ABSTRACT
Introduction
Extracorporeal Photopheresis (ECP) may be considered the unique large-scale cell therapy currently available. It is currently employed mainly as second-line treatment, especially in steroid-resistant or steroid-dependent Graft versus Host Disease (GvHD) with good results and very few limitations.
Areas covered
Many points need to be clarified regarding the ECP mechanism of action, that conditions the lack of uniqueness among the different centers, essentially cycle frequency, treatment duration, and the number of cells to be treated to obtain a response, according to the organs involved. Moreover, reliable biomarkers for prediction of response are lacking, as well as the best pharmacological combination. We will focus on the recent advances concerning ECP for GvHD treatment. We performed a systematic literature research in Pubmed and Embase as of September 2023.
Expert opinion
The recent studies on ECP mechanism of action along with the promising biomarkers of response, and the synergistic benefit of ECP in association with the new drugs render this therapy an important weapon for GvHD resistant to conventional treatment and can be proposed as a valid first-line therapy option with promising results. We believe that it should be used early in all categories of patients, considering its high safety profile.
1. Introduction
Extracorporeal Photopheresis (ECP) may be considered a unique large-scale cell therapy currently available. The history of ECP dates back to the ’80s when Edelson successfully proposed the UV-A irradiation of patients’ mononuclear cells (MNCs) with their consequent apoptosis to cure a neoplastic disease, namely cutaneous T cell lymphoma [Citation1,Citation2]. The story continued after the fortunate observation that UV-A-irradiated MNCs exert an important immunomodulating effect in many immune and autoimmune diseases and, among them, solid organ rejection and acute and chronic graft versus host disease (aGvHD, cGvHD) [Citation3–5]. However, many points remain to be elucidated, including the mechanism of action that still has its mainstay in the induction of apoptosis in virtually all treated MNCs [Citation6,Citation7]. In addition, there are still a number of unresolved issues that account for the variety of treatments among Centers and the variable outcomes: timing of ECP start, optimal frequency of treatments depending on the organ involved and duration of maintenance therapy, optimal timing of ECP discontinuation, and combination therapy. Furthermore, there is no conclusive data on the number of cells to be infused to obtain a response, and identification of valid biomarkers to predict a response. It follows that the main limitations of the procedure are: variability of response rates, the need for adequate resources (equipment and trained personnel), logistical problems for patients who live far from the treating centers, and suitable vascular access. Consequently, the therapy is individualized (decision made case by case by the attending physician) with no definite guidelines. However, ECP demonstrated a clear survival advantage in responders with no adjunctive immunosuppression, augmented risk of infections, or disease relapse. Furthermore, the possibility of reducing or tapering immunosuppressive therapy (including steroids) renders ECP a well-established second-line treatment. In this brief review, we will discuss some recent insights for ECP in GvHD that are considered particularly controversial.
As of September 2023, a search in PubMed and Embase was performed to identify recently published manuscripts (’Photopheresis“[Mesh] OR ”Photochemotherapy“[Mesh] OR ”extracorporeal photopheresis’OR Photochemotherap*) AND (chronic graft versus host disease’ OR cGvHD OR ’acute graft-versus-host disease“ OR aGVHD) AND (”Hematopoietic Stem Cell Transplantation“[Mesh] OR ”Hematopoietic Stem Cell Transplantation’).
2. ECP mechanism of action
ECP mechanism of action has been extensively investigated over the years with the aim of identifying possible markers of response to treatment. MNCs, particularly lymphocytes, play a crucial role in T cell-mediated diseases, including GvHD. Regulatory T cells (Tregs) are a subset of CD4+ cells and their reduced levels are central in the development and maintenance of GvHD. A number of studies demonstrated the role of ECP in increasing the level of functional T-regs in patients with GvHD, by reducing antigen presentation by antigen-presenting cells (APC) and inducing Treg activation with a parallel reduction of pro-inflammatory cytokines [Citation8–10]. The induction of Tregs and downregulation of the inflammation process remains one of the most invoked mechanisms underlying ECP immunomodulation (see ). However, other investigations [Citation11,Citation12] were not able to demonstrate a clear relationship between the frequency of circulating Tregs and clinical response in cGvHD. These controversial findings may be related to the different study designs and subsets of patients. Further data are needed to elucidate the role of Tregs in response to ECP treatment. Many other cells including B lymphocytes, clonal T and NK cells, monocytes/dendritic cells, and naïve T cells in pediatric patients, have been investigated with controversial results [Citation13]. Recently, the interest of research has focused on a set of molecules, including genomics, transcriptomics, proteomics, and cytomics for elucidating the mechanism underlying GvHD and ultimately to help predict disease severity and to choose the best treatment options, including ECP. These studies have been facilitated by the great advances in engineering which have allowed big data collection [Citation14]. In recent years, the role played by micro RNA (miRNAs) (small non-coding RNAs with crucial regulatory functions), in the molecular regulation and modulation of the biological processes underlying aGvHD and cGvHD have been investigated. Changes in miRNAs expression profiles can provide indications of the development, severity, and prognosis of GvHD, also considering that some miRNAs are mainly expressed in certain organs or tissues; miRNA dysregulation may fuel the aberrant immune response observed in GvHD [Citation15]. Lately, miRNAs have been tested in an attempt to unveil their possible role in predicting the response to ECP treatment, since ECP may possibly influence immune regulation through miRNAs-mediated mechanisms [Citation16,Citation17]. If future studies demonstrate the reliability of miRNAs as biomarkers of response to ECP therapy, this would lead to great benefits both in patient management and in optimizing the economic resources considering that they are easily obtained from a small plasma sample.
Figure 1. ECP procedure and hypothesized mechanism of action. Step 1: Mononuclear cells (MNCs) are separated by peripheral blood by an apheresis cell separator. Step 2: MNCs are incubated with 8-methoxy psoralen that cross-links DNA bases after photoactivation by UV-A rays. The treatment induces cell apoptosis, maturation of tolerogenic dendritic cells (DC), and phagocytosis of apoptotic lymphocytes by DC. The process dampens inflammation by reducing pro-inflammatory cytokines, enhancing anti-inflammatory cytokines production, and promoting the expansion of Tregs which, in turn, produce anti-inflammatory cytokines. Step 3: treated cells are returned to the patient and 8-methoxypsoralen is inactiveFootnote1.
