Abstract
Background Abnormalities in distal growth and low levels of insulin–like growth factor (IGF)–I have been reported in children with Perthes' disease. Our aim was to establish whether the acute phase of Perthes' disease is associated with abnormalities of growth, of bone or of collagen turnover.
Methods We performed a cross–sectional study of 15 children (3–11 years of age, 13 boys) at acute presentation and a longitudinal cohort study of 9 children. We measured (1) the lengths of both lower legs (by knemometry) at weeks 1, 2, 6 and 12, (2) height and weight at presentation and at the second–year follow–up, and (3) levels of IGF–I, IGFBP–3, collagen markers and bone alkaline phosphatase at weeks 1 and 12, and in year 2.
Results Height SD scores were normal at presentation but declined thereafter. Lower leg growth was not impaired at presentation but was asymmetrical, ceased during weeks 2–6, and then resumed symmetrically. Patients had persistently low IGF–I, low soft tissue collagen synthesis and enhanced collagen breakdown compared with age– and sex–related reference data. Markers of bone formation increased during follow–up.
Interpretation Acute changes in lower leg growth reflected differential weight bearing, then immobilization and remobilization. Persistently low IGF–I may have contributed to low soft tissue collagen synthesis and growth. Changes in bone formation markers most likely reflected bone healing.
▪
There is increasing evidence that children with Perthes' disease may have a more widespread skeletal disorder involving mild short stature, disproportionate growth retardation and delayed bone age (Burwell et al. Citation1978, Wynne-Davies and Gormley Citation1978, Kristmundsdottir et al. Citation1986). It has been reported that boys with Perthes' disease who have short stature and delayed bone age also show a reduced growth hormone response to insulin-induced hypoglycemia (Rayner et al. Citation1986), but such studies may suffer from selection bias.
Many of the actions of growth hormone are mediated by insulin-like growth factor–I (IGF–I). The bioactivity of circulating IGF–I at target tissue level is, in turn, modulated by its main binding protein, IGFBP-3, and protease activity. There have been conflicting reports regarding circulating levels of IGF-I and IGFBP-3 in children with Perthes' disease (Neidel et al. Citation1992 a, Citationb, Citation1993, Grasemann et al. Citation1996, Matsumoto et al. Citation1998). In addition to hormonal influences, bone growth may be influenced directly by mechanical factors (Golding Citation1994). No studies of short-term changes in bone growth, or of bone and collagen turnover in children with Perthes' disease have been reported.
We conducted a prospective, cross-sectional study of children presenting with Perthes' disease, followed by a longitudinal study on a smaller cohort of these children to the second year of follow-up, with serial measurements of height, weight, lower leg length velocity (LLLV), IGF-I, IGFBP-3 and markers of bone formation (bone alkaline phosphatase (ALP) and procollagen type I C-terminal propeptide (PICP)), bone collagen breakdown (telopeptide of type I collagen (ICTP)) and soft tissue synthesis (procollagen type III N-terminal propeptide (P3NP)). Our aims were (1) to establish whether the acute phase of Perthes' disease was associated with abnormalities of growth or bone and collagen turnover, and (2) to investigate any subsequent changes during treatment and healing.
Patients and methods
Patients
A sequential series of 15 prepubertal children (13 boys and 2 girls, median age 6.2 (3.0–11) years) presenting with acute onset of limp secondary to Perthes' disease (Catterall group 2–4 (Catterall Citation1971), Herring classification A–C (Herring et al. Citation1992)) was eligible to participate in this study (no exclusions). The length of history ranged from < 1 to 12 (median 6) weeks. One boy presenting with Perthes' disease of the right hip had had Perthes' disease of the left hip diagnosed and treated 2 years previously. All other patients in the study had unilateral Perthes' disease. At presentation, all patients had painful restriction of movement of the affected hip. The degree of stiffness was severe in 8/15 (abduction 20° or less, flexion arc 100° or less with flexion deformity, flexion-adduction 0° (Woods and Macnicol Citation2001) and internal rotation 0°–5°) and moderate in 7/15 (abduction 20°–40°, flexion reduced by 20° or less with no flexion deformity, flexion-adduction 1°–10° and internal rotation 5°–10°). 7 children had bone age assessments: there was no significant bone age delay (median difference between bone age and chronological age: 0.0 (−1.5 to +0.8) years).
