ABSTRACT
Introduction: Allergen bioavailability underpins the efficacy and safety of SLIT tablets. Three product-related factors are likely to influence this: tablet potency, formulation and sublingual holding time.
Areas covered: Tablet formulation determines the rate and extent of solubilized allergen release. Using validated in vitro dissolution assays, the two licensed grass pollen SLIT tablets are shown to release ≥85% of their total allergenic activity within several minutes. Sublingual holding time affects the contact duration between solubilized allergens and sublingual tissue. Maximal uptake of allergens by sublingual tissue requires ~5 minutes, with little uptake occurring within the first minute. A higher potency tablet with longer sublingual holding time would provide higher bioavailability, while faster rates of allergen release in vitro are unlikely to translate to a greater increase in bioavailability. Differences in dissolution times cannot serve as a surrogate of in vivo bioavailability, and are not related to differences in efficacy at the marketed tablet dosages. Rapid in vitro dissolution is likely not a key requirement for inducing a potent immune response.
Expert opinion: In vitro dissolution cannot predict the clinical efficacy of SLIT tablets but could be important in immune tolerance and safety. In addition, a discontinuous administration regimen may have benefits for adherence and cost without compromising efficacy.
1. Introduction
Sublingual immunotherapy (SLIT) using natural allergen extracts from grass or tree pollen, or house dust mite (HDM), has become a mainstay of treatment for immunoglobulin (Ig) E-dependent respiratory allergies. Two formulations of grass-pollen SLIT tablets are currently licensed for the treatment of allergic rhinitis (AR) in both Europe and the USA [Citation1]. The 5-grass pollen SLIT tablet (Oralair®; Stallergenes Greer, Antony, France) contains purified, calibrated freeze-dried extract of pollen from sweet vernal grass (Anthoxanthum odoratum), cocksfoot/orchard grass (Dactylis glomerata), perennial rye grass (Lolium perenne), meadow grass (Poa pratensis) and timothy grass (Phleum pratense), and is used at a 300 index of reactivity (IR) dose, equivalent to ~20–25 μg/mL of the group five major allergens [Citation1]. The compressed tablet contains allergen extract plus mannitol, microcrystalline cellulose, croscarmellose sodium, colloidal anhydrous silica, magnesium stearate and lactose monohydrate [Citation1]. The 1-grass pollen SLIT tablet (Grazax®/Grastek®; ALK-Abelló, Hørsholm, Denmark) is a 75,000 SQ-T (standardized quality tablet units) freeze-dried SLIT tablet containing an allergen extract from timothy grass (P. pratense), plus fish gelatin, mannitol and sodium hydroxide [Citation1].
SLIT tablets can be easily administered at home and do not require injections [Citation1]. The sublingual route is often used for the administration of small molecule drugs (e.g. nitroglycerine or opioid analgesics) to achieve fast absorption into the plasma. In contrast, proteins of large molecular size do not cross the mucosa to reach the bloodstream, but instead are captured and processed by oral antigen-presenting cells (APCs) within 15–30 minutes [Citation2], as assessed in vivo in animal models. APCs loaded with allergen-derived peptides migrate to the cervical lymph nodes within 12–24 h, where they interact with naive CD4 + T cells to induce Th1 and regulatory T cells with suppressive activity within 2–5 days. These CD4 + T cells subsequently migrate into the blood and tissues, resulting in long-term allergen-specific tolerance [Citation3]. The sublingual route offers a special advantage over other mucosal routes, because the antigen can be captured and processed by APCs prior to any significant proteolytic degradation occurring, thereby preserving the integrity of T and B cell epitope repertoires [Citation2].
Allergen bioavailability (in this case, the availability to uptake by APCs) underpins the efficacy and safety of SLIT tablets, and at least two product-related factors, in addition to tablet potency, are likely to influence this, namely tablet formulation and sublingual holding time. Here, we examine how the 5- and 1-grass pollen SLIT tablets differ with regards to these factors and other key characteristics, and whether differences between the products and formulations are of clinical relevance.
