?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study aimed to assess the dynamics of rumen methane (CH4) production following the addition of NaNO3. This was done using an in vitro rumen fermentation system that ensures continuous gas and methane assessments. Four different levels of NaNO3 were used to get the final nitrate concentrations of 0.5, 1.0, 1.5, and 2.0 mg/ml of rumen fluid. For each dose, corresponding controls contained sodium chloride and urea were realised to ensure comparable levels of sodium and nitrogen. The addition of nitrates had slight effect on the intensity of fermentation because the total gas produced minus CH4 (total methane-free gas) only went down at the highest dose (2.0 mg/ml), and the final concentrations of SCFA were the same at all doses. The most evident effect was a modification of the SCFA profile (low concentrations of propionate and valerate, progressive increments of acetate, and decreases of butyrate) and a reduction in overall CH4 production. The CH4 yield for the 0.5 mg/ml dose was not different from control in the entire fermentation. Yield of the 1.0 mg/ml dose was significantly lower than the control group (p < 0.05) only within the initial 24-h period, and higher dosages (1.5 and 2.0 mg/ml) were lower during the entire fermentation (p < 0.01). Methane yields were well fitted with the Gompertz model, but only the highest level of nitrate inclusion had a significant impact on the majority of model parameters (p < 0.01). The linear regressions between CH4 yields (y) and the amounts of nitrates (x) at progressive fermentation durations (e.g. 6, 12, 24, and 48 h) produced equations with increasing absolute slopes (from −0.069 to −0.517 ml/mg of nitrate). Therefore, nitrate reduced rumen CH4 yield in a dose-dependent manner: the impact of low doses was primarily observed at the initial stages of fermentation, whereas high doses exhibited effectiveness throughout the entire fermentation process. In conclusion, in batch fermentation systems, the dose effect of nitrates on methane yield was time dependent.
1. Introduction
Nitrates ( added to ruminant diets have anti-methanogenic properties as they represent an alternative electron acceptor, diverting the rumen hydrogen flow away from carbon dioxide reduction (Olijhoek et al. Citation2016; Yang et al. Citation2016). Thermodynamically, the
reduction to NH3 is the preferable pathway when it was compared to methanogenesis due to the lower Gibbs free energy (ΔG0, −599.6 vs −136 kJ/mol, respectively; Latham et al. Citation2016). However, the
supplementation needs to be carefully evaluated due to possible side effects on animals. In fact, during the conversion to NH3, the toxic intermediate nitrite (
) can accumulate and be absorbed, interacting with the haemoglobin and altering its capacity to carry oxygen (Lee and Beauchemin Citation2014). As emerged from the meta-analysis conducted by Feng et al. (Citation2020), who evaluated the possible negative effect of
supplementation in vivo, 1% to 2% of nitrate in the dietary DM was identified as an acceptable dosage, reducing methane production by about 13–14%.
However, there are still important challenges in future research on this topic, mainly connected with the utilisation of the most suitable type of chemical as source (Almeida et al. Citation2022) and the possible attenuation of
toxicity using encapsulation to slow down the release in the rumen (Feng et al. Citation2020). An additional aspect that warrants investigation in future studies is the impact of
as a hydrogen acceptor on rumen fermentation stoichiometry. Studies by Janssen (Citation2010), Wang et al. (Citation2014), and Wenner et al. (Citation2020) emphasised the significance of
in the regulation of hydrogen (H2) balance, consequently affecting methane (CH4) production. Consequently, the utilisation of dietary
could be a useful model for investigating the intricate dynamics of fermentation stoichiometry within the rumen.
In vitro rumen batch fermentation systems are largely used to test a wide number of additives to reduce CH4 yield, including (Yáñez-Ruiz et al. Citation2016). Several in vitro batch studies (Patra and Yu Citation2014; Wu et al. Citation2019) were focused on a single incubation time and specific
dose, preventing the investigation of possible interaction between the fermentation phase and supplementation level. Recently, we have tested (Braidot et al. Citation2022) a new in vitro fermentation system that allows continuous gas and CH4 assessment, and thus main aim of the present research was to evaluate the dynamics of CH4 production for different
dosages over time. Our tentative was to analyse the temporal changes in gas and CH4 yield to better understand the dynamics of CH4 formation.
