ABSTRACT
Background
The only causal treatment for allergic rhinitis (AR) is allergen immunotherapy (AIT) including personalized liquid sublingual AIT (SLIT). We present the methodology for establishing the EfficAPSI cohort to further evaluate the real-life effectiveness and use of SLIT liquid.
Research design and methods
The EfficAPSI cohort was constituted by deterministic linkage of Stallergenes Greer dispensing and nationwide French healthcare insurance system (SNDS) databases. Data from 2006 to 2018 were extracted. All patients who initiated Stallergenes Greer SLIT liquid between 2010 and 2013 were considered as exposed and those dispensed with AR symptomatic treatment only as control. To limit the impact of confounding, the models will be weighted using the inverse probability of treatment weighting (IPTW).
Results
A total of 445,574 patients were included; median age was 38 years; 59.1% were female. Exposed patients (n = 112,492) were significantly younger, more frequently males, and less likely to have comorbidities than controls (n = 333,082). After IPTW, patients’ characteristics from both groups were similar.
Conclusions
To date, the EfficAPSI cohort has the largest number of person-years of follow-up in the field of AIT. The completeness of the data allows to evaluate SLIT liquid effectiveness with rigorous methodology, leading to important insights on personalized medicine in real-life.
1. Introduction
Allergic rhinitis (AR), affecting at least 400 million people worldwide, is a public health concern because of its increasing prevalence, its significant impacts on patients’ quality of life and increasing related burden of disease [Citation1]. In France, AR prevalence ranges from 20% to 30% of the population, with significant regional and age variations. Among them, one-third have an associated allergic asthma [Citation2–4].
Global AR management aims at controlling symptoms and reducing inflammation. International guidelines first recommend allergen avoidance. If not effective or feasible, oral or nasal antihistamines (AH) or intranasal corticosteroids (INCS) are recommended as first-line treatment. Oral corticosteroids (OCS) may be prescribed for very short periods. In case of insufficient or absence of response, allergen immunotherapy (AIT) may be indicated in association with pharmacotherapy [Citation5,Citation6].
AIT is the only causal treatment for respiratory allergies and consists in the repeated administration of an allergen extract to an allergic patient to reduce his/her individual sensitivity by progressively modulating the patient’s immune response to that allergen [Citation7,Citation8]. International guidelines recommend that AIT should be administered for at least three years, either sublingually (SLIT) with tablets or liquid formulations, or by subcutaneous injections (SCIT) [Citation4,Citation7,Citation9]. Both SLIT and SCIT are considered as effective but the latter has an increased frequency of moderate-to-severe systemic adverse reactions compared to the SLIT route [Citation7]. Specifically with SLIT, randomized clinical trials (RCT) and observational studies utilizing standardized allergen extracts have provided evidence of its benefits by demonstrating a sustained reduction in symptoms and in the use of AR and/or asthma medication during treatment, short and long-term follow-up [Citation7,Citation10–15].
Staloral® SLIT liquid (Stallergenes Greer, Antony, France) covers a wide range of allergen extracts. These personalized SLIT liquid formulations are manufactured according to the clinician prescription and the patient’s profile from a main solution obtained by extraction of allergens’ sources which has its own biological activity and is prepared for each individual patient following the named patient product legislation [Citation16,Citation17]. In 2018, the French National Authority for Health (Haute Autorité de Santé, HAS) asked for updated real-life data about personalized SLIT liquid formulations [Citation18]. In France, the recommended database for assessing real-world effectiveness is the public nationwide French healthcare insurance system SNDS (‘Système National des Données de Santé’) [Citation17]. Because of the specific legal status of SLIT liquid formulations in France, details (i.e., allergens, number of vials, concentration) of their dispensations are not recorded in the SNDS but may be available from companies’ dispensing registries.
With the aim of providing robust real-world evidence (RWE) of SLIT liquid treatments to fill an important data gap highlighted by the HAS, it has been thus decided, for the first time ever in the AIT field, to enrich the SNDS with data from the Stallergenes Greer dispensing registry to build the EfficAPSI cohort with the methodology described hereafter. First, a propensity score approach will be applied to address confounding by indication. Then, the EfficAPSI cohort, after inverse probability of treatment weighting (IPTW), will be subsequently exploited to evaluate the real-world effectiveness of personalized SLIT liquid on several endpoints, notably the impact of exposure to SLIT on asthma and on healthcare resource utilization (HCRU) by comparing AR patients treated or not with SLIT.