6 children subsequently withdrew from the study, for either family or social reasons or because they were too young to co-operate with serial knemometry. A cohort of 9 prepubertal children (median age 6.7 (4.3–11) years, 7 boys) was therefore monitored serially during the first 3 months of treatment (). 4 had severe stiffness and 5 moderate stiffness of the affected hip at presentation. 7 boys remained in the study until follow up 12–24 months after initial presentation.
Table 1. Clinical characteristics of patients with Perthes' disease studied longitudinally
Treatment involved a combination of restricted activity using a wheelchair or crutches following an initial period of traction to allow the hip muscle spasm to settle. An arthrogram and examination under anesthesia was undertaken once the spasm had disappeared () (Ismael and Macnicol 1998). The study was approved by the local ethics committee and written informed consent was obtained from the parents.
Investigations
Blood samples were collected during weeks 1 and 12 following initial hospital admission, and once again 12–24 months later, by which time healing had largely taken place. Sampling was standardized by time of day to minimize diurnal variation in markers of bone turnover. The lengths of both lower legs were measured by knemometry at weeks 1, 2, 6 and 12. Knemometry is a sensitive, precise and robust technique that measures knee-heel length under standardized pressure, using a purpose-built measuring device (Wales and Milner Citation1987). Briefly, the subject sits on an adjustable chair, the position of which is customized for each individual, with the position of the feet on the measurement table determined by an individually drawn template used at each visit. The chair mount is moved backwards and forwards and the leg moved slightly from side to side until a stable maximum reading is obtained. Four readings were taken for each leg at each visit, and the mean lower leg length calculated. All measurements were performed by a single trained operator, using the random zero method (Ahmed et al. Citation1995). The technical error was 0.15 mm. Height and sitting height were measured by the same trained operator using a Holtain stadiometer at baseline, and again during the second year after presentation. Weight was measured at the same intervals.
Analytical methods
IGF-I was measured by radioimmunoassay (RIA, Mediagnost, Tübingen, Germany), using an excess of IGF-II to eliminate interference by IGFBPs. IGFBP-3 was also measured by RIA (Blum et al. Citation1990). Between-assay coefficients of variation (CVs) were 8.5%, 6.5% and 8.0% at 69, 140 and 118 μg/L, respectively, for IGF-I and 7.3% and 6.9% at 2772 and 3545 μg/L, respectively, for IGFBP-3. PICP, ICTP and P3NP were measured in duplicate by RIA (Orion Diagnostica, Espoo, Finland). Between-run CVs were 7.8% and 5.2% at 94 and 320 μg/L, respectively, for PICP, 6.3% and 9.2% at 8.7 and 33.8 μg/L, respectively, for ICTP and 5.6% and 6.4% at 4.6 and 10.4 μg/L, respectively, for P3NP. Bone ALP was measured by wheat-germ lectin affinity electrophoresis (Peaston and Cooper Citation1986). Between-run CVs were 2.2%, 3.5% and 1.9% at 251, 349 and 435 U/L, respectively. All measurements met the analytical goal that the analytical CV should not exceed one-eighth of the width of the reference range (Tonks Citation1963).
Data analysis
Height, sitting height and body mass index (BMI) were expressed as age- and sex-specific standard deviation (SD) scores in relation to published data for the UK population (Tanner and Takaishi Citation1965, Cole et al. Citation1995, Freeman et al. Citation1995). SD scores were calculated as (x – population mean for age and sex) / (population SD for age and sex), where x was the measurement in the patient.
For the knemometry data, we calculated LLLV for each time point by subtracting the lower leg length at that time point from the length measured at the previous time point, and dividing by the exact time interval between the two measurements.
The concentrations of IGF-I, IGFBP-3, collagen markers and bone ALP in children are age- and sex-dependent, and (except for bone ALP) log-normally distributed. We calculated age- and sex-specific SD scores (as above), after log-transformation where appropriate, using our own published data in normal children (Crofton Citation1992, Blum Citation1996, Crofton et al. Citation1997).
Data were expressed as mean and 95% confidence intervals (CI) of the mean, except for age, which was expressed as median and range. Comparison with our normal reference populations (mean SD (z) score zero, SD 1.0, by definition) was done by the one-sample t test. Changes with time were evaluated using the Wilcoxon signed rank test. Spearman rank correlations with correction for ties were used to compare variables at each time point.