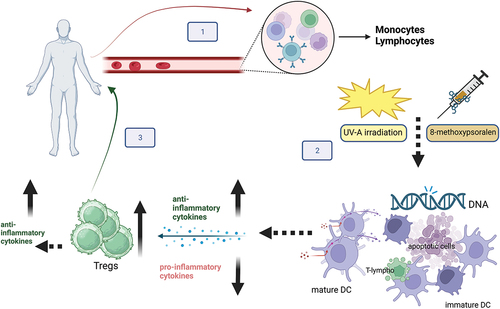
3. Cell dose and treatment schedule
A key point under study is the optimal cell dose to be collected in order to obtain a response and consequently define the treatment schedule [Citation18]. Recent technological advances in the cell separator field allowed the collection of patients’ MNCs with high efficiency and purity. ECP can be performed using the in-line or the off-line system. The most used in-line system (Terakos®) is a closed method approved by FDA only for the ECP procedure that allows integrated and fully automated MNCs separation, 8-methoxy psoralen photoactivation with UV-A rays, and reinfusion of treated cells. The off-line, ‘open’, system is based on MNC collection by apheresis and a second step of UV-A irradiation with a dedicated device. Both systems offer advantages and disadvantages and are valid for performing ECP [Citation19]. In the last years, another online system that integrates MNC collection by apheresis with subsequent irradiation in a closed system has been launched on the market [Citation20]. An important point still under discussion is the quality and quantity of the cells collected. The off-line system permits the collection of a higher number of MNCs than the online system; nevertheless, published results about the response to ECP are similar. Another question is whether the presence of contaminating cells like red blood cells, granulocytes, and platelets may, in some way, influence the UVA irradiation efficacy and/or act differently with the immunomodulatory response [Citation21]. The presence of a high amount of contaminant red blood cells (hematocrit >5%) in the collection bag before irradiation undoubtedly reduces the efficacy of MNC apoptosis induction, since it causes impairment of UV-A irradiation efficacy. In addition, an in vitro study by Franklin and coll demonstrated that chemo-irradiated neutrophils suppress the activation of APCs in the presence of other activating factors, while chemo-irradiated CD3+ T cells activate APCs [Citation22]. These findings suggest that the composition of ECP UVA-treated buffy coat may prove important in its immunomodulatory effect and more attention should be paid by researchers to cells other than MNCs.
The choice of treatment schedule for patients with GVHD depends on various factors, including the type and severity of GVHD, patients’ status, and their response to therapy. The treatment for GVHD remains heterogeneous since no definite guidelines are available; therefore, it is highly individualized, and decisions are made by the attending physician, based on a thorough assessment of the patient’s condition. Lastly, adherence to the treatment schedule, especially during the intensive course, is often challenging for patients living far from the treatment center. Since its inception [Citation1] each ECP cycle for T cell lymphoma treatment consisted of two consecutive procedures (treat as many cells as possible to achieve an anti-cancer effect in cutaneous T cell lymphoma). However, this still represents the standard practice in most centers despite technological improvement (i.e., cell collection efficiency) and the different disease settings (GvHD). Recently, Cid and coll [Citation23]. demonstrated the safety and efficacy in treating patients with GvHD by performing one day ECP, and processing one blood volume by using the off-line method. The therapeutic response was comparable with the traditional 2-procedure schedule. This finding, if confirmed in larger studies, has important implications for many clinical and management aspects such as a positive impact on patients’ quality of life and economic savings. An additional advantage of the ‘one-collection strategy’ would be the reduction of thrombocytopenia, cytopenia, and anemia ocurrence related to the treatment [Citation24]. Indeed, the great number of MNCs collected with high purity supports avoiding a second consecutive procedure to prevent the risk of re-processing MNCs irradiated the previous day. Interestingly, few studies demonstrated a response to ECP also when performed by the so-called ‘mini ECP’ that allows for treating lower cell numbers than the standard procedure [Citation25–27]. At the moment, only a few retrospective observations evidenced a positive trend, not reaching a statistical significance, between response and the number of treated MNCs [Citation28–30]. In a study by Worel et al. in the sertting of steroid-refractory (SR) aGvHD, a correlation between lymphocytes and MNCs/kg body weight treated in a single procedure and response to ECP at 1 month was demonstrated in univariate analyses. The authors concluded that the number of cells infused during each procedure can predict an overall response to ECP at 1 month with approximately 75% sensitivity. The same correlation was retrospectively investigated by our group either in aGvHD and in cGvHD or in pediatric and adult settings. Similarly, we found only a trend, but not a significant association, between cell dose and ECP response. We can speculate that, in responsive patients, ECP is able to prime its immunomodulatory action even via a low number of cells capable of triggering the response against the attacking lymphocytes [Citation31,Citation32].
4. Technical limitations
4.1. Vascular access
Vascular access is one of the main limitations of ECP treatment. In fact, reliable vascular access is crucial for performing ECP, since adequate blood flow is necessary to successfully complete the procedure. Although peripheral access is preferred, these are often insufficient, as are insertions of peripheral cannulae, particularly in patients with a history of repeated venous accesses or vascular complications. Therefore, it is necessary to insert a catheter to enable access to ECP (implantable vascular access device – IVAD-, tunneled central venous catheter – TCVC-, or tunneled central venous catheter with port-port-CVC-). The main risk factors reported with all devices used are clot formation, low flow rate, and bacterial infections. Furthermore, in 2018 an alert of a possible relationship between online ECP and venous thromboembolism was disclosed, although a significant correlation has never been demonstrated [Citation33]. To date, no definitive device has been identified, especially for patients who undergo long-term ECP [Citation34–37]. An unconventional solution to reduce the number of venipunctures for patients with a temporary inability to undergo ECP has been suggested by Pochon and coll., who published the feasibility of using cryopreserved MNCs to allow ECP treatment in patients with GvHD [Citation38]. Nevertheless, in our opinion, this option seems cumbersome and not risk-free, difficult to apply on a large scale.
4.2. Patient’s weight
Low patient weight, in the pediatric setting, has been a technical limitation to ECP due to the relatively high extracorporeal volume of the cell separators. The evolving technology in the last years allows performing safe apheresis procedures in very low-body weight children, as extracorporeal volume has been reduced to less than 200 ml. Indeed, frail pediatric populations also deserve ECP treatment, to induce GvHD response as well as to reduce the side effects of drugs in a growing body. The off-line technique allows the safe treatment of patients weighing less than 20 kg, including very low body weight children (6–8 kgs), provided that priming with irradiated red blood cells is performed and dedicated staff are available to ensure optimal management of the procedure [Citation32,Citation39,Citation40].