2. Patients and methods
2.1. Data sources
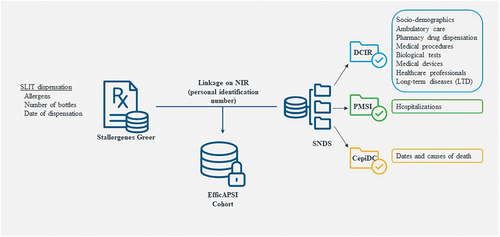
For establishing the EfficAPSI cohort, two databases were linked to constitute a representative nationwide cohort of patients with treated AR with or without asthma ().
The first database was the Stallergenes Greer’s dispensing registry recording prescription and reimbursement details for each dispensation of SLIT liquid for all patients, including dates, number, and composition of vials (i.e., allergen, concentration). The second database was the nationwide French healthcare insurance system SNDS database, including pseudonymized individual information for overall French population such as sociodemographic data, outpatient drug dispensation and other inpatient and outpatient healthcare resources’ reimbursements, hospital discharge summaries, and status for 30 long-term diseases (LTDs). The SNDS covers 98.8% of the French population (over 66 million persons) from birth (or immigration) to death (or emigration). Drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification, hospitalization diagnoses and LTDs according to the International Classification of Diseases 10th revision (ICD-10), and surgical and medical procedures according to the Classification Commune des Actes Médicaux (CCAM) [Citation19].
The linkage process used a deterministic approach based on matching Stallergenes Greer registry data to SNDS data using the unique personal identification number, NIR.
2.2. Ethical issues
Prior to the linkage between the two databases, a protocol validated by a multidisciplinary Scientific Committee was submitted to the Comité d’expertise pour les recherches, les études et les évaluations dans le domaine de la santé (CERESS; file number 790257). This Committee positively evaluated the study objectives and methodology, ethical relevance, scientific quality, and its public health interest. After the approval of cohorts’ details, study design and analyses, the protocol was submitted and approved by the Commission Nationale de l’Informatique et des Libertés (CNIL; file number 919412).
In line with the European General Data Protection Regulation (GDPR), all individuals in the Stallergenes Greer database were informed of the present study and were given the opportunity to opt out from the use of their data. Subsequently, all patients who opted out were excluded from the study. Regarding data from the SNDS database, the Caisse nationale de l’Assurance Maladie (CNAM), as data controller, was responsible for patient information.
2.3. Study population
The EfficAPSI cohort has been constituted from Stallergenes Greer and SNDS databases and all data from 2006 to 2018 were extracted from both databases. All patients with treated AR between 1 January 2010 and 31 December 2013 were identified (inclusion period). Patients who initiated a Stallergenes Greer SLIT liquid treatment were considered as exposed and patients dispensed with AR symptomatic treatment alone as controls. The index date was the date of the first dispensation of SLIT liquid or AR treatment, as appropriate. The look-back period corresponded to the four years before the index date. All patients were followed from the index date until death from any cause, loss to follow-up (defined as absence from reimbursed care for 12 consecutive months), or the end of the study (31 December 2018), whichever came first ().
Figure 2. Study design.
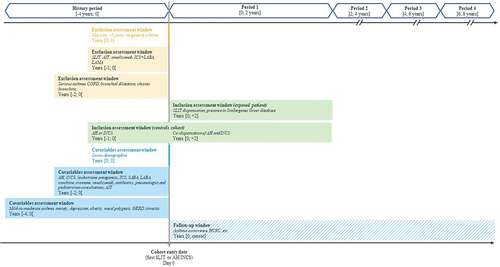
The inclusion criteria were (Appendix Methods S1 for related codes):
For exposed patients:
At least one dispensation of SLIT liquid from January 1st, 2010 to December 31st, 2013 in the Stallergenes Greer database (date of first dispensation = index date);
At least two years of follow-up after the last SLIT dispensation in the Stallergenes Greer database during the study period.
For control patients:
At least two co-dispensations (over a 10-day period) of AH and INCS during the inclusion period (date of first dispensation = index date);
At least one dispensation of AH or INCS in the year before the index date, in the SNDS database.
For all patients:
To be affiliated to the general scheme at least 2 years prior to the index date;
At least 5 years old at the index date.