Results
At presentation with acute onset of Perthes' disease
In the 15 children studied cross-sectionally at baseline, mean (95% CI) SD scores were: height +0.49 (−0.13 to +1.12), sitting height +0.17 (−0.54 to +0.88) and BMI +0.96 (−0.32 to +2.24). Height and sitting height were not significantly different from the reference population. Mean BMI was distorted as a result of one very obese individual with a BMI SD score of +5.6. When this boy was excluded, the mean BMI SD score was +0.54 (−0.44 to +1.51), which is not significantly different from the reference population. The ratio of mean height to sitting height was 1.80 (1.74–1.85).
For the biochemical markers, P3NP and PICP were markedly lower than the reference population, IGF-I was moderately low whilst ICTP was significantly elevated (). Only the two youngest patients (both younger than 4 years) had IGF-I levels greater than the mean for the reference population (+0.04 and +0.14, respectively). In all patients, the SD score for IGF-I was lower or (in one case) equal to the SD score for IGFBP-3.
Table 2. Biochemical markers in 15 children studied cross−sectionally at acute presentation with Perthes' disease, and in 9 of these children studied longitudinally for up to 2 years
There was a strong correlation between IGF-I and BMI at baseline (rs +0.85, p = 0.001) but there were no other significant correlations between any of the markers and anthropometric measurements.
Longitudinal cohort study
For the 7 boys who were followed up to year 2, mean height velocity was 5.0 cm/y (95% CI 3.9–6.1) over this period. The sitting height SD score remained constant but the height SD score declined by –0.11 (−0.25 to +0.03, p = 0.06) and the ratio of height to sitting height also declined slightly (p = 0.06). The BMI SD score remained constant.
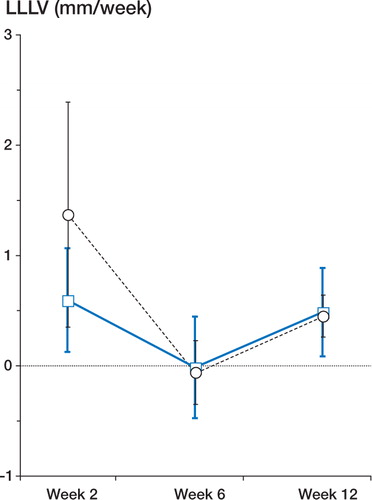
In the 6 patients in whom lower leg measurements were available for both weeks 1 and 2, mean LLLV in the affected leg during the first 2 weeks after clinical presentation was 0.59 mm/week (95% CI 0.12–1.06), which is similar to mean lower leg growth for healthy prepubertal children in our local population (0.5 mm/week, 95% reference interval −0.5 to +1.2, unpublished observations). However, mean LLLV in the unaffected leg was 1.37 mm/week (0.35–2.39), which was significantly elevated compared to the reference population (p < 0.0001). In 5/6 patients, LLLV was greater in the unaffected leg than in the affected leg over this initial period (p = 0.06), the only exception being the boy with bilateral Perthes' disease in whom both lower legs grew at an identical rate (). By week 6, lower leg growth in both legs had virtually stopped (). By week 12, LLLV had increased (p = 0.03 and 0.06 for the unaffected and affected legs, respectively, compared with week 6), was virtually identical in both lower legs, and was similar to that of the reference population ().
Figure 1. LLLV during the first week following presentation in the unaffected leg compared with the affected leg. Only the 6 patients in whom lower leg measurements were available for both weeks 1 and 2 are shown. Circles (unbroken lines): patients with unilateral Perthes' disease. Squares (dashed line): boy with bilateral Perthes' disease (see text).