5. GvHD and organ response to ECP
5.1. Acute GvHD
aGvHD remains a leading cause of death after stem cell transplant and occurs when donor T cells react in response to HLA differences (T cell alloreactivity) on a recipient’s tissue, resulting in inflammatory response and tissue injury [Citation41]. The incidence and severity (graded from I to IV) of the disease are related to a number of risk factors [Citation42]. First-line treatment for aGvHD consists of corticosteroid administration which, however, only achieves a response in about 50% of the patients. Steroid-resistant (SR) or steroid-dependent (SD) patients have poor long-term survival, and infections are the leading cause of death [Citation43]. Indeed, despite the introduction of a number of novel agents in recent years (i.e., monoclonal antibodies, anti IL2-R, ROCK2 inhibitors, methotrexate, mesenchymal stem cells, decidual stromal cells, and fecal microbiota transplantation) only the JAK/STAT pathway inhibitor ruxolitinib has been approved as standard second-line therapy [Citation44]. However, the main side effects associated with ruxolitinib administration are thrombocytopenia, anemia, and CMV reactivations [Citation45]. In a 2002 review of 11 studies employing ECP for the treatment of GvHD, different responses were obtained depending on organ involvement: complete (CR) or partial response (PR) in about 70% of patients with skin involvement and 40% and 55% in liver and gut involvement, respectively. Remarkably, immunosuppressive therapy was discontinued in one-third of cases and reduced in about 50%. No relevant side effects were reported [Citation46]. In prospective studies on SR aGvHD, overall response rates (ORR) of 69% have been reported, with a ORR of 84% for skin, 55% for liver, and 65% for gut aGVHD [Citation4]. Further studies confirmed these results [Citation47,Citation48]. In consideration of the excellent results and safety profile of ECP also in very compromised patients and in low body weight children, it is currently considered a valid second-line treatment (American Society for Apheresis guidelines, ASFA, 2023 class II, 1B) [Citation49]. Promising outcomes were also obtained by employing ECP as a first-line treatment in retrospective studies [Citation48,Citation50]. Furthermore, in a recent randomized phase II trial, the superiority of the association between ECP and steroids as first-line treatment was demonstrated, especially with regard to response in skin involvement. This study also confirmed the steroid tapering in responders [Citation51].
shows the most important studies on aGvHD.
Table 1. Published studies on aGvhd and cGvhd patients treated by ECP.
Undoubtedly, to date, ECP is a recognized treatment that decreases inflammation and induces immune tolerance without affecting the individual immune response to pathogens [Citation52].
5.2. Chronic GvHD
cGvHD is a complex and heterogeneous disease often resembling autoimmune syndromes and is the result of chronic inflammation and dysregulated immunity. It affects about 30–70% of the transplanted patients and it may develop in the skin, gastrointestinal tract, liver, lungs, oropharynx, eyes, genital tract, and/or musculoskeletal systems at any time after allogeneic stem cell transplantation. cGvHD extension and organ disease severity are highly variable, but in almost all patients the quality of life (QoL) is very poor [Citation53]. Notwithstanding the great efforts during these years to treat the disease by using drugs aimed at reducing the pro-inflammatory status and tissue fibrosis, mortality is still high and is mainly related to the development of severe infections and the unavoidable side effects of immunosuppressive therapy. Similarly to aGvHD, first-line therapy consists of steroid administration. Nevertheless, about 50% of patients are SR or SD and second-line treatments include ECP, mTOR inhibitors, calcineurin inhibitors, methotrexate, mycophenolate mofetil, rituximab, infliximab, imatinib, and newer FDA-approved medications: ibrutinib, belumosudil, and ruxolitinib [Citation54].
In a randomized trial conducted in 2008 in 95 patients with cGvHD, ECP in association with conventional treatment was superior compared to conventional treatment alone, in particular for skin (ORR 40% vs. 10%) and oral involvement (ORR 53% vs. 27%). Furthermore, ECP showed a superior steroid-sparing effect (reduction of steroids at least 50%) compared to control arm (21% vs 6% of patients, respectively) [Citation55]. Data were confirmed by other groups, including ourselves [Citation3,Citation11,Citation31,Citation56].
5.2.1. ECP in skin/joint and fascial involvement
In studies conducted so far, cutaneous aGVHD showed a very good response after ECP treatment expecially when it is adopted early in the disease course. Skin, joint, and fascial involvement are frequently observed in cGvHD; they are characterized by heterogeneous manifestations and account for a poor QoL. Severe manifestations are associated with scleroderma and may cause total disability. A number of studies are available on ECP treatment in steroid-refractory cGvHD with skin/joint and fascial involvement and have shown a benefit with a steroid-sparing effect or steroid discontinuation. In the first randomized trial performed in 2008 by Flowers and coll, the assessment of total skin score (TSS) in 10 body regions after at least 12 weeks of ECP treatment in association with SoC documented a clear benefit in ECP treatment arm (CR and PR with steroid reduction) versus the SoC arm [Citation55]. In a prospective, crossover, multicenter study carried out by Greinix and coll. in 2011 on 29 patients with SR or SD cGVHD evaluated after 24 weeks of ECP plus SoC, the advantage in ECP treated group was confirmed, with a significant improvement of TSS, in joint involvement and steroid tapering. Furthermore, the study supported the long-term use of ECP to obtain the best response [Citation57]. Similar results were reported by a number of prospective and retrospective studies with an average overall response rate in skin involvement of 70% and in joint/fascial involvement of 40%.
Notwithstanding a certain variability in the response rate, this data is of considerable interest as the response to ECP was always positive, despite the difference either in patient selection criteria or in the underlying first and second-line therapies and ECP treatment protocol adopted.
Moreover, in a prospective randomized trial evaluating ECP as first-line treatment in association with SoC or SoC alone in patients with new onset cGvHD, ECP was confirmed to give an advantage not only in ORR (74,1% vs 60.9%, respectively) but also in QoL, which remained unchanged contrarily to the decline in SoC alone arm. This study reported also some adverse events related to the ECP procedure [Citation58].