2. Product and formulation characteristics of licensed SLIT tablets for grass pollen-associated AR
The 1- and 5-grass pollen SLIT tablets have several differing characteristics, as illustrated by .
Table 1. Product and formulation characteristics of the 5-grass pollen and 1-grass pollen SLIT tablets
2.1. Tablet potency and administration regimen
The clinical effectiveness of SLIT and wider allergen immunotherapy (AIT) is clearly dose-dependent [Citation6], so clinicians should be able to assess and compare the potency of the various products available. Conversion to the bioequivalent allergy unit (BAU), the only allergen unit approved and recognized by a regulatory agency (the US Food and Drug Administration), permits direct comparison of allergenic potency in this way [Citation7]. The optimal dose of the 5-grass pollen tablet (300 IR) has been confirmed as 9,000 BAU, with higher doses yielding no additional benefit [Citation8,Citation9]. This dose has demonstrated long-term efficacy when prescribed in a discontinuous, pre- and coseasonal regimen (starting approximately 4 months prior to the pollen season) [Citation10], and the high potency of the 5-grass pollen tablet does not appear to be associated with any safety signals or poor tolerability [Citation11].
The approved 75,000 SQ-T dose of the 1-grass pollen tablet equates to 2,800 BAU; this dose is shown to be more effective than the 2,500 and 25,000 SQ-T doses [Citation12], and has also demonstrated long-term efficacy when prescribed continuously during the entire year [Citation13]. In contrast to the clinical development of the 5-grass pollen SLIT tablet, the optimal dose of the 1-grass pollen SLIT tablet has not been confirmed in dose-finding studies. Using the BAU, the 5-grass pollen SLIT tablet appears to have >3-fold greater allergenicity than the 1-grass pollen SLIT tablet.
Economic and cost-effectiveness studies from Germany [Citation14] and Canada [Citation15] have shown that the pre- and coseasonal administration schedule of the 5-grass pollen SLIT tablet appears to be associated with lower annual costs versus perennial administration of the 1-grass pollen SLIT tablet, with annual relative savings estimated at €1,142 and Canadian $1,168, respectively [Citation14,Citation15]. Moreover, compliance with the pre-/coseasonal regimen is high [Citation16]. This has even been demonstrated in a study that administered the 1-grass pollen SLIT tablet to allergic patients according to a pre- and coseasonal schedule instead of the recommended perennial schedule [Citation17]; in this study, compliance ranged from 72% in the first year of treatment to 90% by the third year of treatment [Citation17]. The discontinuous administration regimen of the 5-grass pollen SLIT tablet, in part because of its associated excellent compliance, has therefore been proposed as inherently more effective than perennial SLIT administration regimens [Citation18].
2.2. Grass-pollen epitope coverage
Grass pollen has ubiquitous representation and significant clinical importance worldwide [Citation19]; the distribution of Pooideae species varies across regions of Europe and North America, meaning that pollen allergen exposure to specific grasses differs between countries [Citation20,Citation21]. The pollen allergens from different Pooideae species exhibit some shared T and B cell epitopes, while group 1 and group 5 allergens display species-restricted IgE epitopes [Citation22]. The heterogeneity in grass pollen epitopes across species can be differentiated by the immune system, which may support the use of a mixed pollen allergen extract over a single pollen allergen preparation [Citation21]. The ability of the 5- and 1-grass pollen SLIT tablets to mimic the range of epitopes that may be encountered in Europe has been assessed by testing sera from adult patients living in Spain (n = 19) or Sweden (n = 22) [Citation20]. The 5-grass pollen SLIT tablet demonstrated significantly greater inhibition of IgE binding to pollen allergens from 12 grasses common to Europe (bent grass, Bermuda grass, brome grass, cocksfoot, false oat-grass, meadow fescue, meadowgrass, rye-grass, sweet vernal-grass, timothy, wild oat and Yorkshire fog) than did the 1-grass pollen SLIT tablet (p < 0.0001), both for the total tested population and when stratified by country. This difference between SLIT tablets was significantly greater for Spanish than Swedish patients (p < 0.05). Fewer epitopes associated with grass pollen allergy were present in the 1-grass pollen SLIT tablet, compared with the 5-grass pollen SLIT tablet, and this difference was especially pronounced when comparing sera from patients in the two geographical regions [Citation20]. In agreement with previous research [Citation22], the authors concluded that the 5-grass pollen SLIT tablet has superior epitope coverage to the 1-grass pollen SLIT tablet, especially for patients from Spain and other Southern European countries, who may be less exposed to Timothy grass than other grass species [Citation20].