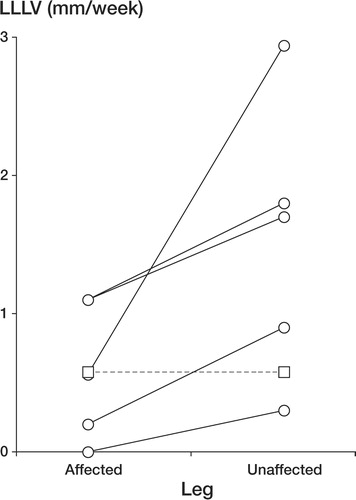
Figure 2. Changes in LLLV during the first 12 weeks following acute onset of Perthes' disease. Week 2 (n = 6), week 6 (n = 7), week 12 (n = 7). Data are plotted as mean and 95% confidence intervals of the mean. Squares (unbroken, thick line): affected leg. Circles (dotted line): unaffected leg.
shows the changes in the biochemical markers in the 9 children studied serially following their acute presentation. IGF-I showed no change with time, remaining moderately low compared to the reference population. This was in contrast to IGFBP-3, which increased during the first 12 weeks. With regard to markers of collagen synthesis, P3NP showed variable and inconsistent changes with time, but nevertheless remained low compared to the reference population (p < 0.01). PICP increased from its initially low levels to reach levels that were indistinguishable from the reference population by 12 weeks. In contrast, the marker of type I collagen breakdown, ICTP, showed no change with time, remaining moderately high compared to the reference population. Bone ALP (like PICP) showed an upward trend, reaching levels higher than those of the reference population by year 2 (p < 0.01). In year 2, there was a correlation between height SD score and IGF-I levels (rs 0.83, p = 0.04), but not between height SD score and any other marker.
Discussion
Several (Goff Citation1954, Burwell et al. Citation1978, Wynn-Davies and Gormley Citation1978), but not all (Girdany and Osman Citation1968, Axer et al. Citation1980) studies have reported mild short stature in children with Perthes' disease. All of these cross-sectional studies included patients at a wide range of stages of the disorder, from the relatively early acute phase to fully healed disease. In our study, children presenting in the early acute phase of Perthes' disease had normal stature and normal sitting height compared with the reference population. However, there was evidence of a decline in height (but not sitting height) SD scores over the two years of follow-up, which is compatible with previous reports.
Knemometry is a highly accurate, sensitive and precise method for assessment of lower leg growth over short time periods (Wales and Milner Citation1987). To our knowledge, there have been no previous reports of lower leg growth in children with Perthes' disease. There was little evidence of impaired growth in either lower leg during the first 2 weeks after diagnosis: LLLV was normal on the affected side but supranormal on the unaffected side. Since there was no systematic difference between lower leg length on the affected and unaffected sides in children with unilateral Perthes' disease at first presentation (data not shown), a long-standing asymmetry in growth is unlikely. Our interpretation is that the limp associated with acute onset of Perthes' disease increases weight-bearing and hence stimulates increased linear growth on the unaffected side. The subsequent almost complete cessation of lower leg growth between weeks 2 and 6 may reflect removal of the weight-bearing stimulus to lower leg growth during traction and bed rest, wheelchair confinement or reduced physical activity. By 3 months after presentation, most children were being mobilized and growth of both lower legs was similar and normal. None of these changes in LLLV were correlated with IGF-I or any of the other markers (data not shown), suggesting that these were localized changes in growth mediated by local mechanical effects rather than by systemic hormonal influences.
At first presentation, IGF-I was moderately low in our study, confirming some (Neidel et al. Citation1992 a, Citationb) but not other (Grasemann et al. Citation1996) previous reports. IGFBP-3 was normal, which also agrees with some (Neidel et al. Citation1993, Grasemann et al. Citation1996) but not other (Matsumoto et al. Citation1998) reports. In almost all patients, we found that IGF-I SD scores were lower than IGFBP-3 SD scores, raising the possibility that levels of bioavailable IGF-I may have been even lower. Only the two youngest patients had (slightly) positive SD scores for IGF-I, which is in agreement with a previous study that reported absent or diminished physiological increase in IGF-I with age in children during early-stage Perthes' disease (Neidel et al. Citation1992a). We found no significant increase in absolute concentrations of IGF-I during the two years of follow-up in our patients (data not shown), which is also in accordance with that study. The cause of the low IGF-I remains unclear. Undernutrition may result in low IGF-I, but the BMI SD score was greater than –1.0 in all patients, indicating good nutritional status. Whatever the underlying cause, our observation that IGF-I was correlated with the declining height SD scores in year 2 suggests that low levels of circulating IGF-I may be an etiological factor in the mildly short stature that has been described in children with Perthes' disease. The absence of a correlation between IGF-I and height SD scores at first presentation (when height was normal and lower leg growth was not impaired) suggests that the low IGF-I may have been of relatively recent onset and had not yet had an influence on stature.