5.2.2. ECP in lung cGvhd
Bronchiolitis Obliterans Syndrome (BOS) BOS is the manifestation of cGvHD in the lung. It is a relatively new disease entity; the incidence rate reported is heterogeneous and ranges from 4% to 26%; it is usually diagnosed after one-year post-transplant, with a significant mortality of approximately 50% at 5 years [Citation59]. In patients affected by cGvHD experiencing lung dysfunction, once infectious complications are ruled out, BOS must be considered in the differential diagnosis. Indeed, BOS is the most frequent late-onset non-infectious pulmonary complication after allogeneic stem cell transplantation [Citation60,Citation61]. Notwithstanding the revised consensus criteria for diagnosing BOS released by the National Institute of Health (NIH) in 2014, the diagnosis of BOS after allogeneic stem cell transplantation may be difficult and has several points to be clarified. A cough, not otherwise explicable accompanied by dyspnea and a progressive exercise limitation, can be diagnostic even if these nonspecific symptoms make it difficult to make an early diagnosis that is fundamental to contain morbidity and mortality. Therefore, the diagnosis is based essentially on spirometric measurements defining pulmonary function changes (basically Forced Expiratory Volume in 1 second-FEV1) rather than histology. Treatment attempts are varied (corticosteroids, fluticasone, imatinib, montelukast, azithromycin, etc.) and largely unsatisfactory. The philosophy behind the treatment strategy for BOS is to stabilize the decline of lung function, as, once BOS is established, reversal of respiratory function negative trend is very difficult to achieve. ECP treatment has proved a good option to stabilize or ameliorate lung function, having a high level of safety and tolerability, also in very compromised patients. Studies on the use of ECP in BOS are few and report results only on case series or case controls. Lucid and coll [Citation62]. reported lung function stabilization in 6/9 patients who previously had a rapid decline of lung function. The treatment lasted a maximum of 12 months and was followed by steroid tapering. Favorable results were also reported by Brownback and coll [Citation63]. on 8 patients treated for a maximum of 2 years. However, the authors observed a reduction in response after 1 year of treatment. Our group also confirmed the improvement in FEV1 in a cohort of 13 patients. Of note, a subgroup of 6 patients developed BOS while under delayed ECP treatment (2 sessions/month) for cGvHD with other organ involvement. All patients underwent the same intensified schedule and ECP was able to reverse or slow lung function decline in most patients, even in those treated at more advanced stages of BOS [Citation64]. Finally, Hefazi and coll [Citation65]. retrospectively, analyzed 26 patients undergoing ECP for more than 3 months. They did not observe a change in FEV1 but rather an improved survival, probably related to the reduction of steroids in the ECP cohort.
5.3. ECP for GvHD prophylaxis
Considering the high safety profile and proven efficacy reported in studies to date, ECP has been used in an attempt to prevent acute and chronic GvHD. Conflicting results were shown when ECP was adopted as part of the GvHD prophylaxis; in fact, a recent randomized trial did not demonstrate any beneficial effect of ECP as adjunctive treatment to the standard GvHD prophylaxis [Citation66]. On the contrary, in other prospective studies, ECP was a valuable adjunctive treatment for both aGvHD and cGvHD prophylaxis, without impacting on graft versus leukemia effect (GvL).
In a study conducted in 2010, ECP showed a benefit in improving survival and delaying aGvHD when used before myeloablative conditioning [Citation67]. In another investigation, ECP was used early in patients undergoing allogeneic transplantation after a reduced-intensity conditioning regimen; this approach showed lower GvHD incidence (either acute or chronic) with no impact on GvL [Citation68].
Kitko and coll. employed ECP post-transplant combined with TNF alpha inhibitor and demonstrated an improved response to GVHD therapy [Citation69]. Finally, Abdelhakim and coll. investigated the role of ECP in a pilot study before myeloablative conditioning and in post-engraftment settings for GvHD prevention. Incidence of any grade GvHD was comparable to the literature, even though the more severe forms of the disease seemed to be lower [Citation70]. In two multicenter trials, ECP was also investigated as part of a reduced-intensity conditioning regimen before allogeneic stem cell transplantation in 23 patients with myelodysplastic syndrome or aggressive lymphoma. Preliminary results confirmed the excellent safety profile of ECP and the absence of interference with neutrophils or platelet engraftment [Citation71].
6. ECP in combination with the new drugs
Studies on new agents for the treatment and prevention of GvHD have been facilitated after the release of the 2014 NIH consensus criteria that allow for conducting investigations in more homogenous populations. Three new agents for cGvHD have been approved in the last few years (ibrutinib, ruxolitinib, belumosudil), in an attempt to reduce steroid administration and related side effects, ultimately improving QoL and OS [Citation72]. Each drug acts by inhibiting distinct pathways in alloreactive T and B cells, favoring tolerance [Citation7]. Ruxolitinib is a JAK1/2 inhibitor: it stops the activation of T cells, neutrophils, and DC that cooperate with GvHD development. Therefore, ruxolitinib inhibits the signaling involved in the immune response and inflammation. It is approved also for aGvHD treatment with encouraging results [Citation45]. In consideration of the synergistic effect on the immune system exerted by ruxolitinib and ECP, lately, some studies evaluated the combination of the two approaches in an attempt to obtain a better response in patients not responsive to conventional treatment. Modemann and coll [Citation73]. showed the efficacy of ECP in treating patients with severe SR-aGVHD of the lower gastrointestinal tract. In their single-center study, the combination of ruxolitinib and ECP showed a better response (CR 44%) than the two separate approaches, with a low incidence of severe infections and a reduction of steroid dosage. Other studies in SR aGVHD demonstrated a CR of 4.8% with ECP alone [Citation3] vs 46.3% with ruxolitinib alone [Citation74]. An investigation in refractory cGVHD showed that ruxolitinib plus ECP obtained an ORR of 74% with most patients obtaining a PR. Gut and skin cGVHD manifestations responded better (54% and 44%) than liver, eye, and lung involvement (21%, 20%, and 13%, respectively) [Citation75]. The reported response rate in patients treated with ruxolitinib for SR-cGVHD was 85% [Citation74], while ECP response rates for patients with SR-cGVHD are between 50% and 65% [Citation76]. Very recently, two pilot studies reported the administration of Interleukin-2 (IL-2) in combination with ECP for the treatment of steroid-refractory cGvHD [Citation77,Citation78]. The rationale is to obtain a synergistic effect by allowing the expansion of Tregs in response to IL-2 (growth factor able to induce Tregs expansion) and ECP. Belizaire and coll. obtained a 62% clinical response by introducing IL-2 after an initial treatment with ECP [Citation77]. Thereafter, in a phase II study on 12 patients, Salhotra and coll. reported an ORR of 80% (all PR) following low-dose IL-2 in combination with ECP. Furthermore, 70% of patients were able to taper steroids [Citation78].
ECP is a good therapeutic approach as a single therapy with a beneficial effect in SR or SD GvHD; all the more so the combination of ECP and novel, more sophisticated, immunosuppressive drugs seems promising in the near future with the intriguing possibility of building up tailored therapies for the diverse patient populations.
7. Conclusion
The recent studies on ECP mechanism of action along with promising biomarkers of response, and the synergistic benefit of ECP in association with new drugs, render this therapy an important weapon for GvHD resistant to conventional treatment, and hopefully, it could be proposed as first-line treatment. We believe that it should be used as soon as possible in all categories of patients, considering its high safety profile.
