumpler, W. V., Strycula, P., Najjar, S. S., Ferrucci, L., Ingram, D. K., Longo, D. L., & Mattson, M. P. (2007). A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. American Journal of Clinical Nutrition, 85(4), 981–988. https://doi.org/https://doi.org/10.1093/ajcn/85.4.981
- Striegel-Moore, R. H., Franko, D. L., Thompson, D., Affenito, S., May, A., & Kraemer, H. C. (2008). Exploring the typology of night eating syndrome. International Journal of Eating Disorders, 41(5), 411–418. https://doi.org/https://doi.org/10.1002/eat.20514
- Sun, M., Feng, W., Wang, F., Li, P., Li, Z., Li, M., Vlaanderen, J., Vermeulen, R., & Tse, L. A. (2017). Meta-analysis on shift work and risks of specific obesity types. Obesity Reviews, 19(1), 28–40. https://doi.org/https://doi.org/10.1111/obr.12621
- Sutton, E. F., Beyl, R., Early, K. S., Cefalu, W. T., Ravussin, E., & Peterson, C. M. (2018). Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabolism, 27(6), 1212–1221.e3. https://doi.org/https://doi.org/10.1016/j.cmet.2018.04.010
- Tahara, Y., Otsuka, M., Fuse, Y., Hirao, A., & Shibata, S. (2011). Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. Journal of Biological Rhythms, 26(3), 230–240. https://doi.org/https://doi.org/10.1177/0748730411405958
- Taheri, S., Lin, L., Austin, D., Young, T., & Mignot, E. (2004). Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Medicine, 1(3), e62. https://doi.org/https://doi.org/10.1371/journal.pmed.0010062
- Taylor, B. J., Matthews, K. A., Hasler, B. P., Roecklein, K. A., Kline, C. E., Buysse, D. J., Kravitz, H. M., Tiani, A. G., Harlow, S. D., & Hall, M. H. (2016). Bedtime variability and metabolic health in midlife women: The SWAN sleep study. Sleep, 39(2), 457–465. https://doi.org/https://doi.org/10.5665/sleep.5464
- Tinsley, G. M., Forsse, J. S., Butler, N. K., Paoli, A., Bane, A. A., La Bounty, P. M., Morgan, G. B., & Grandjean, P. W. (2017). Time-restricted feeding in young men performing resistance training: A randomized controlled trial. European Journal of Sport Science, 17(2), 200–207. https://doi.org/https://doi.org/10.1080/17461391.2016.1223173
- Tremblay, M. S., Aubert, S., Barnes, J. D., Saunders, T. J., Carson, V., Latimer-Cheung, A. E., Chastin, S. F. M., Altenburg, T. M., & Chinapaw, M. J. M. (2017). Sedentary behavior research network (SBRN) – terminology consensus project process and outcome. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 75. https://doi.org/https://doi.org/10.1186/s12966-017-0525-8
- Tuomi, T., Nagorny, C. L. F., Singh, P., Bennet, H., Yu, Q., Alenkvist, I., Isomaa, B., Östman, B., Söderström, J., Pesonen, A.-K., Martikainen, S., Räikkönen, K., Forsén, T., Hakaste, L., Almgren, P., Storm, P., Asplund, O., Shcherbina, L., Fex, M., … Mulder, H. (2016). Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metabolism, 23(6), 1067–1077. https://doi.org/https://doi.org/10.1016/j.cmet.2016.04.009
- Van Cauter, E., & Copinschi, G. (2006). Endocrine and other biological rhythms. In L. DeGroot, & J. Jameson (Eds.), Endocrinology (pp. 235–256). Saunders.