2. Material and methods
2.1. Experimental design
The experiment consisted of four fermentation runs conducted over consecutive periods (weeks), utilising NaNO3 (Sigma Aldrich, Milan, Italy) as the source of nitrate ions.
The NaNO3 was added to the substrate to achieve a final concentration of 0.5, 1.0, 1.5, and 2.0 mg/ml of incubated rumen fluid. For each dosage, a respective control was prepared with the addition of sodium chloride (Sigma Aldrich, Milan, Italy) and urea (Carlo Erba, Milan, Italy) to guarantee a comparable amount as mg of sodium and nitrogen (ratio 1:1,95) ().
Table 1. Dosages of the chemicals used in the experiment.
2.2. In vitro experiment
The in vitro apparatus is composed of eight fermentation glass bottles with a total available capacity of 750 ml. The gas originated during the fermentation process flows from the fermenters to the gas counter and then to the infrared gas analyser sensor, which continuously detects and registers gas volume and methane concentration (Braidot et al. Citation2022).
The fermenters were filled with 500 ml of buffered rumen fluid (rumen fluid: buffer, 1:2, v/v) following the methodology proposed by Menke et al. (Citation1979). The rumen fluid was collected in the same slaughterhouse from culled dairy productive cows and was delivered within half an hour after collection to the laboratory in airtight bottles refluxed with CO2 and maintained at 39°C.
A total mixed ration for ruminants based on corn silage (crude protein: 14% DM; neutral detergent fibre: 33% DM) was used as substrate (3300 mg of DM/fermentation glass bottle) in all experiments. The substrate was grounded at 0.5 mm length, weighted, and introduced in each bottle as dry material. The fermenters were hermetically sealed and incubated in a water bath at 39°C for 48 h.
2.3. Sampling of fermentation fluid and analysis
At the end of the incubation, pH was directly measured (GLP 22, Crison Instruments, S.A. Barcelona, Spain), while samples of fermentation fluid were collected for NH3 (10 ml) and short-chain volatile fatty acid (SCFA, 5 ml of rumen fluid added with 5 ml of H2SO4 0.01 N) determinations and were stored at −20°C until being analysed. Rumen fluid for protozoa count (5 ml) was also collected at the end of incubation and added with an 18.5% formaldehyde solution (1:1, v/v). Protozoa were quantified using a microscope as described by Dehority (Citation2003). The NH3 concentration was determined using an Ammonia Gas Sensing Combination Electrode (Hach Company, Colorado, USA). The SCFA samples were centrifuged at 20,000 g for 20 min at 4°C, and the supernatant was filtered using a polypore filter (RC 0.45 µm, 25 mm, DTO Servizi Srl, Venice, Italy). The filtrate was transferred into autosampler vials, and 20 μl were injected into HPLC (Shimadzu Corporation, Kyoto, Japan). The instrument was composed of an LC-20AT pump, a vacuum degasser, a Prominence SPD-M20A photodiode-array detector, a Prominence SIL-20AC HT autosampler, and a Prominence CTO-20AC column oven set at 40°C. The HPLC separations were obtained using an Aminex HPX-87 H column (300 mm x 7.8 mm) with a pre-column (Bio-Rad, Hercules, California, USA). Sulphuric acid 0.008 N was used as the mobile phase at a flow rate of 0.6 ml/min. Full spectra were recorded in the range of 190–400 nm, and the optimum wavelength detection for all SCFA was found to be 220 nm. Peaks of analyses were compared with the retention times of a standard mixture, and quantification was based on the external standard method. SCFA standards for acetic acid, propionic acid, butyric acid, iso-butyric acid, iso-valeric acid, and valeric acid were obtained from Merck (Darmstadt, Germany). From the SCFA profile composition (in mM), the net hydrogen produced was estimated as reported by Wang et al. (Citation2014):
H2 mM = 2[acetate + n-butyrate + iso-butyrate] – [propionate + iso-valerate + valerate].
2.4. Calculations and statistical analysis
Cumulative methane production was recorded continuously for 48 h and measurements at each hour were fitted using the Gompertz equation:
where is the CH4 produced (ml) at a specific time (t), B is the asymptotic methane volume (ml), C is the specific CH4 production rate (1/h) dependent on time (t), A is a constant that describes the decline of production rate. From the equation, other parameters have been calculated: the lag phase (Lag), the maximum fermentation rate (MFR), and the time needs to reach the maximum fermentation rate (TMFR) as reported by Lavrenčič et al. (Citation2015).
