Abstract
Background Fat embolism (FE), the release of bone marrow contents into the circulation and the subsequent cardiovascular changes, is still a potentially fatal com-plication during orthopedic surgery. Different causative factors have been suggested, but the exact pathomechanism of FE still remains unclear. We investigated the role of polymethylmethacrylate (PMMA) in FE during vertebroplasty in sheep.
Methods In 8 sheep, two vertebral bodies were augmented alternatively with PMMA or bone wax. Pulmonary and cardiovascular parameters were monitored during the procedure.
Results The peak response was similar for both groups and characterized by hypotension, a drop in cardiac output and pulmonary hypertension. However, the recovery in pulmonary arterial pressure and pulmonary vascular resistance was quicker in the wax group.
Interpretation The injection of PMMA may cause prolonged pulmonary hypertension during vertebro-plasty and also arthroplasty. Surgeons should be aware of this potential cardiovascular complication, especially in patients with impaired pulmonary and cardiovascular function.
▪
Fat embolism (FE) can be defined as a release of bone marrow contents (including bone cement and its components) into the circulation and the ensuing cardiovascular changes (Aebli et al. Citation2002). Manipulation of the bone marrow cavity (i.e. reaming, cement injection or implant insertion) releases bone marrow contents which cause microembolization of the lung vessels within seconds after starting the manipulation. Cardiovascular deterioration during FE includes hypotension, hypoxemia, arrhythmia, cardiac arrest and sudden death. Fat embolism is observed intraoperatively during cemented and cementless hip and knee arthroplasty (Herndon et al. Citation1974, Fahmy et al. Citation1990, Ereth et al. Citation1992, Parmet et al. Citation1993, Christie et al. Citation1994, Morawa et al. Citation1996), during intramedullary nailing of fractured long bones (Talucci et al. Citation1983, Pell et al. Citation1993) and also during vertebroplasty (VP) (Aebli et al. Citation2002, Chen et al. Citation2002). Intraoperative cardiac arrest during hip arthroplasty has been noted in 0.6–10% of patients and intraoperative deaths have been reported in 0.02–0.5% (Coventry et al. Citation1974, Duncan Citation1989, Patterson et al. Citation1991, Hofmann et al. Citation1999, Parvizi et al. Citation1999). Cardiac arrest appears to be more common during hemiar-throplasties for femoral neck fractures than during total hip arthroplasty (Christie et al. Citation1994). Further-more, cementless prostheses have been reported to cause less severe hypotension and fewer cardiac arrests than cemented ones (Ereth et al. Citation1992).
Even after many studies, the exact pathomechanism of FE is unclear. Firstly, the chemical and thermal effects of the polymethylmethacrylate (PMMA) bone cement and methylmethacrylate monomer have been accused of being responsible for the cardiovascular changes during FE (Homsy et al. Citation1972, Peebles et al. Citation1972, Berman et al. Citation1974). It has been suggested that the monomer causes sys-temic vasodilatation (Peebles et al. Citation1972, Berman et al. Citation1974, Samii et al. Citation1980). However, the currently accepted mechanism is the microembolisa-tion of the arterioles and capillaries in the lungs due to the extravasation of bone marrow particles, leading to cardiovascular deterioration (Breed Citation1974, Orsini et al. Citation1987, Wheelwright et al. Citation1993, Schemitsch et al. Citation1998, Aebli et al. Citation2002). Other explanations have included a nerve reflex trig-gered by the increase in pressure within the bone marrow cavity during manipulations (i.e. reaming, cement injection or implant insertion), similar to the carotid sinus reflex (Rudigier and Ritter Citation1983). Furthermore, cardiovascular changes during FE may be a result of vasoactive mediators from the bone marrow cavity gaining access to the circula-tion (Byrick et al. Citation1991, Wheelwright et al. Citation1993).
In the past, different animal models have been used to investigate FE (Breed Citation1974, Rudigier and Ritter Citation1983, Orsini et al. Citation1987, Wheelwright et al. Citation1993, Schemitsch et al. Citation1998). Recently, an animal model of vertebroplasty has been developed to investigate FE (Aebli et al. Citation2002). Reproducible cardiovascular reactions during FE have been observed in several studies using this animal model (Aebli et al. Citation2003a, Citationb).
We investigated the cardiovascular reactions to injection of PMMA during the first minutes after the initiation of FE during vertebroplasty in sheep.