2.3. Tablet dissolution and allergen release
Absorption of small molecules through biological membranes, including the transmucosal membrane, is positively related to lipophilicity and negatively related to ionization and molecular size. Transmucosal formulations of the small molecule, fentanyl, achieve rapid dissolution and absorption across the mucosa and are therefore more likely to match the time-course of transitory flares of breakthrough pain than oral formulations in patients with stable, controlled persistent pain [Citation23]. Conversely, the superior immunogenicity of a novel intranasal influenza vaccine, based on a novel polycationic lipid-ceramide carbamoyl-spermine, is thought to be attributed to long retention of the vaccine at the administration site, eliciting strong local and systemic immune responses [Citation24]. Pharmacokinetic and biodistribution studies following intranasal administration of the vaccine in mice showed that both the lipids and antigens were retained in the nose and lung for at least 24 h, and this retention appeared to be correlated with enhanced immunogenicity [Citation24]. Therefore, rapid dissolution is not necessarily a key factor for induction of a potent immune response to medications administered via the nasal route. This might also apply to oral administered agents.
SLIT relies on the efficient delivery of soluble allergens to the sublingual mucosa, in a form that is appropriate for capture by the immunocompetent cells that reside there. After entering the oral mucosa, allergens are taken up by resident APCs, which subsequently migrate to the draining cervical lymph nodes; here, they elicit allergen specific Th1/Treg responses, thus suppressing the established allergic Th2 response, and leading to the gradual development of immune tolerance [Citation3,Citation25]. As mentioned previously, allergen bioavailability within the sublingual mucosa is the foundation of both the safety and efficacy of AIT products. The European Medicines Agency defines bioavailability as ‘the rate and extent to which the active substance or active moiety is absorbed from a pharmaceutical form and becomes available at the site of action’ [Citation26]. Therefore, any rigorous assessment of allergen bioavailability from SLIT tablets should consider several parameters, including allergen content and release kinetics after tablet dissolution, the effect of salivary components on allergen degradation, and the extent of allergen uptake by epithelial cells and APCs in the sublingual mucosa [Citation27].
Depending on the method used, in vitro dissolution assays of SLIT tablets can produce dramatically different results. Using a method advocated by the Japanese Pharmacopoeia, the 1-grass pollen SLIT tablet was shown to dissolve completely within 1 s versus incomplete dissolution of the 5-grass pollen SLIT tablet after 30 s, and this difference between dissolution times was significant (p < 0.001) [Citation28]. Grass group 5 major allergen content was measured by enzyme-linked immunosorbent assay using a proprietary monoclonal antibody against Phl p 5, after dissolution of either grass pollen SLIT tablet in a phosphate buffer approximating human saliva in pH, ionic strength and total protein content (but not containing salivary enzymes or any other components of saliva). Full recovery of the 1-grass pollen SLIT tablet allergen content was achieved after ~15 s, whereas only ~16% of Phl p 5 content was released from the 5-grass pollen SLIT tablet after 120 s (p < 0.0001 for comparison of released Phl p 5) [Citation28]. It should be remembered that quantification of Phl p 5 can be affected by the specificity and sensitivity of the antibodies used for detection, and/or whether they are mono- or polyclonal antibodies; moreover, quantification of Phl p 1/grass group 1 major allergens has not been assessed for either SLIT tablet formulation.