- Van Cauter, E., Desir, D., Decoster, C., Fery, F., & Balasse, E. G. (1989). Nocturnal decrease of glucose tolerance during constant glucose infusion. The Journal of Clinical Endocrinology & Metabolism, 69(3), 604–611. https://doi.org/https://doi.org/10.1210/jcem-69-3-604
- Van Drongelen, A., Boot, C. R., Merkus, S. L., Smid, S., van der Beek, T., & J, A. (2011). The effects of shift work on body weight change – a systematic review of longitudinal studies. Scandinavian Journal of Work, Environment, and Health, 37(4), 263–275. https://doi.org/https://doi.org/10.5271/sjweh.3143
- van Moorsel, D., Jansen, J., Havekes, B., Scheer, F. A. J. L., Jörgensen, J. A., Hoeks, J., Schrauwen-Hinderling, V. B., Duez, H., Lefebvre, P., Schaper, N. C., Hesselink, M. K. C., Staels, B., & Schrauwen, P. (2016). Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Molecular Metabolism, 5(8), 635–645. https://doi.org/https://doi.org/10.1016/j.molmet.2016.06.012
- Van Proeyen, K., Szlufcik, K., Nielens, H., Pelgrim, K., Deldicque, L., Hesselink, M., Van Veldhoven, P. P., & Hespel, P. (2010). Training in the fasted state improves glucose tolerance during fat-rich diet. The Journal of Physiology, 588(Pt. 21), 4289–4302. https://doi.org/https://doi.org/10.1113/jphysiol.2010.196493
- Versteeg, R. I., Ackermans, M. T., Nederveen, A. J., Fliers, E., Serlie, M. J., & la Fleur, S. E. (2018). Meal timing effects on insulin sensitivity and intrahepatic triglycerides during weight loss. International Journal of Obesity, 42(2), 156–162. https://doi.org/https://doi.org/10.1038/ijo.2017.199
- Vollmers, C., Gill, S., DiTacchio, L., Pulivarthy, S. R., Le, H. D., & Panda, S. (2009). Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences, 106(50), 21453–21458. https://doi.org/https://doi.org/10.1073/pnas.0909591106
- Wakui, T., Shirono, S., Takahashi, S., Fujimura, T., & Harada, N. (2002). Physical activity, energy expenditure and work intensity of care-workers on shift work in a special nursing home for the elderly. Journal of Occupational Health, 44(1), 8–14. https://doi.org/https://doi.org/10.1539/joh.44.8
- Westerterp-Plantenga, M. S., IJedema, M. J., & Wijckmans-Duijsens, N. E. (1996). The role of macronutrient selection in determining patterns of food intake in obese and non-obese women. European Journal of Clinical Nutrition, 50, 580–591.
- Wilkinson, M. J., Manoogian, E. M. C., Zadourian, A., Lo, H., Fakhouri, S., Shoghi, A., Wang, X., Fleischer, J. G., Navlakha, S., Panda, S., & Taub, P. R. (2020). Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metabolism, 31(1), 92–104.e5. https://doi.org/https://doi.org/10.1016/j.cmet.2019.11.004
- Willis, E. A., Creasy, S. A., Honas, J. J., Melanson, E. L., & Donnelly, J. E. (2020). The effects of exercise session timing on weight loss and components of energy balance: Midwest exercise trial 2. International Journal of Obesity (London), 44(1), 114–124. https://doi.org/https://doi.org/10.1038/s41366-019-0409-x
- Wittmann, M., Dinich, J., Merrow, M., & Roenneberg, T. (2006). Social jetlag: Misalignment of biological and social time. Chronobiology International, 23(1-2), 497–509. https://doi.org/https://doi.org/10.1080/07420520500545979
- Wong, P., Manuck, S. B., DiNardo, M. M., Korytowski, M., & Muldoon, M. F. (2015). Shorter sleep duration is associated with decreased insulin sensitivity in healthy white men. Sleep, 38(2), 223–231. https://doi.org/https://doi.org/10.5665/sleep.4402
- Woods, S. C., Seeley, R. J., Porte Jr, D., & Schwartz, M. W. (1998). Signals that regulate food intake and energy homeostasis. Science, 280(5368), 1378–1383. https://doi.org/https://doi.org/10.1126/science.280.5368.1378
- Zerón-Rugerio, M., Cambras, T., & Izquierdo-Pulido, M. (2019). Social jetlag associates negatively with the adherence to the Mediterranean diet and body mass index among young adults. Nutrients, 11(8), 1756. https://doi.org/https://doi.org/10.3390/nu11081756
- Zitting, K.-M., Vujovic, N., Yuan, R. K., Isherwood, C. M., Medina, J. E., Wang, W., Buxton, O. M., Williams, J. S., Czeisler, C. A., & Duffy, J. F. (2018). Human resting energy expenditure varies with circadian phase. Current Biology, 28(22), 3685–3690.e3. https://doi.org/https://doi.org/10.1016/j.cub.2018.10.005