To our knowledge, there have been no previous reports concerning bone and collagen turnover in children with Perthes' disease. P3NP, a marker of type III collagen synthesis in soft tissue, reflects overall somatic growth (Trivedi et al. Citation1989). Its very low level at diagnosis, persisting throughout follow-up, is consistent with a widespread deficit in soft tissue collagen deposition accompanying the overall suboptimal growth in these children.
Bone ALP is a product of differentiated osteoblasts and of hypertrophic chondrocytes in the epiphyseal growth plate, and reflects bone formation and growth (Crofton and Kelnar Citation1998). It was normal at diagnosis but subsequently showed an upward trend, unrelated to growth. This increased osteoblastic activity is most likely to have been associated with healing of the capital epiphyseal infarct.
PICP is a product of the proliferative osteoblast and, as such, reacts more rapidly to clinical events than bone ALP. Although often considered (in adults) to be almost exclusively a marker of bone collagen matrix formation, it also reflects type I collagen synthesis in soft tissue. PICP, like P3NP, reflects overall growth in children (Crofton and Kelnar Citation1998). At diagnosis, it is likely that the moderately low circulating PICP reflects a combination of very low rates of type I collagen synthesis in soft tissue (in keeping with very low P3NP) but normal rates in bone (in keeping with normal bone ALP). During bone healing, circulating PICP increased, reflecting increased osteoblast proliferation.
ICTP reflects type I collagen degradation (Crofton and Kelnar Citation1998). Its moderately increased levels at diagnosis suggest increased type I collagen degradation at a time when type I collagen synthesis is low, presumably resulting in a negative balance of collagenous matrix in soft tissue and/or bone which then persists throughout follow-up. It remains unresolved whether the low IGF-I levels, low rates of collagen synthesis and increased rates of collagen breakdown observed at presentation are part of the etiology of Perthes' disease or, conversely, whether Perthes' disease may, directly or indirectly, result in suppression of circulating IGF-I and whole-body collagen synthesis.
In summary, we have shown in a small longitudinal cohort study that children with Perthes' disease were of normal stature at the onset of symptoms, but that height SD scores declined thereafter. Lower leg growth was not impaired at presentation but was asymmetrical, reflecting differential weight bearing. Subsequent cessation followed by resumption of symmetrical lower leg growth probably reflected our treatment of immobilization followed by gentle remobilization. At presentation, the patients already had low circulating IGF-I, low rates of soft tissue collagen synthesis and enhanced rates of collagen breakdown which persisted over the next 2 years. Low circulating IGF-I may have contributed to declining height SD scores. We consider that this study has provided us with further insights into the pathophysiology of the growth abnormalities associated with the fragmentation and early healing phases of Perthes' disease.
This study was supported by a project grant from the Edinburgh Sick Children's NHS Trust. We wish to thank Ms Jean Wade, Ms Nancy Evans and Ms Karin Weber for their expert technical assistance.