Animals and methods
Animal model
We conducted experiments on 8 skeletally mature mixed-bred ewes (3–5 years old, mean body weight 64 kg) which were subjected to unilateral augmen-tation of 2 vertebral bodies (L1 and L2), using PMMA or bone wax. The study was approved by our local Animal Ethics Committee.
Preparation of animals and monitoring
Anesthesia was induced with thiopental (bolus of 1.0 g/sheep) and maintained with halothane (1–2%) in a mixture of nitrous oxide (0.5 L/min) and oxygen (1.5 L/min). The animals underwent positive-pressure ventilation; the rate and end tidal pressure were adjusted to maintain a normal end tidal pCO2 (40 (SD 2) mm Hg) prior to inject-ing augmentation material into the first vertebral body.
We placed catheters into both carotid arteries and attached them to Statham pressure transducers (P23AC) to measure arterial blood pressure (diameter 1.5 mm; length 3.8 cm) and left ventricular pressure (diameter 1.5 mm; length 30.5 cm). The correct position of the ventricular catheter was established by observing the pressure readings. An introducer (Intro-Flex, 8.5F, Edwards Critical-Care Division, Irvine, CA) for a Swan-Ganz catheter was inserted into a jugular vein. Subsequently, a Swan-Ganz catheter (Model CCO/SvO2, Edwards Critical-Care) was floated into the pulmonary artery to measure pulmonary arterial pressure (PAP), central venous pressure (CVP), cardiac output (Q) and venous oxygen saturation (SvO2). A saphenous vein was cannulated and a saline drip infusion established.
Arterial blood pressure, PAP, CVP and left ventricular pressure were digitized at 100 Hz using a MacLab A/D converter (ADI Instruments, Castle Hill, NSW, Australia) and displayed on a Macintosh LCIII computer. The heart rate (HR) was derived from the arterial pulse. Cardiovascular variables were recorded continuously. We collected cardiac output data with a Vigilance monitor (Model VM/E, Edwards Critical-Care) which was connected to a Macintosh LCIII. The thermodilution technique using a thermal filament enabled continuous monitoring of Q (periodic measurements). In order to obtain Q data at certain time points (i.e. 0.5, 1, 3, 5 and 10 min postinjection), we used the bolus thermodilution method. Iced saline solution (0–5°C; 10.0 mL) was injected through the Swan-Ganz catheter and the Q value was calculated by the Vigilance monitor. We analyzed cardiovascular data in 2-min time intervals for 10 min before the first injection of augmentation material. These data were averaged and represented pre-VP data. Cardiovascular data were also analyzed at 0.5, 1, 3, 5 and 10 min postinjection, for each VP. The values at 0.5 min postinjection represented peak response (maximum or minimum change) because a previous study had shown that the maximum or minimum changes in cardiovascular parameters were reached approximately 30 sec after starting the injection (Aebli et al. Citation2002). Vertebral bodies were augmented at 10 min intervals because in the same earlier study (Aebli et al. Citation2002), it had been noted that mean arterial blood pressure (MABP) had recovered to a new steady state—which was within 10% of the original baseline—5 min after the injection of one vertebral body. We calculated pulmonary vascular resistance (PVR) and systemic vascular resistance (SVR) according to the following formula: PVR = 80 × (MPAP–LVDP)/Q (dyne s/cm5) and SVR = 80 × (MABP–MCVP)/Q (dyne s/cm5) where LVDP is left ventricular diastolic pressure.
At the end of the protocol, the animal was killed with an overdose of barbiturates.
Surgical procedure and augmentation
With the sheep in a sternal position, we made a 20 cm midline incision over the vertebral spinal processes of T11–L4. The paravertebral muscles were then detached dorsoventrally, exposing the intervertebral joint line. A 3.2-mm hole was drilled through the right pedicle into each vertebral body to a depth of 10 mm, so that a syringe would fit tightly into it.
Medium viscosity bone cement (CMW3 DePuy New Zealand Ltd., NZ) was used for PMMA injec-tion. The base (16 g) and monomer (9 mL) were mixed for 1 min and then drawn into 3-mL syringes with reinforced plungers. To achieve a pasty con-sistency, the cement was allowed to cure at room temperature for 4–6 min prior to injection. Bone wax (Ethicon, Johnson and Johnson Medical NZ, Auckland, New Zealand) was heated in a water bath (46–47°C) to achieve similar viscosity to the cement. Bone wax was also injected using 3.0-mL syringes. In both groups, 6.0 mL was injected unless injection pressure was perceived as being too high—meaning that filling of the vertebral body had been achieved. As the first augmentation (L1) could alter the cardiovascular reaction to the second augmentation (L2), we alternated PMMA and wax injections such that each type was evalu-ated for first and second injection.