Comparable assays to those used for grass pollen have been used to evaluate SLIT tablets containing house dust mite (HDM) allergens (12 SQ HDM or 300 IR HDM). Disintegration times were ~1 s versus 45 s for the 12 SQ HDM and 300 IR HDM SLIT tablets, respectively (p < 0.0001). Complete release of Der f 1, Der p 1 and Der 2 from the 12 SQ-HDM SLIT tablet was obtained within 30 s, whereas for the 300 IR HDM SLIT tablet, soluble HDM major allergen content continued to increase over time, but did not reach complete release, even after 10 minutes [Citation29,Citation30]. In contrast, using an in vitro dissolution assay endorsed by the European Pharmacopoeia (Ph. Eur. 2.9.3 dissolution test for solid dosage forms, apparatus 4 flow-through cell [Citation31]), ≥85% of total allergenic activity is shown to be released from 300 IR and 100 IR HDM and 5 grass SLIT tablets within 2 minutes () [Citation27].
Figure 1. Kinetics of allergen activity release and dissolution of 300 IR and 100 IR HDM and 5 grass SLIT tablets in vitro.
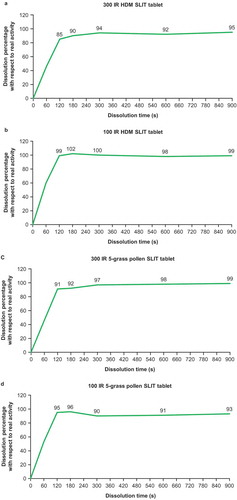
Rapid complete release of grass pollen or HDM major allergens (e.g. within seconds) may contribute to a faster allergen saturation of APCs and prevent optimal allergen targeting [Citation27]. The kinetics of allergen uptake by APCs in the sublingual mucosa are dose-dependent up to the point of saturation, and therefore are an important contributor to allergen bioavailability [Citation32]. The 5-grass pollen and 300 IR HDM SLIT tablets have been designed to sustain allergen release over 2–3 minutes, with the aim of promoting allergen uptake by APCs without triggering rapid activation of local pro-inflammatory mast cells [Citation33]. It is unclear whether the rapid release of allergen from the 1-grass pollen SLIT tablet offers an advantage over the more gradual release from the 5-grass pollen SLIT tablet, because the production of high local concentrations of proteins may overwhelm immune cell uptake mechanisms. Unlike small molecule therapeutics (e.g. fentanyl, nitroglycerin), allergen uptake into sublingual tissue is comparatively slow and can be saturated, because it involves the transport of bulky proteins across cellular barriers.
Proteins that are swallowed are rapidly degraded by digestive enzymes and absorbed [Citation34,Citation35], and this process may alter the T and B cell epitope repertoire from that seen with oromucosal or nasal uptake. In a study that investigated the in vivo distribution kinetics of radiolabelled Parietaria judaica allergen after sublingual, oral and intranasal administration in healthy, non-allergic humans, no degradation of the labelled allergen occurred in the sublingual space for up to 20 minutes after sublingual administration, and there was no absorption of the radiolabelled allergen through the sublingual mucosa into the bloodstream for up to 30 minutes, but swallowed labelled allergen underwent rapid hydrolysis and absorption in the gastrointestinal tract [Citation34]. These findings are consistent with the large size and relatively high molecular weight of the protein, which also possesses a hydrophilic surface [Citation34]. Studies in allergic subjects using the radiolabeled allergens, Par j 1 and Der p2, administered sublingually have demonstrated that sensitization to the allergen affects at some extent its biodistribution and kinetics, especially at mucosal level [Citation35,Citation36].
The sublingual holding (swallowing) time affects the time of contact between solubilized allergens and sublingual tissue, because swallowing will remove the majority of unabsorbed allergens from the sublingual space, preventing further uptake. It takes 5 minutes for maximal uptake of allergens by the sublingual tissue, with little uptake within the first minute [Citation32] (). In light of this, the sublingual holding time should be at least several minutes. Early swallowing (e.g. at 1 minute) may reduce the uptake of allergens.