8. Expert opinion
To date, the cornerstones on which ECP choice is based for the treatment of GvHD are: the safety profile with only minimal side effects (mainly related to citrate toxicity), absence of organ toxicity, no increased risk of disease relapse, and no adjunctive immune suppression. Although ECP mechanism of action has not yet been fully understood, its ability to boost natural mechanisms of immune tolerance is undeniable. All these characteristics taken together render ECP an appealing treatment option, particularly in frail patients.
New technical developments and advances have substantially shortened the cycle duration and qualified ECP for use in children, even if no randomized clinical trials are available to date in this setting. Initially, ECP had only been used empirically in GvHD clinical settings both in adults and children but over the years it has gained importance step by step, notwithstanding a few randomized trials. The high number of patients treated with favorable results, makes ECP highly recommended as a second-line treatment, immediately after steroid failure [Citation49]. Recent preclinical and clinical research activities are throwing more light on the complexities of its mechanisms of action. Moreover, promising data on the identification of potential surrogate markers that are considered predictive of clinical response to ECP therapy are emerging.
Besides, the recent available studies confirm that ECP is safe and tolerable in GvHD setting with very few limitations (venous accesses and mild anemia or thrombocytopenia) that do not discourage its use even in the pediatric setting. Importantly, ECP allows tapering/weaning of steroids in responder patients, with evident advantages. Considering the heterogeneous results reported so far on the number of cells treated with different protocols, we can assume that a minimum number of cells for an effective ECP treatment was always collected and the cell dose infused to the patients was above the minimum threshold. Even if patients with a low peripheral blood MNC count (200/microliter) can benefit from ECP treatment, in the case of severe cytopenia (as in severe cases of acute GVHD or in patients with poor graft function) with MNCs < 200/microliter ECP cannot be performed. Currently, the limitations of the published studies (retrospective analyses, heterogeneous patient cohorts, different types of transplants, conditioning regimens, and immunosuppressive therapies) do not allow definitive conclusions to be drawn. Several questions remain opened regarding the best treatment schedule and how long and when to discontinue the treatment, taking into consideration the related costs [Citation32,Citation79–81]. These issues are closely related either to the type of organ involved and GvHD (acute or chronic) [Citation82]. Based on the published experience, we are convinced that ECP treatment must be tailored to the clinical and biological findings of each patient. The mechanism of response to ECP can be triggered independently from the stage of the disease and we, therefore, suggest starting the treatment at any time. Nevertheless, in our opinion, ECP has to be introduced early, in the attempt to obtain the maximum response. cGvHD with lung involvement is challenging due to the difficulties in early diagnosis and treatment. Therefore, in consideration of the heterogeneity of BOS, having more reliable diagnostic criteria to better detect the early phase of the disease is desirable. ECP was shown to slow down lung function decline in preliminary studies. The irreversible tissue damage and the rapid evolution that accounts for the high mortality rate as well as the lack of effective treatments in this specific setting, render ECP an adjunctive treatment that should be hopefully performed following an intensive course. Once the response is obtained (stop/improvement of lung function decline) ECP may be maintained long-term to trigger a recall. In fact, based on the preliminary positive results obtained in the field of chronic lung rejection [Citation83], long-term ECP courses (maintenance treatment) in responder patients may improve survival [Citation84]. Larger studies are needed to assess the optimal ECP treatment schedule.
The recent introduction of new promising drugs for both acute and chronic GvHD and the preliminary favorable reports on the use of ECP in association with ruxolitinib in SR patients render ECP an adjunctive treatment potentially able to enhance the response and, in parallel, to help in expediting the drug withdrawal (including ruxolitinib) with the aim of limiting toxicity. Therefore, ECP should be adopted early in association with novel drugs, in consideration of its established safety profile.
The clarification of the ECP mechanism of action along with the role of promising new biomarkers (e.g. miRNAs) is of paramount importance for the prediction of response to ECP and deserves further studies. In fact, it would allow a treatment tailored to the patient with the obvious advantages of reducing the patient’s discomfort and costs. For these reasons, new biomarkers, even if not well defined at the moment, must be the subject of entry in the near future into the decision-making algorithm for ECP use.
Article highlights
ECP is feasible, safe, and effective in the treatment of acute and chronic GvHD with no adjunctive infectious risk or disease relapse.
Optimal timing and frequency of ECP need to be defined to tailor the procedure based on the type of disease (acute or chronic GvHD) and organ involvement.
Patients with lung cGvHD have poor survival. ECP seems to be a promising adjunctive treatment to hinder, and, at best, reverse lung function decline.
Biological markers for the prediction of response to ECP are lacking; nevertheless, some promising biomarkers are emerging (e.g. miRNAs).
ECP in association with newly available drugs (e.g. ruxolitinib) may be a promising option to obtain a better response.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Aknowledgments
The authors thank the Librarian Valeria Scotti for their help in the literature search.