Statistics
Data were calculated and presented as mean (SE). We used mixed model analysis to account for inter-group and intragroup variability relating to pre- and postinjection periods. Two-way ANOVA was used to test for significance regarding the changes of cardiovascular data from preinjection value (PreVP) to peak value and values at 1, 3, 5 and 10 min for both groups. We used simple regression analysis to test for any correlation between dif-ferent cardiovascular variables. Post hoc analyses were achieved using the paired t-test. Statistical comparison was also done between the PMMA and wax group at preinjection and for peak response using ANOVA and unpaired t-tests. A p-value of < 0.05 was considered significant for all statisti-cal analyses. The 95% confidence intervals (CIs) were calculated for the cardiovascular parameters with significant changes. Statistical analyses were performed using Microsoft Excel XP.
Results
Augmentation
On average, 5.7 (SD 0.1) mL PMMA or 5.8 (SD 0.2) mL bone wax was injected into each vertebral body over a period of 35–60 sec. At postmortem, PMMA and bone wax leakage into paravertebral veins was discovered in all animals.
Hemodynamic changes
Baseline cardiovascular data collected before VP (Pre-VP) are presented in and . There was no significant difference between the two groups before VP.
Table 1. Cardiovascular data for the PMMA and wax groups prior to vertebroplasty (pre-VP) and following vertebro-plasty (peak at 0.5 min to 10 min). Values are mean (SE)
Table 2. The 95% confidence intervals for selected cardiovascular parameters of the PMMA and wax groups prior to vertebroplasty (pre-VP) and following vertebroplasty (peak at 0.5 min to 10 min)
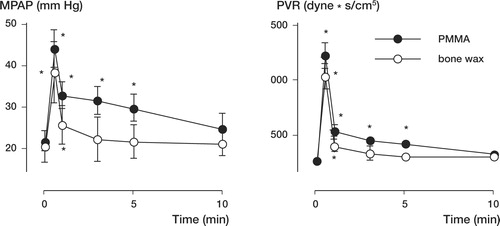
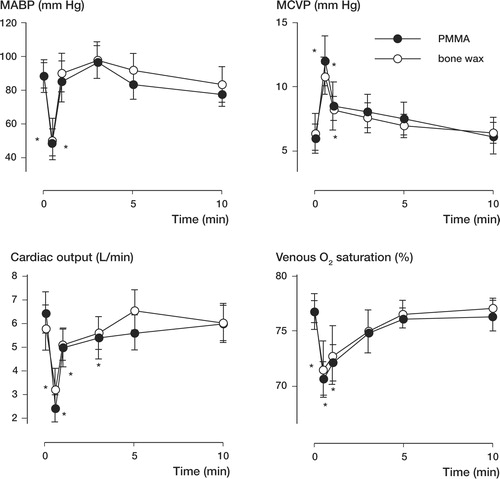
We found no significant difference between the cardiovascular responses to the first and second injection in both groups. Thus, we pooled the data for the two injections, for each group separately, and statistical analysis was performed for the PMMA group and the bone wax group. Injection of both PMMA and bone wax resulted in cardiovascular changes ( and ). The peak response was similar for both groups (no statistically significant difference between PMMA and bone wax) and characterized by a drop in MABP, Q and SvO2 as well as an increase in MPAP and MCVP. There was no statistically significant change in HR and LVDP. Mean arterial blood pressure dropped by 47% and 40% for the PMMA and wax groups respectively. The drop in Q was of similar magnitude (PMMA: 61%, and bone wax: 39%). The increase in MPAP was the most prominent change: 103% and 96% for the PMMA and bone wax groups, respectively.
Hypotension had ceased after 1 min in both groups ( and ). Cardiac output values had recovered after 1 min and 3 min for the bone wax and PMMA groups, respectively. The major difference between the cardiovascular response of the PMMA group and that of the bone wax group was the recovery in MPAP ( and ). 3 min postinjection, MPAP had fully recovered to baseline values in the bone wax group, whereas in the PMMA group MPAP had only recovered by 52%. Mean pulmonary artery pressure had recovered to baseline values after 10 min in the PMMA group.
Figure 1. Mean arterial blood pressure (MABP), mean central venous pressure (MCVP), cardiac output and venous O2saturation recorded prior to vertebroplasty (time 0) and 0.5, 1, 3, 5, and 10 min following vertebroplasty with PMMA and bone wax. Asterisks indicate values that are significantly different from the preinjection (time 0) value (p < 0.05).