The exclusion criteria were (Appendix Methods S1 and S2 for related codes):
Affiliated to Mayotte health assurance scheme at index date;
History of severe asthma, chronic obstructive pulmonary disease (COPD), bronchial dilatation and chronic bronchitis in the four years prior to the index date;
A least one dispensation of omalizumab or AIT in the year prior to the index date;
At least three dispensations of ICS combined with a long-acting β2-agonists (LABA) in the year prior to the index date;
At least two dispensations of a long-acting muscarinic antagonist (LAMA, alone or in combination with a LABA) in the year before the index date;
For control patients, being recorded in the Stallergenes Greer database.
The following variables were extracted from either Stallergenes Greer’s registry or SNDS database for each enrolled patient: demographic data, hospitalizations in public and private hospitals, LTD, outpatient medical expenses and drugs (). Except for demographic characteristics analyzed at baseline, all the above information was analyzed at both baseline and during follow-up.
Table 1. Available variables extracted from both databases.
2.4. Pre-specified study endpoints
The first endpoint will be the evaluation of the impact of SLIT on the occurrence of asthma event (prevention of asthma onset or worsening in patients without or with asthma, respectively) over the study period. To minimize bias that could be related to this ‘a priori’ definition and reinforce consistency and robustness of the analyses, several more or less stringent definitions of events based on LTD, hospitalizations, drugs and GINA steps will be used in the analyses.
The secondary endpoint will be the HCRU for AR and asthma. Different periods will be defined to allow on-treatment and post-treatment periods evaluation. For each period, specific drugs dispensation, medical procedures, consultations, hospitalizations, and sick leave will be considered.
2.5. Proposal of statistical approaches
To limit the impact of confounding factors and to account for the likely indication bias in comparative analyses, all models will be weighted using the IPTW. The probability of being treated with Staloral® will be determined for each patient from the propensity score estimated using a logistic regression adjusted for all the following patients’ characteristics at baseline: year of the index date, demographic data (age, sex, CMUc, department of residence), comorbidities (mild-to-moderate asthma, anxiety, depression, obesity, nasal polyposis, gastroesophageal reflux disease and sinusitis), history of hospitalization for asthma dispensation of antibiotics for respiratory infection, dispensation of AR and asthma treatments stratified into four time periods during the look-back period (]0 to −6 months],]-6 to −12 months],]-12 to −18 months], and]-18 to −24 months]). Models will be adjusted for unbalanced variables after weighting (standardized mean differences > 0.1), number of AH and INCS in the year preceding the index date, and on treatment dispensation and medical consultations as time-varying variables (by year): treatment dispensation (AH, INCS, leukotriene antagonists, ICS, SABA, LABA, LAMA, xanthine, cromone, omalizumab, and oral corticosteroids); number of pulmonologists and pediatrician consultations; dispensation of SLIT liquid. For outcomes that included one or more of the preceding time-varying variables, the corresponding variables will be excluded from the adjustment.
To describe the study population, quantitative variables will be reported as mean, standard deviation, median, and interquartile range [IQR] and categorical variables will be reported as number and percentage.
All comparative analyses will be adjusted using the IPTW. To assess pre-specified endpoints, several statistical approaches will be performed, such as Cox proportional hazard regression models and generalized estimating equations (GEE) [Citation20].
All analyses will be performed with SAS statistical software (version 9.4; SAS Institute, Cary, NC, U.S.A.).
3. Results
3.1. Constitution of the cohort
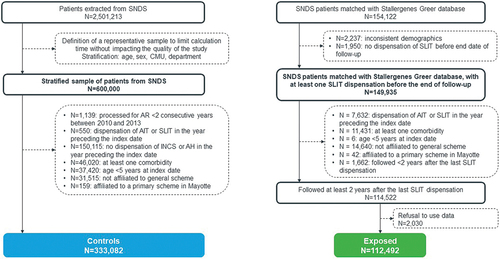
Between 2010 and 2013, 154,122 patients initiating a SLIT liquid treatment recorded in Stallergenes Greer database were linked with the SNDS database. After applying exclusion criteria 39,600 patients were excluded, mainly because they were not covered by the general health insurance scheme (n = 14,640). In addition, 2,030 patients opted out of their data being analyzed. Therefore, a total of 112,492 patients were included in the SLIT liquid exposed cohort. Simultaneously, 2,501,213 patients with AR with or without asthma, who have initiated AR symptomatic drug were identified from SNDS database from 2010 to 2013. In the stratified sample (n = 600,000), 266,918 patients were excluded including 150,115 for absence of INCS or AH dispensation in the year before the index date, and a total of 333,082 patients were included in the control cohort. Therefore, the EfficAPSI cohort included a total of 445,574 AR patients with or without asthma either treated with SLIT liquid or symptomatic drugs only in France ().