Figure 2. Kinetics of grass pollen allergen binding by oromucosal Langerhans cells ex vivo.
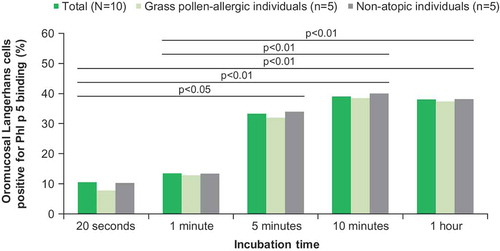
Human saliva contains >3,000 distinct proteins, including enzymes, hormones, antibodies and cytokines [Citation37], any of which may promote allergen degradation, decrease allergen potency, and preclude optimal targeting onto epithelial cells and APCs. Such effects are difficult, if not impossible, to model and assess using in vitro dissolution assays. For these reasons, although differences in dissolution times can be demonstrated using in vitro dissolution assays, these cannot serve as a surrogate of bioavailability, because they lack any direct correlation with an in vivo sustained effect [Citation27].
3. Clinical efficacy and safety of the 1- and 5-grass pollen SLIT tablets
The optimal allergen dose for SLIT should achieve the highest therapeutic effect without compromising safety or leading to unacceptable allergen-mediated discomfort during treatment [Citation29]. Both the 1- and 5-grass pollen SLIT tablets have been extensively studied during clinical development, and their favorable efficacy and safety profiles are well established (). Perennial treatment with the 1-grass pollen SLIT tablet leads to clinical improvement in adults suffering from rhinoconjunctivitis that is sustained over the long term [Citation13,Citation38]. The 5-grass pollen SLIT tablet, administered in a pre-coseasonal schedule, is effective in reducing allergic symptoms and symptomatic medication use, and improving a patient’s quality of life [Citation1,Citation39]. Improvement in symptoms upon allergen exposure is detectable after 1 month of treatment with the 5-grass pollen SLIT tablet [Citation40], demonstrating that although it may have a slower in vitro dissolution time than the 1-grass pollen SLIT tablet, allergen release is sufficient to achieve clinical efficacy within only 1 month. The clinical onset of action has not been determined for the 1-grass pollen SLIT tablet.
The safety profiles of both SLIT tablet products in clinical trials are similar, with the majority of adverse events being local, mild-to-moderate reactions that resolve without sequelae in the first 1–2 weeks of treatment. Neither SLIT tablet products caused anaphylactic shock or fatalities in clinical trials, although some grade 1–3 systemic AEs were observed. Notably, while no use of epinephrine was documented in trials of the 5-grass pollen SLIT tablet, 4 cases of epinephrine autoinjector use occurred during clinical trials of the 1-grass pollen SLIT tablet conducted either with adult or pediatric patients [Citation40–Citation42].
4. Conclusion
The optimal dose of the 5-grass pollen tablet (300 IR; 9000 BAU) has demonstrated long-term efficacy when prescribed in a discontinuous, pre- and coseasonal regimen. Importantly, the high potency of the 5-grass pollen tablet does not appear to be associated with any safety signals or poor tolerability. The optimal dose of the 1-grass pollen SLIT tablet has not been confirmed, but the approved dose (75,000 SQ-T; 2800 BAU) has demonstrated long-term efficacy when prescribed continuously during the entire year. The pre-/coseasonal regimen of the 5-grass pollen SLIT tablet appears to be associated with excellent adherence, and lower annual costs versus the 1-grass pollen SLIT tablet.
Differences between the licensed 1- and 5-grass pollen SLIT products are evident. A recent report has implied that in vitro dissolution kinetics of SLIT tablets correlate with bioavailability and clinical efficacy [Citation28]; however, it is clear that this is not the case, and in vitro dissolution rate cannot be used as a reliable indicator of clinical efficacy. Based on the current knowledge of the oral immune response, a formulation encompassing a very rapid dissolution may not be optimal for sublingual administration of allergens. A direct head-to-head comparison between the two grass pollen SLIT tablet products, to assess any differences in the extent of their efficacy, has yet to be performed.