Additional information
Funding
Notes
1. Figure 1 was created with BioRender.com.
References
- Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary Results N Engl J Med. 1987;316(6):297–303. doi: 10.1056/NEJM198702053160603
- Tatsuno K, Yamazaki T, Hanlon D, et al. Extracorporeal photochemotherapy induces bona fide immunogenic cell death. Cell Death Dis. 2019;10(8):578. doi: 10.1038/s41419-019-1819-3
- Sakellari I, Gavriilaki E, Batsis I, et al. Favorable impact of extracorporeal photopheresis in acute and chronic graft versus host disease: prospective single-center study. J Clin Apheresis. 2018;33(6):654–660. doi: 10.1002/jca.21660
- Abu-Dalle I, Reljic T, Nishihori T, et al. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies. Biol Blood Marrow Transplant. 2014;20(11):1677–1686. doi: 10.1016/j.bbmt.2014.05.017
- Knobler R, Arenberger P, Arun A, et al. European dermatology forum - updated guidelines on the use of extracorporeal photopheresis 2020 - part 1. J Eur Acad Dermatol Venereol. 2020;34(12):2693–2716. doi: 10.1111/jdv.16890
- Berger C, Hoffmann K, Vasquez JG, et al. Rapid generation of maturationally synchronized human dendritic cells: contribution to the clinical efficacy of extracorporeal photochemotherapy. Blood. 2010;116(23):4838–4847. doi: 10.1182/blood-2009-11-256040
- Salhotra A, Sandhu K, O’Hearn J, et al. A critical review of belumosudil in adult and pediatric patients with chronic graft-versus-host disease. Expert Rev Clin Immunol. 2023;19(3):241–251. doi: 10.1080/1744666X.2023.2152330
- Biagi E, Di Biaso I, Leoni V, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplant. (abbreviation). 2007;84(1):31–39. doi: 10.1097/01.tp.0000267785.52567.9c
- Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112(4):1515–1521. doi: 10.1182/blood-2007-11-125542
- Lopes SM, Roncon S, Pinho AC, et al. Should we use extracorporeal photopheresis more often? evidence from graft-versus-host disease patients monitored with treg as a biomarker. Future Sci OA. 2020;6(8):FSO623–FSO. doi: 10.2144/fsoa-2020-0107
- Gandelman JS, Song DJ, Chen H, et al. A prospective trial of extracorporeal photopheresis for chronic graft-versus-host disease reveals significant disease response and no association with frequency of regulatory T cells. Biol Blood Marrow Transplant. 2018;24(12):2373–2380. doi: 10.1016/j.bbmt.2018.06.035
- Denney HA, Whittle RJ, Lai J, et al. Regulatory T cells in chronic graft-versus-host disease after extracorporeal photopheresis: correlation with skin and global organ responses, and ability to taper steroids. Transplant. (abbreviation). 2017;101(1):204–211. doi: 10.1097/TP.0000000000001165
- Wang L, Ni M, Hückelhoven-Krauss A, et al. Modulation of B cells and homing marker on NK cells through extracorporeal photopheresis in patients with steroid-refractory/Resistant graft-vs.-host disease without hampering anti-viral/Anti-leukemic effects. Front Immunol. 2018 Oct 8;9:2207. doi: 10.3389/fimmu.2018.02207
- Ren HG, Adom D, Paczesny S. The search for drug-targetable diagnostic, prognostic and predictive biomarkers in chronic graft-versus-host disease. Expert Rev Clin Immunol. 2018;14(5):389–404. doi: 10.1080/1744666X.2018.1463159
- Vajari MK, Moradinasab S, Yousefi AM, et al. Noncoding RNAs in diagnosis and prognosis of graft-versus-host disease (GVHD). J Cell Physiol. 2022;237(9):3480–3495. doi: 10.1002/jcp.30830
- Montoya RT, López-Godino O, Garcia-Barbera N, et al. Identification of circulating microRNA signatures as potential noninvasive biomarkers for prediction to response to extracorporeal photoapheresis in patients with graft versus host disease. Blood. 2019;134(Supplement_1):4466. doi: 10.1182/blood-2019-121655
- Amat P, López-Corral L, Goterris R, et al. Biomarker profile predicts clinical efficacy of extracorporeal photopheresis in steroid-resistant acute and chronic graft-vs-host disease after allogenic hematopoietic stem cell transplant. J Clin Apheresis. 2021;36(5):697–710. doi: 10.1002/jca.21918
- Piccirillo N, Putzulu R, Massini G, et al. Mononuclear cell collection for extracorporeal photopheresis: concentrate characteristics for off-line UV-A irradiation procedure. J Clin Apheresis. 2018 Jun;33(3):217–221. Epub 2017 Aug 22. PMID: 28833438. doi: 10.1002/jca.21574
- Helmberg W, Sipurzynski S, Groselje-Strehle A, et al. Does offline beat inline treatment: investigation into extracorporeal photopheresis. Transfus Med Hemother. 2020;47(3):198–204. doi: 10.1159/000506750
- Radwanski K, Burgstaler E, Weitgenant J, et al. Pilot study of a new online extracorporeal photopheresis system in patients with steroid refractory or dependent chronic graft vs host disease. J Clin Apheresis. 2020;35(4):342–350. doi: 10.1002/jca.21804
- Laulhé M, Lefebvre S, Le Broc-Ryckewaert D, et al. A standardized methodical approach to characterize the influence of key parameters on the in vitro efficacy of extracorporeal photopheresis. PLoS One. 2019 1;14(3):e0212835. doi: 10.1371/journal.pone.0212835
- Franklin C, Bruderek K, Schilling B, et al. Chemoirradiated neutrophils and T cells differentially affect immune functions of APCs. J Leukocyte Biol. 2019;106(2):481–493. doi: 10.1002/JLB.5A0618-242R
- Cid J, Carbassé G, Suárez-Lledó M, et al. Efficacy and safety of one-day offline extracorporeal photopheresis schedule processing one total blood volume for treating patients with graft-versus-host disease. Transfus (Abbreviation). 2019;59(8):2636–2642. doi: 10.1111/trf.15384
- Gambichler T, Chatzipantazi M, Stranzenbach R, et al. Impact of extracorporeal photopheresis on blood and coagulation parameters. Dermatol Ther. 2022;35(5):e15366. doi: 10.1111/dth.15366
- Hackstein H, Amoros JJ, Bein G, et al. Successful use of miniphotopheresis for the treatment of graft-versus-host disease. Transfus (Abbreviation). 2014;54(8):2022–7. doi: 10.1111/trf.12596
- Verdú-Amorós J, Woessmann W, Maecker-Kolhoff B, et al. Mini photopheresis for refractory chronic graft-versus-host disease in children and adolescents. Transfus (Abbreviation). 2018;58(11):2495–2500. doi: 10.1111/trf.14880
- Matic T, Bojanic I, Mazic S, et al. An automated mini buffy coat preparation method for use in mini extracorporeal photopheresis treatment of graft-vs-host-disease in a low body weight pediatric patient. J Clin Apheresis. 2019;34(4):468–473. doi: 10.1002/jca.21700