The kinetics parameters derived from the Gompertz model and fermentation data measured at the end of incubation (total gas, methane-free gas, CH4 percentage, protozoa count, total SFCA content, SCFA composition, ammonia content, net H2 produced) were statistically analysed with SAS software (Version 9.4, SAS Institute Inc., USA) and the following multifactorial model:
where is the experimental data, μ is the overall mean, αi is the random effect (block) of the fermentation run (i = 1,4); βj is the fixed effect of the dose (j = 1,4); γk is the effect of the type of addition (k = 1,2;
or urea and NaCl) and εijk is the residual error.
The CH4 produced at 6-h progressive times (e.g. at 6, 12, 18, 24, 30, 36, 42, and 48 h) was analysed with the previous model added with the fixed effect of time (σz; z = 1,8) in a factorial model with repeated measures, taking in account all possible interactions between factors. The MIXED procedure of SAS Software was used for the analysis.
The linear relationship between the amount of added (X) and the methane yield (Y) was tested at 4 selected times of fermentation (6, 12, 24, and 48 h) considering the effect of the separate fermentation runs. The MIXED procedure of SAS Software was used as described by St-Pierre (Citation2001) and the model applied was the following:
Where Yij is the CH4 produced for the inclusion level i of the j run, a represents the overall intercept, b is the overall regression coefficients, Xi is the level of inclusion expressed in total mg (i = 82, 165, 247, and 330 mg),
is the random effect of the run (j = 1,4), and
is the residual error. The coefficient of determination (R2) was obtained from a single-factor regression analysis between the adjusted values derived from the mixed model and the measured values.
3. Results
reports the main fermentative traits at the end of the fermentative process (48 h). Ammonia, total SCFA concentration, and protozoa count in the fermentation fluid were not changed by factors considered, whereas there was an increment in final pH with the increasing dosages (p < 0.01) regardless of the type of chemical addition. For the total gas, the total methane-free gas and the CH4 concentration, the interaction of the model was significant (p < 0.01). The additions of urea and NaCl never determined significant variations, while doses of 1.5 and 2.0 mg/ml lowered (p < 0.01) total gas and the total methane-free gas compared with other doses. Only the highest
addition determined a significant reduction in the CH4 concentration compared with lower doses.
Table 2. Main fermentative traits at the end of the fermentative process (48 h) for the different inclusion levels of sodium nitrate (NaNO3) or other chemicals (NaCl + urea)a.
The supplementation had a significant impact on the SCFA profile. The interaction between factors was significant (p < 0.01) for the concentration of acetate and butyrate, that showed no significant variations for the NaCl and urea additions, while they were modified by nitrate additions. The acetate showed higher concentrations than the respective controls at 1.0 (67.8 vs 62.4% of total SCFA concentration), 1.5 (67.9 vs 62.9% of total SCFA concentration), and 2.0 mg/ml (68.1 vs 61.9% of total SCFA concentration). The lowest nitrate dosage showed similar concentrations as the respective control (63.9 vs 62.6% of total SCFA concentration). On the contrary, the butyrate showed a lower concentration with respect to the controls at the
doses of 1.0, 1.5 and 2.0 mg/ml. Furthermore, the
addition resulted in lower propionate and valerate concentrations and an increase in the acetate: propionate ratio than in controls (16.2 vs 17.5%, 1.12 vs 1.24%, 4.16 vs 3.62, p < 0.01, respectively), with no effects caused by increasing dosages.
The net H2 availability expressed as mol/100 mol of SCFA, calculated by a stoichiometric model, was higher in the added bottles (136 vs 132 mol/100 mol, p < 0.01).
reports the CH4 yield at progressive incubation times (6 h intervals) and the kinetic parameters of the Gompertz model used to fit the CH4 production. For CH4, the interaction between fermentation time, type of chemical addition, and dose was significant (p < 0.01). The CH4 yield was never influenced by the addition of NaCl and urea, whereas in the added fermenters the CH4 yield was reduced in a dose-dependent manner. The lowest of
dose did not affect CH4 production during the fermentative process. The dose of 1.0 mg/ml resulted in lower CH4 yields compared to the corresponding control (p < 0.05) only within the first 24 h, while the higher two dosages showed differences (p < 0.01) at all times considered. For the Gompertz parameters, the interaction between dose and chemical addition was significant for most parameters except the MFR. The NaCl and urea additions did not significantly change any parameter, while
nitrate additions reduced (at the highest dose) the asymptotic CH4 yield, the lag phase, and the TMFR, and there was a significant increment in the A parameter. Finally, MFR was lower for
additions (15.6 vs 19.7 ml/h) and declined with increasing dosages (19.6, 17.1, 16.8, and 17.1 for 0.5, 1.0, 1.5, and 2.0 mg/ml of chemicals added).