5. Expert opinion
The biovailability of sublingual AIT in tablets is certainly of central importance, and likely related also to efficacy. It is true that the unique mode of action of SLIT (modulation of the immune response) and the complexity of events following sublingual administration make it difficult to study in detail all of the aspects involved. Nonetheless, immunological studies on allergen uptake by APCs in relation to the dissolution of tablets would allow us to further improve the efficacy of SLIT.
It is clear that the primary endpoint to be evaluated when comparing different SLIT formulations should be clinical efficacy supported by analysis of systemic biomarkers (for example, IgG4 or IgE), but this will require expensive and complex head-to-head trials, and this is not immediately feasible.
As already discussed, it is impossible to draw conclusions on the bioavailability and clinical efficacy of SLIT tablets, based on their in vitro dissolution time. However, the different dissolution times between the grass pollen SLIT tablet products could potentially influence administration.
Further investigation of the relationship between allergen retention at the administration site, and the elicited local and systemic immune responses, as well as the optimal frequency and duration of administration to gain an appropriate and sustained immune response is needed. Additionally, a better understanding of the possible differences in, and magnitude and extent of, humoral and mucosal immune responses provided by monovalent or multivalent formulations may pave the way for improving and optimizing the efficacy of future SLIT. One can speculate that tablets designed to sustained allergen release over 2–3 minutes, without triggering rapid activation of local proinflammatory mast cells, are behind good safety profile.
Real-life data collected from registries of patients using SLIT and other treatments for grass pollen allergy are starting to provide more insights about the long-term effects of SLIT, and in the near future, will likely play a greater role in guiding treatment, especially regarding the most appropriate and effective administration regimen. Pre- and coseasonal administration appears to have advantages over perennial administration in terms of a reduced frequency and duration of administration, and the benefits for adherence and cost savings arising from this.
Article highlights
Differences in AIT tablet dissolution times cannot be assumed as a surrogate of in vivo bioavailability.
Clinical efficacy of AIT tablets is unlikely related to a very rapid in vitro allergen release at the marketed tablet dosages.
The current knowledge of the oral immune response supports tablet formulations with dissolution times superior to one minute.
The relevant aspect for allergists is 6 versus 12 months of treatment which is very important for long term compliance.
Pre- and co-seasonal administration scheme of AIT tablets appears to be associated with lower annual costs compared to perennial administration.
Declaration of interest
GW Canonica has received grants and consultancy fees from A.Menarini, Alk-Abello’, Allergy Therapeutics, AstraZeneca-Medimmune, Boehringer lngelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, HalAllergy, Merck Sharp & Dome, Mundipharma, Novartis, Orion, Sanofi-Aventis, SanofiGenzyme/Regeneron, Stallergenes-Greer, Uriach Pharnta, Teva, Valeas, and ViforPharma. L Mascarell, C Bos, and E Karagiannis are employees of Stallergenes Greer. P Devillier reports consulting fees, honoraria for lectures, and/or research funding from ALK-Abellô, Stallergenes Greer, Astra Zeneca, Chiesi, Boehringer-lngelheim, GlaxoSmithKline, Mylan, Novartis, Sanofi and Yslab. P Demoly has received consultancy fees from ALK, Stallergenes Greer, Allergy Therapeutics, YS/ab, Sanofi, Bausch & Lomb, AstraZeneca, and ThermoFisher Scientific; has received fees for participation in review activities from BTT; and has received lecture fees from MYLAN, Chiesi, ALK, and Stallergenes Greer. TB Casale was involved in several clinical trials including industry-sponsored trials and reports consulting fees from Stallergenes Greer, and has received research support from Stallergenes Greer Novartis, Genentech and Merck, and has been an investigator and consultant for Novartis and Genentech with all fees to his university employer. G Passalacqua reports past consultancy for Stallergenes Greer. U Wahn reports consulting fees from Allergopharma, Danone, Hipp, Merck, Novartis, IMS Health GmbH & Co and Stallergenes Greer; honoraria for lectures from ALK-Abellô, Allergopharma, Allergy Therapeutics, LETI, MSD, Nestlé, Novartis, Nutricia and Stallergenes Greer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Review of Clinical Immunology for their review work, but have no other relevant financial relationships to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgements
Medical writing support in the development of this manuscript was provided by James Reed, PhD of Newmed Publishing Services and funded by Stallergenes Greer in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Additional information
Funding
References
- Didier A, Wahn U, Horak F, et al. Five-grass-pollen sublingual immunotherapy tablet for the treatment of grass-pollen-induced allergic rhinoconjunctivitis: 5 years of experience. Expert Rev Clin Immunol. 2014;10(10):1309–1324.