3.2. Description of the cohort characteristics
Among the 445,574 patients enrolled, a majority were female (59.1%), the median age was 38 years with 15.7% aged under 18 years old, and 11.8% benefited from the ‘Couverture Maladie Universelle complémentaire’ (CMUc, a complementary health insurance allowing full health cost recovery). About one-third (29.7%) of the patients had a history of mild-to-moderate asthma, and 14.8% a history of gastroesophageal reflux disease (GERD). During the 2 years prior to the index date, they had received a median of four dispensations (IQR: [2–8]) of AH or INCS.
Compared to all patients treated with SLIT liquid, the 2,030 patients who opted out of their data being analyzed were 4 years younger (median 34 years [IQR: 28–43] vs. 38 years [30–44]), were more frequently from the Ile-de-France region, had a greater history of AH dispensation (mean 3.9 vs. 5 dispensations) and had more frequently mild-to-moderate asthma (36.4% vs. 30.8%).
Exposed patients were significantly younger than controls (respective mean 37.8 vs. 38.8 years) and were more frequently male (44.0% vs. 39.9%). They were less likely to benefit from CMUc (5.3% vs. 13.3%) and less likely to have comorbidities, particularly anxiety (6.7% vs. 14.2%), depression (6.9% vs. 11.0%) and GERD (8.2% vs. 17.1%). On the other hand, in both groups, one-third of patients presented mild-to-moderate asthma (30.8% vs. 29.3%). With regards to AR treatment, exposed patients had a lower mean number of AH or INCS dispensed or AH and INCS co-dispensed (). After IPTW weighing, patients’ characteristics from both cohorts were similar (Appendix Figure S1).
Table 2. Description of the population at baseline, before weighting.
4. Discussion
The incidence of AR and asthma is increasing worldwide. The large scope of allergy phenotypes, associations of allergy phenotypes, and global patient management, in particular for those with preexisting asthma, leads to an unmet medical need that is addressed by the development of personalized medicine. SLIT liquid is a targeted treatment, specifically developed and adapted for a patient to treat his/her specific allergies taking into account his own immunologic (allergen sensitizations) profile and response to treatment. Precision dosing may optimize the overall benefit-risk profile of SLIT liquid for patients throughout their treatment, enabling them to achieve both short- and long-term treatment goals, while ensuring safety [Citation21,Citation22]. The evaluation of this promising personalized treatment to treat one of the most prevalent and impactful disease worldwide is necessary and essential to make public decision makers aware of their public health interest and maintain their public coverage for patients. To our knowledge, the EfficAPSI cohort is the first and unique cohort of patients created by linking the nationwide French health insurance database with a Company dispensing registry. To date, it is the largest cohort in terms of number of patients, representativeness, completeness of data and long-term follow-up.
4.1. Baseline characteristics of EfficAPSI patients
The baseline characteristics of EfficAPSI patients were consistent with the published epidemiologic data, our algorithm identified about 2.5 million of patients with treated AR (before randomized sampling) [Citation2,Citation23]. Patients who opted out from the use of their data appeared to be younger, with more AR treatment and with more frequent mild-to-moderate asthma. However, they represented only 1.8% of the SLIT liquid cohort and are unlikely to influence the results of this large cohort study. The EfficAPSI cohort is representative of the French population of patients treated for AR, with or without SLIT liquid treatment and with or without an history of mild-to-moderate asthma.
4.2. Methodological considerations
The increasing value of RWE for decision making is incontestable: RWE confirms and complements results from RCTs; particularly for an etiologic treatment such as AIT or personalized medications [Citation24,Citation25]. RWE studies (or RWS) on AIT make it possible to obtain results from a larger and more representative population than in RCTs, and to provide information on endpoints requiring large cohorts of patients with long-term follow-up to assess the post-treatment effect and the potential for prevention.