Table 3. Methane yield at progressive incubation times (6 h intervals) and kinetic parameters of the fitting Gompertz model of the methane yield for the different inclusion levels of sodium nitrate (NaNO3) or other chemicals (NaCl + urea)a.
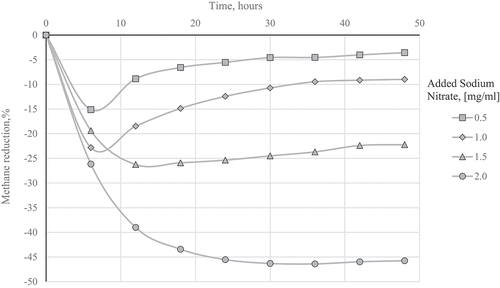
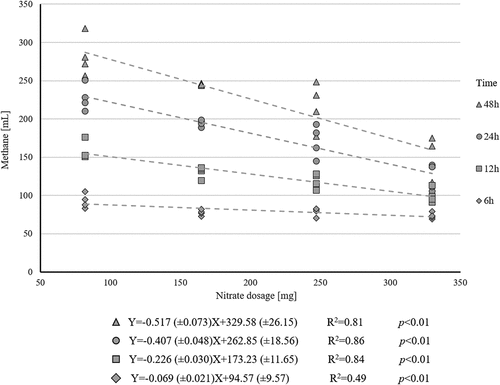
describes the CH4 reductions for the various inclusion levels throughout the fermentation while shows the results of the linear regression analysis, calculated within the fermentation runs at selected incubation times (6, 12, 24, and 48 h) between the CH4 production and of
dosages. Regressions were significant for all the fermentation length (R2 ranging from 0.49 to 0.86) with slopes between −0.069 and −0.517 ml of CH4 per mg of
added.
4. Discussion
4.1. Total gas and fermentative parameters
In control fermenters, the progressive addition of chemicals had no relevant effects on fermentation and only caused small increases in the final pH of the fermentation fluid due to the buffering effect of ammonia generated by urea.
In fermenters treated with progressive doses of , in addition to an increase in pH in the fermentation fluid, there were quantitative and qualitative impacts on fermentation. The most evident effect was a progressive decrease in overall CH4 yield. However, under our conditions, the intensity of fermentation measured by the total gas produced minus the CH4 (e.g. total methane-free gas) decreased only at the highest
dose (2.0 mg/ml). Also, the final SCFA concentrations were similar between doses. Overall, these results did not support a substantial modification in fermentability caused by
addition, while previous articles (Sakthivel et al. Citation2012; Patra and Yu Citation2013; Yang et al. Citation2016) described a decrease in fermentation activity due to the detrimental effect of
and its redox intermediate
on the rumen microbiota. Finally,
addition did not appear to depress protozoa populations, despite contradictory data in the literature. Protozoa counts were reduced in goats (Asanuma et al. Citation2015), but not in dairy cows (van Zijderveld et al. Citation2010) or in vitro rumen continuous fermenters (Wenner et al. Citation2020). The maintenance of the rumen protozoa population is important for nitrate metabolism because it appears to speed the conversion of
to
(Yang et al. Citation2016).
Typically, the process of reduction generate an increase in NH3 concentration in fermentation fluid (Božic et al. Citation2009; Patra and Yu Citation2015; Nguyen et al. Citation2016). In the literature, it has been suggested that some factors, such as the incomplete conversion of
into NH3 with the accumulation of the intermediate
(Yang et al. Citation2016) or the presence of nitro-reducing bacteria (Latham et al. Citation2016) could reduce the expected increment in NH3. In our conditions, a numerical increment in NH3 concentration was measured from the lowest to the highest addition of
(from 66.9 to 81.4 mg/dl), but this increase did not reach statistical significance. The expected increment of NH3-N due to the maximum addition of
(54 mg of N corresponding to 448 mg of NaNO3) is comparable to the observed increment of ammoniacal nitrogen (47 mg of NH3-N).