Discussion
The augmentation of vertebral bodies resulted in cardiovascular changes regardless of the material used. However, our findings indicate that PMMA may have an adverse effect on pulmonary arte-rial vessels, causing prolonged pulmonary arterial hypertension.
The main observation in our study was that the recovery time of PVR was shorter in the wax group than in the PMMA group. The methylmethacrylate monomer may have directly caused vasoconstric-tion of the pulmonary arteries (Johansen and Benu-mof Citation1977, Fairman et al. Citation1984) and consequently delayed recovery of MPAP in the PMMA group. Alternatively, the monomer or other cement com-ponents may have stimulated the formation of thrombi (Hallin et al. Citation1974, Dahl et al. Citation1992) or the agglutination of plasma lipids (Dustmann et al. Citation1972). It has also been suggested that PMMA increases systemic levels of histamine during arthroplasty (Tryba et al. Citation1991), but to date, the release of vasoactive cytokines due to PMMA has not been fully investigated.
In our study, the injection of PMMA or an inert material (i.e. bone wax) into a lumbar vertebral body resulted in pulmonary vasoconstriction 30 seconds after starting the injection. The increase in PVR caused a forward failure of the heart, with subsequent hypotension and decrease in cardiac output in the presence of unchanged left ventricular diastolic pressure. The increase in PVR may have been due to pulmonary vasoconstriction triggered by a reflex reaction to the embolization (Malik Citation1983, Dalen et al. Citation1967). Dalen et al. (Citation1967) demonstrated that the intravenous injection of silicon beads of diameter of 50–250 μm caused a reflex increase in PVR within a few seconds after the injection. The purpose of such a reflex vasoconstriction most likely prevents microembolization of the brain, with its disastrous consequences (Dalen et al. Citation1967, Malik Citation1983). Alternatively, pulmonary vasoconstriction may have been caused by vasoactive cytokines (Malik and van der Zee Citation1977, Binder et al. Citation1980). It is unlikely that the increase in PVR was due mechanical occlusion of the pulmonary vessels by emboli or thrombi, because blockage of more than 50% of all pulmonary arteries would be necessary to increase PVR (Dalen et al. Citation1967, Ohkuda et al. Citation1978).
Other experimental studies on FE have also described an increase in PVR with forward failure of the heart (Wheelwright et al. Citation1993, Woo et al. Citation1995). Wheelwright et al. (Citation1993) pressurized the distal femur of dogs with a piston, and observed a 400% increase in PVR within the first 3 min after starting the pressurization. The authors only observed a fall in arterial blood pressure of 13%, which contrasts with a drop of almost 50% in our study. Breed (Citation1974) pressurized the distal femur of rabbits with PMMA and bone wax and observed a fall in blood pressure in both groups within the first few seconds after starting the injection. Breed concluded that the cardiovascular disturbance observed in his study could not be fully explained by an adverse effect of the bone cement alone. He therefore suggested that more than one pathological mechanism may be involved. This is in accor-dance with our results. The first mechanism may be reflex pulmonary vasoconstriction due to microembolization. The second mechanism may be chemically (cement monomer) induced pulmonary vasoconstriction. The existence of these two mech-anisms would explain why FE occurs during both cemented and cementless arthroplasty, but is less severe during cementless arthroplasty (Ereth et al. Citation1992). This is why medullary lavage and venting (with or without applied vacuum) during cemented hip arthroplasty can reduce but not eliminate FE (Wheelwright et al. Citation1993, Pitto et al. Citation1998).
Differences between clinical vertebroplasty and this animal model have been discussed elsewhere (Aebli et al. Citation2002). The volume of ovine lumbar vertebral bodies is smaller than for their human counterparts. According to the L4 measurements of McLain et al. (Citation2002), ovine L4 volumes (∼15 cm3) are about one quarter of those of human L4 (∼57 cm3). The volume of injected material (6 cm3) therefore represents 40% of the verte-bral body volume. However, we did not intend to achieve a vertebral filling that would be equivalent to the clinic situation. In investigating FE during VP, it was imperative to inject a similar amount of material and thus replace a similar amount of bone marrow during the experiment as in the clini-cal situation (Cotten et al. Citation1998, Deramond et al. Citation1998, Heini et al. Citation2000). A second limitation of the present study pertains to the lack of histological evaluation of the lungs for fat emboli. However, a previous study had shown that the injection of PMMA and bone wax resulted in similar quantities of fat emboli in the lungs (Aebli et al. Citation2003b).