The purpose of the linkage is to combine data related to an individual or event from one source with additional data on the same individual or event from another source, in order to generate synergy and improve quality and completeness of data [Citation26]. In EfficAPSI cohort, the Stallergenes Greer registry provides valuable information on SLIT liquid treatment details as received by the patients (date, type of allergen, etc.) which could not be identified solely based on the SNDS medical-administrative database. Conversely, the SNDS provides information of all in- and out-patients reimbursements, without clinical related data. Therefore, this study can benefit from comprehensive data of a large representative nationwide cohort reflecting actual clinical practice.
However, RWS also face limitations including less robust methodology (notably lack of randomization), less structured and controlled data, and presence of confounding factors leading to the inability to match the reliability and accuracy of RCTs [Citation27,Citation28]. As with all studies using a medico-administrative or claim database such as SNDS, the absence of clinical data may be a limitation of the study. For instance, information on subtypes of allergen sensitizations is not available in the SNDS but only in Stallergenes Greer’s database, which limits the interpretation when comparing the SLIT and control groups. However, the identification of AR and the diagnosis of asthma in the SNDS are validated and all drugs are available which allows us to identify all patients, treatment modifications, exacerbation, or improvement of asthma disease. Therefore, to ensure comparability between patients receiving SLIT liquid and control patients, we applied relevant inclusion criteria based on several dispensations of AR treatment, identifying only moderate to severe AR patients who were likely to be treated with SLIT liquid. In EfficAPSI study, certain methodological aspects mirroring those from RCTs were considered to address some of these limitations and provide a high-quality RWE as recommended [Citation29,Citation30]. An example of a good-quality retrospective database study (REACT study) on AIT was recently published [Citation14,Citation30]. Similarly, our study was conducted following guidelines to conduct qualitative observational studies [Citation31,Citation32]. Propensity score-based methods, several definitions and sensitivity analyses were performed to reinforce the consistency and robustness of the initial analyses.
Baseline characteristics of the cohort are not similar between ‘SLIT liquid exposed patients’ and ‘control patients,’ with, as expected, younger ‘SLIT liquid exposed’ patients having fewer comorbidities compared to controls. However, the high statistical power of our cohort must be taken into account for all these analyses at baseline: a difference of 1 dispensation over 2 years or of 1% for mild-to-moderate asthma history could not be interpreted as a significant difference between groups. Furthermore, the construction of a propensity score, in collaboration with five clinicians specialized in allergology, clinical pharmacology and pneumology, allows us to have comparable groups of patients and to adjust for measured and unmeasured confounders. Propensity score-based methods (propensity score matching [PSM] and IPTW) are increasingly popular methods used to address confounding by indication in real-world studies and aimed to achieve a balanced distribution of confounders across treatment groups, thereby more closely emulating the properties of a RCT [Citation33]. From a statistical standpoint, the IPTW method we used has been shown to lead to more robust and less biased estimations of the treatment effect when there are few outcome events relative to the number of potential confounders (i.e., fewer than eight events per confounder) [Citation33]. When comparing SLIT liquid exposed and control patients, the weight for each patient is calculated by inverting the probability of receiving the treatment that the patient did in fact receive. IPTW provides an estimation of the Average Treatment Effect (ATE) because the study population is re-weighted to assess the effects of the treatment in the scenario that it was offered to all patients within the population. On the other hand, the PSM, used in the REACT study [Citation14,Citation30], estimates the Average Treatment effect for the Treated (ATT), excluding all unmatched patients. Consequently, results only reflect the effect of treatment for patients actually treated [Citation33].
The linkage between the two databases will allow to evaluate the impact of SLIT liquid treatment in terms of asthma occurrence, of HCRU, and of health-economic burden. The significance of this cohort, which contains all healthcare consumption data for the whole population, is that it enables the development of robust algorithms to define the events of interest, particularly asthma occurrence. Furthermore, the inclusion of all types of healthcare consumption, both specific and nonspecific of AR or asthma, allows for comprehensive analyses.
5. Conclusions
The EfficAPSI cohort shows the potential to link a patient dispensing registry to the SNDS, to constitute a registry and claims cohort enriched database. To date, the EfficAPSI cohort has the largest number of person-years of follow-up in the field of AIT. The completeness of the data, during treatment and follow-up, will allow to evaluate all patients’ care, including the care pathway, in the French healthcare system to evaluate SLIT liquid and provide important insights about this personalized medicine in real-life. Based on a rigorous methodology ensuring consistency and robustness of the results, the EfficAPSI cohort will enable to substantiate the SLIT liquid evidence established in RCTs during treatment and after treatment cessation on patients treated for AR with and without asthma.