The SCFA concentration in the fermentation fluid was not affected by additions but there were relevant modifications of the SCFA profile. We obtained a decrement in propionate and valerate caused by the type of chemical addition without any variation due to the dose, while there was an increment in acetate and a decrement in butyrate reached in response to the progressive
additions. These results are consistent with previous findings both from in vitro and in vivo studies (Nolan et al. Citation2010; Lin et al. Citation2011; Li et al. Citation2012). The
conversion to NH3 is favoured over propionogenesis because it is thermodynamically more favourable than the reduction of fumaric acid to succinic acid (van Zijderveld et al. Citation2010; Yang et al. Citation2016). Moreover, the increment in acetate production generates a further decrease in the H2 available for propionate production (Nolan et al. Citation2010). A similar effect was also observed for butyrate concentration. The changes in individual SCFA content can be summarised in terms of net H2 production, according to the stoichiometric model described by Wang et al. (Citation2014). An increase in acetate, reductions in propionate and valerate as well as a change in butyrate were reflected in a significant increment of H2 net production with the
addition. These results indicate that the inclusion of
favours fermentation pathways that produce more H2.
4.2. Methane dynamics
Different trends in CH4 production were observed for the various dosages applied (). For the lowest dose, the CH4 did not change significantly in the entire fermentation when compared to the respective control while reductions occurred for 1.0 mg/ml dose only within the first 24 h. For the higher doses, the differences with respective controls progressively increased up to 30 h and then were stable. shows the CH4 reductions expressed as a percentage of the relative controls observed for the various
inclusion levels at different fermentation lengths. Overall, these variations could be explained taking into account the balance between the H2 generated during fermentation and the available
: a dose of 1.0 mg/ml consumes all the H2 available in 24 h while doses of 1.5 and 2.0 mg/ml require longer time. It means that at short times the limiting factor is the H2 availability but as fermentation proceeds, the limitation becomes the
shortage. However, the fermentation time at which the limitation shifts from H2 to
is dose-dependent: it is included within about 12 h of fermentation for the two lowest dosages, while it requires 24 h for the 1.5 mg/ml dose and 30 h for the inclusion level of 2.0 mg/ml.
The Gompertz model was previously employed for the kinetic study of data from in situ degradability or gas production (Susmel et al. Citation1999; Sarnataro et al. Citation2020) and produced satisfactory results also in fitting CH4 data in the current study (R2 > 0.95). However, the predominant effect of the addition to the kinetic parameters was a significant reduction in the asymptotic values and the MFR. For the other parameters, the type of chemical addition was never significant. Despite the goodness of fit obtained with the Gompertz equation, the kinetics parameters did not accurately describe the evolution of CH4 production during the fermentative process. Even if the kinetics approach allowed for the investigation of specific CH4 production metrics such as the MFR and TMFR, this solution did not guarantee an accurate differentiation of
effects for the various dosages used.
Often, the use of various doses in experimental work can be useful to generate equations (by regression approach) to predict the effects of untested dosages (e.g. “dose-response” experiments). Considering this, the relationship between the four additions and the CH4 production was assessed at four different durations of fermentation (i.e. 6, 12, 24, and 48 h, as shown in ). The predictive equations obtained were different, showing an increment in the dose effect according to the fermentation time considered. From a stoichiometric calculation, 1 mg of
completely transformed NH3 allows a reduction in CH4 yield of 0.409 ml (Beauchemin et al. Citation2020). Considering the presented equations, the slope was much lower than that expected for the 6-h fermentation (−0.069 ml/mg). The slope increased to −0.226 ml/mg at 12 h, and at 24 h it was close to what was expected (−0.407 ml/mg). A further increase in fermentation time overestimated the CH4 reduction (−0.517 ml/mg).
As a result, in batch rumen systems, the fermentation length affects the predictive equations derived from different doses and this was consistent with our prior observations on the time dependency between additions and CH4 production. In order to analyse this phenomenon, it has to be considered that in the initial stages of fermentation when larger doses are administered, the limiting component is H2, and therefore the available
cannot express its full potential, requiring long fermentation times to cause an overall methane drop. On the contrary, for low doses, the limit is represented by
availability and the antimethanogenic potential is consumed in relatively short times of fermentation. This aspect causes a variation in the regression-line slope, which results time dependent.