- Ahmed S F, Wallace W H B, Kelnar C J H. Knemometry in childhood: a study to compare the precision of two different techniques. Ann Hum Biol 1995; 22: 247–52
- Axer A, Gershuni D H, Hendel D, Mirovski Y. Indications for femoral osteotomy in Legg-Calvé-Perthes disease. Clin Orthop 1980, 150: 78–87
- Blum W F. Insulin-like growth factors and their binding proteins. Diagnostics of endocrine function in children and adolescents, M B Ranke. Barth, Edition J&J, Heidelberg 1996; 190–218, etc:
- Blum W F, Ranke M B, Kietzmann K, Gauggel E, Zeisel J H, Bierich J R. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab 1990; 70: 1292–7
- Burwell R G, Dangerfield P H, Hall D J, Vernon C L, Harrison M H M. Perthes' disease. An anthropometric study revealing impaired and disproportionate growth. J Bone Joint Surg (Br) 1978; 60: 461–77
- Catterall A. The natural history of Perthes' disease. J Bone Joint Surg (Br) 1971; 53: 37–52
- Cole T J, Freeman J V, Preece M A. Body mass index reference curves for the UK, 1990. Arch Dis Child 1995; 73: 25–9
- Crofton P M. Wheat-germ lectin affinity electrophoresis for alkaline phosphatase isoforms in children: age-dependent reference ranges and changes in liver and bone disease. Clin Chem 1992; 38: 663–70
- Crofton P M, Kelnar C J H. Bone and collagen markers in paediatric practice. Int J Clin Pract 1998; 52: 557–65
- Crofton P M, Wade J C, Taylor M R H, Holland C V. Serum concentrations of the carboxyterminal propeptide of type I procollagen, the aminoterminal propeptide of type III procollagen, the cross-linked carboxyterminal telopeptide of type I collagen and their interrelationships in school-children. Clin Chem 1997; 43: 1577–81
- Freeman J V, Cole T J, Chinn S, Jones P R M, White E M, Preece M A. Cross-sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 1995; 73: 17–24
- Girdany B R, Osman M Z. Longitudinal growth and skeletal maturation in Perthes' disease. Radiol Clin North Am 1968; 6: 245–51
- Goff C. Legg-Calvé-Perthes Syndrome. Charles C Thomas, Toronto 1954
- Golding J S R. The mechanical factors which influence bone growth. Eur J Clin Nutr 1994; 48(Suppl 1)S178–85
- Grasemann H, Nicolai R D, Hauffa B P, Reinhardt W, Nicolai H, Hovel M. Skeletal immaturity, IGF-I and IGFBP-3 serum concentrations in Legg-Calve-Perthes disease. Klin Padiatr 1996; 208: 339–43
- Herring J A, Neustadt J B, Williams J J, Early J S, Brown R H. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop 1992; 12: 143–50
- Ismail A M, Macnicol M F. Prognosis in Perthes' disease. J Bone Joint Surg (Br) 1998; 80: 310–4
- Kristmundsdottir F, Burwell R G, Hall D J, Marshall W A. A longitudinal study of carpal bone development in Perthes' disease: its significance for both radiologic standstill and bilateral disease. Clin Orthop 1986, 209: 115–23
- Matsumoto T, Enomoto H, Takahashi K, Motokawa S. Decreased levels of IGF binding protein-3 in serum from children with Perthes' disease. Acta Orthop Scand 1998; 69: 125–8
- Neidel J, Zander D, Hackenbroch M H. No physiologic agerelated increase of circulating somatomedin-C during early stage of Perthes' disease: a longitudinal study in 21 boys. Arch Orthop Surg 1992a; 111: 171–3
- Neidel J, Zander D, Hackenbroch M H. Low plasma levels of insulin-like growth factor in Perthes' disease. A controlled study of 59 consecutive children. Acta Orthop Scand 1992b; 63: 393–8
- Neidel J, Schönau E, Zander D, Rutt J, Hackenbroch M H. Normal plasma levels of IGF binding protein in Perthes' disease. Follow-up of previous report. Acta Orthop Scand 1993; 64: 540–2
- Peaston R T, Cooper J. Affinity electrophoresis of alkaline phosphatase isoenzymes. Clin Chem 1986; 32: 235–6
- Rayner P H W, Schwalbe S L, Hall D J. An assessment of endocrine function in boys with Perthes' disease. Clin Orthop 1986, 209: 124–8
- Tanner J M, Takaishi M. Standards from birth to maturity for height, weight, height velocity and weight velocity in British children. Arch Dis Child 1965; 41: 613–5
- Tonks D B. A study of the accuracy and precision of clinical chemistry determinations in 170 Canadian laboratories. Clin Chem 1963; 9: 217–33
- Trivedi P, Hindmarsh P, Risteli J, Risteli L, Mowat A P, Brook C G D. Growth velocity, growth hormone therapy, and serum concentrations of the amino-terminal propeptide of type III procollagen. J Pediatr 1989; 114: 225–30
- Wales J K H, Milner R D G. Knemometry in assessment of linear growth. Arch Dis Child 1987; 62: 166–71
- Woods D, Macnicol M. The flexion-adduction test: an early sign of hip disease. J Pediatr Orthop B 2001; 10: 180–5
- Wynn-Davies R, Gormley J. The aetiology of Perthes' disease. J Bone Joint Surg (Br) 1978; 60: 6–14