Declaration of interest
Financial and competing interests disclosure: P Devillier reports fees for advisory boards, lectures, consulting, or support for attending meetings from ALK-Abelló, AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, IQVIA, Menarini, Mylan/Meda Pharma, Procter & Gamble, Stallergenes Greer and Viatris; M Molimard reports fees for consulting from ALK-Abelló, Novartis and Stallergenes Greer; JF Bergmann reports fees for advisory boards and consulting from Amgen, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, IQVIA, Janssen, Lilly, Novartis, Pfizer, Sanofi, Roche and Takeda;
A Gouverneur, J Vadel and C Collin are employees of IQVIA; L Girard and S Scurati are employees of Stallergenes Greer; P Demoly reports fees for research, teaching or consulting purposes from ALK-Abelló, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Puressentiel, Stallergenes Greer, ThermoFisherScientific, Viatris and Zambon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Metabolism and Toxicology for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors were involved in the conception and design, A Gouverneur, J Vadel and C Collin in analysis, all authors in the interpretation of the data; A Gouverneur, J Vadel and C Collin in drafting of the paper or revising it critically for intellectual content; all authors approved the final version of the manuscript; and all authors are agreeing to be accountable for all aspects of the work.
Geolocation information
France, Europe
Supplemental Material
Download MS Word (356.5 KB)Data availability statement
According to the principles of data protection and French regulations, the authors cannot publicly release the data from the ‘Système National des Données de Santé’ (SNDS). However, any person or structure, public or private, for-profit or nonprofit can access SNDS data upon authorization from the ‘Commission Nationale de l’Informatique et des Libertés’ (i.e., the French Data Protection Office) to carry out a study, research, or an evaluation of public interest (https://www.snds.gouv.fr).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/1744666X.2023.2294040.
Additional information
Funding
References
- Sánchez-Borges M, Martin BL, Muraro AM, et al. The importance of allergic disease in public health: an iCAALL statement. World Allergy Organ J. 2018;11(1):8. doi: 10.1186/s40413-018-0187-2
- Klossek J, Annesi-Measano I, Boucot I, et al. Prevalence, severity and impact of allergic rhinitis in the French community survey instant 2006. Allergy. 2008;63(s88):158–611. doi: 10.1111/j.1398-9995.2008.01760.x
- Beasley R, The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The Lancet. 1998;351(9111):1225–1232. doi: 10.1016/S0140-6736(97)07302-9
- Brożek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi: 10.1016/j.jaci.2017.03.050
- Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, asthma & Immunology/European Academy of Allergy and clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–1296.e3. doi: 10.1016/j.jaci.2013.01.049
- Wise SK, Damask C, Roland LT, et al. International consensus statement on allergy and rhinology: allergic rhinitis - 2023. Int Forum Allergy Rhinol. 2023;13(4):293–859. doi: 10.1002/alr.23090
- Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi: 10.1111/all.13317
- Larsen JN, Broge L, Jacobi H. Allergy immunotherapy: the future of allergy treatment. Drug Discov Today. 2016;21(1):26–37. doi: 10.1016/j.drudis.2015.07.010
- Wallace DV, Dykewicz MS, Oppenheimer J, et al. Pharmacologic treatment of seasonal allergic rhinitis: synopsis of guidance from the 2017 joint task force on practice parameters. Ann Intern Med. 2017;167(12):876–881. doi: 10.7326/M17-2203
- Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: House dust mite-driven allergic asthma. Allergy. 2019;74(5):855–873. doi: 10.1111/all.13749
- Zielen S, Devillier P, Heinrich J, et al. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. 2018;73(1):165–177. doi: 10.1111/all.13213
- Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: a retrospective database study in France. Allergy. 2019;74(7):1317–1326. doi: 10.1111/all.13705
- Wahn U, Bachert C, Heinrich J, et al. Real-world benefits of allergen immunotherapy for birch pollen-associated allergic rhinitis and asthma. Allergy. 2019;74(3):594–604. doi: 10.1111/all.13598