5. Conclusion
The amounts of nitrogen and sodium equivalents to NaNO3 additions did not influence rumen fermentation in batch in vitro systems. The experiment confirmed the potential of NaNO3 as source to mitigate rumen methane yield and modify patterns of fermentation. However, the dose-effect depends also on the fermentation time. In batch fermentation systems, the impact of low doses was appreciable only at the beginning of the fermentation, while high doses demonstrated a complete effect only at the end of the fermentation process. In conclusion in batch fermentation systems the dose effect of nitrates on methane yield resulted time dependent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Almeida K, Santos G, Daniel J, Nino-de-Guzman C, Amaro F, Sultana H, Arriola K, Araujo R, Vyas D. 2022. Effects of nitrate sources on in vitro methane production and ruminal fermentation parameters in diets differing in starch degradability. Anim Feed Sci Technol. 292:115437. doi: 10.1016/j.anifeedsci.2022.115437.
- Asanuma N, Yokoyama S, Hino T. 2015. Effects of nitrate addition to a diet on fermentation and microbial populations in the rumen of goats, with special reference to selenomonas ruminantium having the ability to reduce nitrate and nitrite. Anim Sci J. 86:378–384. doi: 10.1111/asj.12307.
- Beauchemin KA, Ungerfeld EM, Eckard RJ, Wang M. 2020. Review: fifty years of research on rumen methanogenesis: lessons learned and future challenges for mitigation. Animal. 14:2–16. doi: 10.1017/S1751731119003100.
- Božic AK, Anderson RC, Carstens GE, Ricke SC, Callaway TR, Yokoyama MT, Wang JK, Nisbet DJ. 2009. Effects of the methane-inhibitors nitrate, nitroethane, lauric acid, Lauricidin® and the Hawaiian marine algae Chaetoceros on ruminal fermentation in vitro. Bioresour Technol. 100:4017–4025. doi: 10.1016/j.biortech.2008.12.061.
- Braidot M, Sarnataro C, Romanzin A, Spanghero M. 2022. A new equipment for continuous measurement of methane production in a batch in vitro rumen system. J Anim Physiol Anim Nutr. 107:747–753. doi: 10.1111/jpn.13780.
- Dehority BA. 2003. Rumen Microbiology. Nottingham University Press.
- Feng XY, Dijkstra J, Bannink A, van Gastelen S, France J, Kebreab E. 2020. Antimethanogenic effects of nitrate supplementation in cattle: a meta-analysis. J Dairy Sci. 103:11375–11385. doi: 10.3168/jds.2020-18541.
- Janssen PH. 2010. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol. 160:1–22. doi: 10.1016/j.anifeedsci.2010.07.002.
- Latham EA, Anderson RC, Pinchak WE, Nisbet DJ. 2016. Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Front Microbiol. 7:228. doi: 10.3389/fmicb.2016.00228.
- Lavrenčič A, Levart A, Košir IJ, Čerenak A. 2015. In vitro gas production kinetics and short-chain fatty acid production from rumen incubation of diets supplemented with hop cones (Humulus lupulus L.). Animal. 9:576–581. doi: 10.1017/S1751731114002936.
- Lee C, Beauchemin KA. 2014. A review of feeding supplementary nitrate to ruminant animals: nitrate toxicity, methane emissions, and production performance. Can J Anim Sci. 94:557–570. doi: 10.4141/cjas-2014-069.
- Li L, Davis J, Nolan J, Hegarty R. 2012. An initial investigation on rumen fermentation pattern and methane emission of sheep offered diets containing urea or nitrate as the nitrogen source. Anim Prod Sci. 52:653. doi: 10.1071/AN11254.
- Lin M, Schaefer DM, Guo WS, Ren LP, Meng QX. 2011. Comparisons of in vitro nitrate reduction, methanogenesis, and fermentation acid profile among rumen bacterial, protozoal and fungal fractions. Asian-Aust J Anim Sci. 24:471–478. doi: 10.5713/ajas.2011.10288.
- Menke KH, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. 1979. The estimation of the digestibility and metabolisable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor. J Agric Sci. 93:217–222.
- Nguyen SH, Li L, Hegarty RS. 2016. Effects of rumen protozoa of Brahman heifers and nitrate on fermentation and in vitro methane production. Asian-Aust J Anim Sci. 29:807–813. doi: 10.5713/ajas.15.0641.