- Moingeon P, Mascarell L. Induction of tolerance via the sublingual route: mechanisms and applications. Clin Dev Immunol. 2012;2012:623474.
- Moingeon P. Update on immune mechanisms associated with sublingual immunotherapy: practical implications for the clinician. J Allergy Clin Immunol Pract. 2013;1(3):228–241.
- ALK-Abelló/Merck. US prescribing information: Grastek® (Timothy grass pollen allergen extract) tablet for sublingual use; 2014 Available from: http://www.merck.com/product/usa/pi_circulars/g/grastek/grastek_pi.pdf
- Stallergenes-Greer. US prescribing information: Oralair® (Sweet Vernal, Orchard, Perennial Rye, Timothy, and Kentucky blue grass mixed pollens allergen extract) tablet for sublingual use; 2014 Available from: http://oralair.com/docs/ORALAIR%20Prescribing%20Information-Med%20Guide.pdf
- Calderón MA, Larenas D, Kleine-Tebbe J, et al. European academy of allergy and clinical immunology task force report on ‘dose-response relationship in allergen-specific immunotherapy’. Allergy. 2011;66(10):1345–1359.
- Passalacqua G, Sastre J, Pfaar O, et al. Comparison of allergenic extracts from different origins: the value of the FDA’s bioequivalent allergy unit (BAU). Expert Rev Clin Immunol. 2016;12(7):733–739.
- Didier A, Malling HJ, Worm M, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120(6):1338–1345.
- Demoly P, Passalacqua G, Calderon MA, et al. Choosing the optimal dose in sublingual immunotherapy: rationale for the 300 index of reactivity dose. Clin Transl Allergy. 2015;5:44.
- Didier A, Malling HJ, Worm M, et al. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12.
- Didier A, Bons B. Safety and tolerability of 5-grass pollen tablet sublingual immunotherapy: pooled analysis and clinical review. Expert Opin Drug Saf. 2015;14(5):777–788.
- Durham SR, Yang WH, Pedersen MR, et al. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(4):802–809.
- Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–25 e5.
- Westerhout KY, Verheggen BG, Schreder CH, et al. Cost effectiveness analysis of immunotherapy in patients with grass pollen allergic rhinoconjunctivitis in Germany. J Med Econ. 2012;15(5):906–917.
- Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. J Eval Clin Pract. 2014;20(3):225–238.
- Schäfer U, Kienle-Gogolok A, Hadler M, et al. Treatment satisfaction during sublingual immunotherapy with a five-grass pollen tablet for allergic rhinoconjunctivitis: a prospective, non-interventional study. Drugs Real World Outcomes. 2017;4(2):109–117.
- Wessel F, Chartier A, Meunier JP, et al. Safety and tolerability of an SQ-standardized GRAss ALlergy immunotherapy tablet (GRAZAX(R)) in a real-life setting for three consecutive seasons - the GRAAL trial. Clin Drug Investig. 2012;32(7):451–463.
- Demoly P, Calderon MA, Casale TB, et al. The value of pre- and co-seasonal sublingual immunotherapy in pollen-induced allergic rhinoconjunctivitis. Clin Transl Allergy. 2015;5:18.
- Popescu FD. Molecular biomarkers for grass pollen immunotherapy. World J Methodol. 2014;4(1):26–45.