- Fritzsching B, Contoli M, Porsbjerg C, et al. Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: results from the REACT study, a retrospective cohort study. Lancet Reg Health Eur. 2022;13:100275. doi: 10.1016/j.lanepe.2021.100275
- Bozek A, Foks A, Trzaska K, et al. Long-term effects of allergen sublingual immunotherapy. Adv Dermatol Allergol. 2020;37(6):943–947. doi: 10.5114/ada.2019.85365
- Caimmi D, Demoly P. L’immunothérapie allergénique par APSI : place et perspectives pour le traitement et la prévention des allergies respiratoires ; le cas des pollinoses. Rev Fr Allergol. 2019;59(8):617–623. doi: 10.1016/j.reval.2019.07.007
- Haute Autorité de Santé. Études en vie réelle pour l’évaluation des médicaments et dispositifs médicaux. 2023. [cited 2023 Dec 15]. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2021-06/guide_etude_en_vie_reelle_medicaments__dm.pdf
- Haute Autorité de Santé. Décision n° 2018.0028/DC/SEM du 21 février 2018 du collège de la Haute Autorité de santé portant adoption d’une recommandation sur les allergènes préparés spécialement pour un seul individu. 2022 [cited 2023 Dec 15]. Available from: https://www.has-sante.fr/jcms/c_2832239/fr/decision-n-2018-0028/dc/sem-du-21-fevrier-2018-du-college-de-la-haute-autorite-de-sante-portant-adoption-d-une-recommandation-sur-les-allergenes-prepares-specialement-pour-un-seul-individu-apsi
- Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–962. doi: 10.1002/pds.4233
- Ballinger GA. Using Generalized Estimating Equations for Longitudinal Data Analysis. Organ Res Methods. 2004;7(2):127–150. doi: 10.1177/1094428104263672
- Incorvaia C, Ridolo E, Bagnasco D, et al. Personalized medicine and allergen immunotherapy: the beginning of a new era? Clin Mol Allergy CMA. 2021;19(1):10. doi: 10.1186/s12948-021-00150-z
- Thétis-Soulié M, Hosotte M, Grozelier I, et al. The MaDo real-life study of dose adjustment of allergen immunotherapy liquid formulations in an indication of respiratory allergic disease: reasons, practices, and outcomes. Front Allergy. 2022;3:971155. doi: 10.3389/falgy.2022.971155
- Demoly P, Allaert FA, Lecasble M, et al. PRAGMA. Validation of the classification of ARIA (allergic rhinitis and its impact on asthma). Allergy. 2003;58(7):672–675. doi: 10.1034/j.1398-9995.2003.t01-1-00202.x
- Devillier P, Demoly P, Molimard M. Allergen immunotherapy: what is the added value of real-world evidence from retrospective claims database studies? Expert Rev Respir Med. 2020;14(5):445–452. doi: 10.1080/17476348.2020.1733417
- Vogelberg C, Klimek L, Brüggenjürgen B, et al. Real-world evidence for the long-term effect of allergen immunotherapy: Current status on database-derived European studies. Allergy. 2022;77(12):3584–3592. doi: 10.1111/all.15506
- Scailteux LM, Droitcourt C, Balusson F, et al. French administrative health care database (SNDS): the value of its enrichment. Therapie. 2019;74(2):215–223. doi: 10.1016/j.therap.2018.09.072
- Passalacqua G, Bagnasco D. Real-life studies in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2021;21(4):361–367. doi: 10.1097/ACI.0000000000000757
- Calderon MA, Demoly P. Integrating the evidence in allergen immunotherapy: why real-world data should be important for the practicing clinician? Allergy Asthma Proc. 2022;43(4):305–309. doi: 10.2500/aap.2022.43.220006
- Paoletti G. Editorial: pharmacotherapy and evidence based medicine. Curr Opin Allergy Clin Immunol. 2022;22(4):257–258. doi: 10.1097/ACI.0000000000000831
- Fritzsching B, Contoli M, Porsbjerg C, et al. Real-world evidence: methods for assessing long-term health and effectiveness of allergy immunotherapy. J Allergy Clin Immunol. 2022;149(3):881–883. doi: 10.1016/j.jaci.2021.12.781
- Campbell JD, Perry R, Papadopoulos NG, et al. The REal life EVidence AssessmeNt tool (RELEVANT): development of a novel quality assurance asset to rate observational comparative effectiveness research studies. Clin Transl Allergy. 2019;9(1):21. doi: 10.1186/s13601-019-0256-9
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919
- Allan V, Ramagopalan SV, Mardekian J, et al. Propensity score matching and inverse probability of treatment weighting to address confounding by indication in comparative effectiveness research of oral anticoagulants. J Comp Eff Res. 2020;9(9):603–614. doi: 10.2217/cer-2020-0013