- Nolan JV, Hegarty RS, Hegarty J, Godwin IR, Woodgate R. 2010. Effects of dietary nitrate on fermentation, methane production and digesta kinetics in sheep. Anim Prod Sci. 50:801. doi: 10.1071/AN09211.
- Olijhoek DW, Hellwing ALF, Brask M, Weisbjerg MR, Højberg O, Larsen MK, Dijkstra J, Erlandsen EJ, Lund P. 2016. Effect of dietary nitrate level on enteric methane production, hydrogen emission, rumen fermentation, and nutrient digestibility in dairy cows. J Dairy Sci. 99:6191–6205. doi: 10.3168/jds.2015-10691.
- Patra AK, Yu Z. 2013. Effective reduction of enteric methane production by a combination of nitrate and saponin without adverse effect on feed degradability, fermentation, or bacterial and archaeal communities of the rumen. Bioresour Technol. 148:352–360. doi: 10.1016/j.biortech.2013.08.140.
- Patra AK, Yu Z. 2014. Combinations of nitrate, saponin, and sulfate additively reduce methane production by rumen cultures in vitro while not adversely affecting feed digestion, fermentation or microbial communities. Bioresour Technol. 155:129–135. doi: 10.1016/j.biortech.2013.12.099.
- Patra AK, Yu Z. 2015. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J Appl Microbiol. 119:127–138. doi: 10.1111/jam.12819.
- Sakthivel PC, Kamra DN, Agarwal N, Chaudhary LC. 2012. Effect of sodium nitrate and nitrate reducing bacteria on in vitro methane production and fermentation with buffalo rumen liquor. Asian-Aust J Anim Sci. 25:812–817. doi: 10.5713/ajas.2011.11383.
- Sarnataro C, Spanghero M, Lavrenčič A. 2020. Supplementation of diets with tannins from chestnut wood or an extract from stevia rebaudiana bertoni and effects on in vitro rumen fermentation, protozoa count and methane production. J Anim Physiol Anim Nutr. 104:1310–1316. doi: 10.1111/jpn.13414.
- St-Pierre NR. 2001. Invited review: integrating quantitative findings from multiple studies using mixed model methodology. J Dairy Sci. 84:741–755. doi: 10.3168/jds.S0022-0302(01)74530-4.
- Susmel P, Spanghero M, Stefanon B. 1999. Interpretation of rumen degradability of concentrate feeds with a Gompertz model. Anim Feed Sci Technol. 79:223–237. doi: 10.1016/S0377-8401(99)00016-4.
- van Zijderveld SM, Gerrits WJ, Apajalahti J, Newbold JR, Dijkstra J, Leng RA, Perdok HB. 2010. Nitrate and sulfate: effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J Dairy Sci. 93:5856–5866. doi: 10.3168/jds.2010-3281.
- Wang M, Sun XZ, Janssen PH, Tang SX, Tan ZL. 2014. Responses of methane production and fermentation pathways to the increased dissolved hydrogen concentration generated by eight substrates in in vitro ruminal cultures. Anim Feed Sci Technol. 194:1–11. doi: 10.1016/j.anifeedsci.2014.04.012.
- Wenner BA, Wagner BK, St-Pierre NR, Yu ZT, Firkins JL. 2020. Inhibition of methanogenesis by nitrate, with or without defaunation, in continuous culture. J Dairy Sci. 103:7124–7140. doi: 10.3168/jds.2020-18325.
- Wu H, Meng Q, Zhou Z, Yu Z. 2019. Ferric citrate, nitrate, saponin and their combinations affect in vitro ruminal fermentation, production of sulphide and methane and abundance of select microbial populations. J Appl Microbiol. 127:150–158. doi: 10.1111/jam.14286.
- Yáñez-Ruiz DR, Bannink A, Dijkstra J, Kebreab E, Morgavi DP, O’Kiely P, Reynolds CK, Schwarm A, Shingfield KJ, Yu Z, et al. 2016. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants-A review. Anim Feed Sci Technol. 216:1–18. doi: 10.1016/j.anifeedsci.2016.03.016.
- Yang C, Rooke JA, Cabeza I, Wallace RJ. 2016. Nitrate and inhibition of ruminal methanogenesis: microbial ecology, obstacles, and opportunities for lowering methane emissions from ruminant livestock. Front Microbiol. 12:132. doi: 10.3389/fmicb.2016.00132.