- Perseghin P, Galimberti S, Balduzzi A, et al. Extracorporeal photochemotherapy for the treatment of chronic graft-versus-host disease: trend for a possible cell dose-related effect? Ther Apher Dial. 2007;11(2):85–93. doi: 10.1111/j.1744-9987.2007.00421.x
- Bertani G, Santoleri L, Ferri U, et al. Response of steroid-refractory chronic graft-versus-host disease to extracorporeal photopheresis correlates with the dose of CD3+ lymphocytes harvested during early treatment cycles. Transfus (Abbreviation). 2016;56(2):505–510. doi: 10.1111/trf.13369
- Worel N, Lehner E, Führer H, et al. Extracorporeal photopheresis as second-line therapy for patients with acute graft-versus-host disease: does the number of cells treated matter? Transfus (Abbreviation). 2018 Apr;58(4):1045–1053. doi: 10.1111/trf.14506
- Del Fante C, Scudeller L, Viarengo G, et al. Response and survival of patients with chronic graft-versus-host disease treated by extracorporeal photochemotherapy: a retrospective study according to classical and national institutes of health classifications. Transfus (Abbreviation). 2012;52(9):2007–15. doi: 10.1111/j.1537-2995.2011.03542.x
- Perotti C, Del Fante C, Tinelli C, et al. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfu (Abbreviation). 2010;50(6):1359–1369. doi: 10.1111/j.1537-2995.2009.02577.x
- FDA Examining Patient Deaths Associated With Extracorporeal Photopheresis Treatment. ASH clinical news. 2018 Apr.Available from: https://ashpublications.org/ashclinicalnews/news/3778/FDA-Examining-Patient-Deaths-Associated-With-Extracorporeal-Photopheresis
- Schwede K, Nagel S, Simon JC, et al. How to perform extracorporeal photopheresis via port catheter. Transfu (Abbreviation). 2017;57(11):2567–2570. doi: 10.1111/trf.14265
- Adamski J. Vascular access considerations for extracorporeal photopheresis. Transfu (Abbreviation). 2018;58(Suppl1):590–597. doi: 10.1111/trf.14500
- Szymanski J, Shah P, Dynis M, et al. An ex vivo comparison of vascular access devices used in extracorporeal photopheresis. Transfu (Abbreviation). 2018;58(Suppl 1):609–613. doi: 10.1111/trf.14482
- Söderström A, Nørgaard MS, Thomsen AE, et al. Ultrasound-guidance of peripheral venous catheterization in apheresis minimizes the need for central venous catheters. J Clin Apheresis. 2020;35(3):200–205. doi: 10.1002/jca.21780
- Pochon C, Reppel L, Halle P, et al. Cryopreservation as a way to maintain extracorporeal photopheresis regimen for GvHD treatment while circumventing patient temporary inability to undergo apheresis. Bone Marrow Transplant. 2017;52(1):167–170. doi: 10.1038/bmt.2016.240
- Asensi Cantó P, Sanz Caballer J, Fuentes Socorro C, et al. Role of extracorporeal photopheresis in the management of children with graft-vs-host disease. J Clin Apheresis. 2022;37(6):573–583. doi: 10.1002/jca.22012
- Sebastián E, Andrés Esteban EM, González-Vicent M, et al. Extracorporeal photopheresis in paediatric patients: a retrospective comparison between different ‘off-line’ protocols. Vox Sang. 2022;117(10):1220–1229. doi: 10.1111/vox.13346
- Malard F, Holler E, Sandmaier BM, et al. Acute graft-versus-host disease. Nat Rev Dis Primers. 2023 8;9(1):27. doi: 10.1038/s41572-023-00438-1
- Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012 5;119(1):296–307. doi: 10.1182/blood-2011-06-364265
- Gooptu M, Koreth J. Translational and clinical advances in acute graft-versus-host disease. Haematologica. 2020;105(11):2550–2560. doi: 10.3324/haematol.2019.240309
- Patel DA, Crain M, Pusic I, et al. Acute Graft-versus-Host Disease: An Update on New Treatment Options. Drugs. 2023;83(10):893–907. doi: 10.1007/s40265-023-01889-2
- Zeiser R, von Bubnoff N, Butler Jet al.REACH2 Trial GroupRuxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host DiseaseN Engl J Med. 2020 7; 382191800–1810. doi: 10.1056/NEJMoa1917635
- Dall’amico R, Messina C. Extracorporeal photochemotherapy for the treatment of graft-versus-host disease. Ther Apher. 2002;6(4):296–304. doi: 10.1046/j.1526-0968.2002.00448.x
- Berger M, Albiani R, Sini B, et al. Extracorporeal photopheresis for graft-versus-host disease: the role of patient, transplant, and classification criteria and hematologic values on outcome-results from a large single-center study. Transfu (Abbreviation). 2015;55(4):736–47. doi: 10.1111/trf.12900
- Sestili S, Eder S, Belhocine R, et al. F. Extracorporeal photopheresis as first-line strategy in the treatment of acute graft-versus-host disease after hematopoietic stem cell transplantation: a single-center experience. Cytotherapy. 2020;22(8):445–449. doi: 10.1016/j.jcyt.2020.03.003
- Connelly-Smith L, Alquist CR, Aqui NA, et al. Guidelines on the use of therapeutic Apheresis in clinical practice - evidence-based approach from the writing committee of the american society for Apheresis: the ninth special issue. J Clin Apheresis. 2023;38(2):77–278. doi: 10.1002/jca.22043
- Castagna L, Morabito L, Mauro E, et al. First-line extracorporeal photochemotherapy for acute GVHD after unmanipulated haploidentical BMT following nonmyeloablative conditioning and post transplantation CY. Bone Marrow Transplant. 2014;49(2):317–318. doi: 10.1038/bmt.2013.174
- Mehta RS, Bassett R, Rondon G, et al. Randomized phase II trial of extracorporeal phototherapy and steroids vs. steroids alone for newly diagnosed acute GVHD. Bone Marrow Transplant. 2021;56(6):1316–1324. doi: 10.1038/s41409-020-01188-4
- Nygaard M, Karlsmark T, Andersen NS, et al. Extracorporeal photopheresis is a valuable treatment option in steroid-refractory or steroid-dependent acute graft versus host disease-experience with three different approaches. Bone Marrow Transplant. 2019 Jan;54(1):150–154. doi: 10.1038/s41409-018-0262-x
- Zeiser R, Blazar BR, Longo DL. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–2579. doi: 10.1056/NEJMra1703472
- Saidu NEB, Bonini C, Dickinson A, et al. New approaches for the treatment of chronic graft-versus-host disease: Current status and future directions. Front Immunol. 2020 9;11:578314. doi: 10.3389/fimmu.2020.578314
- Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112(7):2667–74. doi: 10.1182/blood-2008-03-141481
- Dignan FL, Greenblatt D, Cox M, et al. Efficacy of bimonthly extracorporeal photopheresis in refractory chronic mucocutaneous GVHD. Bone Marrow Transplant. 2012;47(6):824–830. doi: 10.1038/bmt.2011.186