- Batard T, Sanjuan A, Denis L, et al. Two grass pollen tablets commercially available for allergy immunotherapy display different IgE epitope repertoires. Clin Transl Allergy. 2019;9:13.
- Moingeon P, Cox L. Relevance of a 5-grass sublingual tablet for immunotherapy of patients with grass pollen allergy in North America. Expert Rev Clin Immunol. 2016;12(6):617–623.
- Chabre H, Gouyon B, Huet A, et al. Molecular variability of group 1 and 5 grass pollen allergens between Pooideae species: implications for immunotherapy. Clin Exp Allergy. 2010;40(3):505–519.
- Moore N, Darwish M, Amores X, et al. A review of the pharmacokinetic profile of transmucosal fentanyl formulations. Curr Med Res Opin. 2012;28(11):1781–1790.
- Even-Or O, Joseph A, Itskovitz-Cooper N, et al. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine. 2011;29(13):2474–2486.
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133(3):621–631.
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP), Note for guidance on the investigation of bioavailability and bioequivalence (EMEA/CPMP/EWP/1401/98); 2000 Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf
- Mascarell L, Batard T, Cuine JF, et al. The bioavailability of allergens in allergy tablets depends on several factors. Int Arch Allergy Immunol. 2018;175(4):252–253.
- Lund K, Kito H, Skydtsgaard MB, et al. The importance of tablet formulation on allergen release kinetics and efficiency: comparison of freeze-dried and compressed grass pollen sublingual allergy immunotherapy tablet formulations. Clin Ther. 2019;41(4):742–753.
- Ohashi-Doi K, Kito H, Du W, et al. Bioavailability of house dust mite allergens in sublingual allergy tablets is highly dependent on the formulation. Int Arch Allergy Immunol. 2017;174(1):26–34.
- Kito H, Du W, Nakazawa H, et al. The effective allergenic reactivity of house dust mite sublingual immunotherapy tablets is determined by tablet formulation. Biol Pharm Bull. 2019;42(6):1030–1033.
- European Pharmacopoeia. 2.9.3 Dissolution test for solid dosage forms; 2008 Available from: http://www.uspbpep.com/ep60/2.9.%203.%20dissolution%20test%20for%20solid%20dosage%20forms%2020903e.pdf
- Allam JP, Wurtzen PA, Reinartz M, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126(3):638–45 e1.
- Mascarell L, Rak S, Worm M, et al. Characterization of oral immune cells in birch pollen-allergic patients: impact of the oral allergy syndrome and sublingual allergen immunotherapy on antigen-presenting cells. Allergy. 2015;70(4):408–419.
- Bagnasco M, Mariani G, Passalacqua G, et al. Absorption and distribution kinetics of the major Parietaria judaica allergen (Par j 1) administered by noninjectable routes in healthy human beings. J Allergy Clin Immunol. 1997;100(1):122–129.
- Passalacqua G, Altrinetti V, Mariani G, et al. Pharmacokinetics of radiolabelled Par j 1 administered intranasally to allergic and healthy subjects. Clin Exp Allergy. 2005;35(7):880–883.
- Bagnasco M, Passalacqua G, Villa G, et al. Pharmacokinetics of an allergen and a monomeric allergoid for oromucosal immunotherapy in allergic volunteers. Clin Exp Allergy. 2001;31(1):54–60.
- Jagr M, Eckhardt A, Pataridis S, et al. Proteomics of human teeth and saliva. Physiol Res. 2014;63(Suppl 1):S141–54.
- Durham SR, Emminger W, Kapp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125(1):131–8 e1-7.
- Devillier P, Dreyfus JF, Demoly P, et al. A meta-analysis of sublingual allergen immunotherapy and pharmacotherapy in pollen-induced seasonal allergic rhinoconjunctivitis. BMC Med. 2014;12:71.
- Larenas-Linnemann D. How does the efficacy and safety of Oralair((R)) compare to other products on the market? Ther Clin Risk Manag. 2016;12:831–850.
- Canonica GW, Baena-Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62(3):317–324.
- Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy. 2014;69(7):854–867.