- UVADEX Chronic GVHD Study GroupGreinix HT, van Besien K, Elmaagacli, AH, et al. Progressive improvement in cutaneous and extracutaneous chronic graft-versus-host disease after a 24-week course of extracorporeal photopheresis–results of a crossover randomized study. Biol Blood Marrow Transplant. 2011;17(12):1775–82. doi: 10.1016/j.bbmt.2011.05.004
- Jagasia M, Scheid C, Socié G, et al.Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019, 23;314: 2218–2229. doi: 10.1182/bloodadvances.2019000145
- Bergeron A, Chevret S, Peffault de Latour R, et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2018 3;51(5):1702617. doi: 10.1183/13993003.02617-2017
- Grønningsæter IS, Tsykunova G, Lilleeng K, et al. Bronchiolitis obliterans syndrome in adults after allogeneic stem cell transplantation-pathophysiology, diagnostics and treatment. Expert Rev Clin Immunol. 2017;13(6):553–569. doi: 10.1080/1744666X.2017.1279053
- Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(7):1072–8. doi: 10.1016/j.bbmt.2010.11.018
- Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant. 2011;46(3):426–429. doi: 10.1038/bmt.2010.152
- Brownback KR, Simpson SQ, Pitts LR. Effect of extracorporeal photopheresis on lung function decline for severe bronchiolitis obliterans syndrome following allogeneic stem cell transplantation. J Clin Apheresis. 2016;31(4):347–352. doi: 10.1002/jca.21404
- Del Fante C, Galasso T, Bernasconi P, et al. Extracorporeal photopheresis as a new supportive therapy for bronchiolitis obliterans syndrome after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(5):728–731. doi: 10.1038/bmt.2015.324
- Hefazi M, Langer KJ, Khera N, et al. Extracorporeal photopheresis improves survival in hematopoietic cell Transplant patients with bronchiolitis obliterans syndrome without significantly impacting measured pulmonary functions. Biol Blood Marrow Transplant. 2018;24(9):1906–1913. doi: 10.1016/j.bbmt.2018.04.012
- Ali MM, Gedde-Dahl T, Osnes LT, et al. Extracorporeal photopheresis as graft-versus-host disease prophylaxis: a randomized controlled trial. Transplant Cell Ther. 2023;29(6):364.e1–364.e11. doi: 10.1016/j.jtct.2023.02.023
- Shaughnessy PJ, Bolwell BJ, van Besien K, et al. Extracorporeal photopheresis for the prevention of acute GVHD in patients undergoing standard myeloablative conditioning and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(6):1068–1076. doi: 10.1038/bmt.2009.307
- Michallet M, Sobh M, Garban F, et al. Extracorporeal photopheresis for GVHD prophylaxis after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation: a prospective multicenter phase 2 study. Leuk Lymphoma. 2018;59(2):372–380. doi: 10.1080/10428194.2017.1334120
- Kitko CL, Braun T, Couriel DR, et al. Combination therapy for graft-versus-host disease prophylaxis with etanercept and extracorporeal photopheresis: results of a phase II clinical trial. Biol Blood Marrow Transplant. 2016;22(5):862–868. doi: 10.1016/j.bbmt.2015.11.002
- Abdelhakim H, Accurso A, Merkel D, et al. Peri-transplant extracorporeal photopheresis to mitigate GVHD- a pilot clinical trial. Bone Marrow Transplant. 2021;56(4):980–982. doi: 10.1038/s41409-020-01142-4
- FM F, XV W, SM L, et al. Incorporation of extracorporeal photopheresis into a reduced intensity conditioning regimen in myelodysplastic syndrome and aggressive lymphoma: results from ECOG 1402 and 1902. Transfu (Abbreviation). 2020;60(8):1867–1872. doi: 10.1111/trf.15798
- Malard F, Mohty M. Updates in chronic graft-versus-host disease management. Am J Hematol. 2023;22(10):1637–1644. Epub ahead of print. doi: 10.1002/ajh.27040
- Modemann F, Ayuk F, Wolschke C, et al. Ruxolitinib plus extracorporeal photopheresis (ECP) for steroid refractory acute graft-versus-host disease of lower GI-tract after allogeneic stem cell transplantation leads to increased regulatory T cell level. Bone Marrow Transplant. 2020;55(12):2286–2293. doi: 10.1038/s41409-020-0952-z
- Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212
- Maas-Bauer K, Kiote-Schmidt C, Bertz H, et al. Ruxolitinib-ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021;56(4):909–916. doi: 10.1038/s41409-020-01122-8
- Pierelli L, Perseghin P, Marchetti M, et al. Extracorporeal photopheresis for the treatment of acute and chronic graft-versus-host disease in adults and children: best practice recommendations from an Italian society of Hemapheresis and cell manipulation (SIdEM) and Italian group for Bone Marrow transplantation (GITMO) consensus process. Transfu (Abbreviation). 2013;53(10):2340–2352. doi: 10.1111/trf.12059
- Belizaire R, Kim HT, Poryanda SJ, et al. Efficacy and immunologic effects of extracorporeal photopheresis plus interleukin-2 in chronic graft-versus-host disease. Blood Adv. 2019;3(7):969–979. doi: 10.1182/bloodadvances.2018029124
- Salhotra A, Talley M, Wu X, et al. Clinical and immunologic responses to extracorporeal photopheresis and low-dose IL-2 in patients with steroid refractory chronic graft-versus host disease. Bone Marrow Transplant. 2022;57:1045–47. doi: 10.1038/s41409-022-01671-0
- Yalniz FF, Murad MH, Lee SJ, et al. Steroid refractory chronic graft-versus-host disease: cost-effectiveness analysis. Biol Blood Marrow Transplant. 2018;24(9):1920–1927. doi: 10.1016/j.bbmt.2018.03.008
- Lopes SM, Roncon S, Pinho AC, et al. Should we use extracorporeal photopheresis more often? Evidence from graft-versus-host disease patients monitored with Treg as a biomarker. Future Sci OA. 2020 10;6(8):FSO623. doi: 10.2144/fsoa-2020-0107
- Buchele V, Hackstein H. A simplified extracorporeal photopheresis procedure based on single high-dose ultraviolet a light irradiation shows similar in vitro efficacy. Transfu (Abbreviation). 2021 Mar;61(3):883–893. Epub 2020 Dec 10. PMID: 33300629. doi: 10.1111/trf.16209
- Reschke R, Zimmerlich S, Döhring C, et al. Effective extracorporeal photopheresis of patients with transplantation induced acute intestinal GvHD and bronchiolitis obliterans syndrome. Biomedicines. 2022 4;10(8):1887. doi: 10.3390/biomedicines10081887
- Del Fante C, Scudeller L, Oggionni T, et al. Long-term off-line extracorporeal photochemotherapy in patients with chronic lung allograft rejection not responsive to conventional treatment: a 10-year single-centre analysis. Respiration. 2015;90(2):118–128. doi: 10.1159/000431382
- Del Fante C, Perotti C. Extracorporeal photopheresis for bronchiolitis obliterans syndrome after allogeneic stem cell transplant: an emerging therapeutic approach? Transfus Apher Sci. 2017;56(1):17–19. doi: 10.1016/j.transci.2016.12.010