Surgical measures for minimizing FE during vertebroplasty and arthroplasty may include avoid-ing excessive bone marrow pressurization (Orsini et al. Citation1987, Hofmann et al. Citation1999, Aebli et al. Citation2003a), medullary canal lavage (Sherman et al. Citation1983, Byrick et al. Citation1989, Wheelwright et al. Citation1993) and the use of a venting hole (Herndon et al. Citation1974, Jones Citation1975). The anesthetic management consists of cardiovascular support by administrating 100% inspired oxygen and aggressive volume support (Jones Citation1975, Fallon et al. Citation2001). However, the increase in PVR we observed may increase right ventricular afterload, and thus lead to acute right heart failure–especially in patients with preexisting pulmonary and cardiovascular impairment. Exces-sive fluid treatment may aggravate right heart fail-ure. Fluid therapy should thus be monitored care-fully. The placement of a pulmonary artery catheter may be needed in order to utilize selective pulmo-nary vasodilators, especially if PMMA is used. The use of non-PMMA cements for vertebroplasty may alleviate the cardiovascular changes during FE, but will not prevent them.
We are indebted to Joanne Draffin for her valuable technical assistance. We thank Edwards Lifesciences Pty Ltd., Auck-land, New Zealand, for providing the Vigilance Cardiac Output Monitor. We also thank Mr. John North and Profes-sor Rocco Pitto for reviewing the manuscript.
- Aebli N, Krebs J, Davis G, Walton M, Williams M J.A. Theis J. C. Fat embolism and acute hypotension during verte-broplasty: an experimental study in sheep. Spine 2002; 27(59)460–6
- Aebli N, Krebs J, Schwenke D, Davis G, Theis J C. Cardio-vascular changes during multiple vertebroplasty with and without vent-hole: an experimental study in sheep. Spine 2003a; 28(14)1504–11
- Aebli N, Krebs J, Schwenke D, Davis G, Theis J C. Pressurization of vertebral bodies during vertebroplasty causes cardiovascular complications: an experimental study in sheep. Spine 2003b; 28(14)1513–20
- Berman A T, Price H L, Hahn J F. The cardiovascular effects of methylmethacrylate in dogs. Clin Orthop 1974, 100: 265–9
- Binder A S, Kageler W, Perel A, Flick M R, Staub N C. Effect of platelet depletion on lung vascular permeability after microemboli in sheep. J Appl Physiol 1980; 48(3)414–20
- Breed A L. Experimental production of vascular hypoten-sion, and bone marrow and fat embolism with methyl-methacrylate cement. Traumatic hypertension of bone. Clin Orthop 1974, 102: 227–44
- Byrick R J, Bell R S, Kay J C, Waddell J P, Mullen J B. High-volume, high-pressure pulsatile lavage during cemented arthroplasty. J Bone Joint Surg (Am) 1989; 71(9)1331–6
- Byrick R J, Mullen J B, Wong P Y, Kay J C, Wigglesworth D, Doran R J. Prostanoid production and pulmonary hyper-tension after fat embolism are not modified by methyl-prednisolone. Can J Anaesth 1991; 38(5)660–7
- Chen H L, Wong C S, Ho S T, Chang F L, Hsu C H, Wu C T. A lethal pulmonary embolism during percutaneous verte-broplasty. Anesth Analg 2002; 95(4)1060–2
- Christie J, Burnett R, Potts H R, Pell A C. Echocardiography of transatrial embolism during cemented and uncemented hemiarthroplasty of the hip. J Bone Joint Surg (Br) 1994; 76(3)409–12
- Cotten A, Boutry N, Cortet B, Assaker R, Demondion X, Leblond D, Chastanet P, Duquesnoy B, Deramond H. Per-cutaneous vertebroplasty: state of the art. Radiographics 1998; 18(2)311–20
- Coventry M B, Beckenbaugh R D, Nolan D R, Ilstrup D M. 2,012 total hip arthroplasties. A study of postoperative course and early complications. J Bone Joint Surg (Am) 1974; 56(2)273–84
- Dahl O E, Johnsen H, Kierulf P, Molnar I, Ro J S, Vinje A, Mowinckel P. Intrapulmonary thrombin generation and its relation to monomethylmethacrylate plasma levels during hip arthroplasty. Acta Anaesthesiol Scand 1992; 36(4)331–5
- Dalen J E, Haynes F W, Hoppin F G, Jr., Evans G L, Bhard-waj P, Dexter L. Cardiovascular responses to experimen-tal pulmonary embolism. Am J Cardiol 1967; 20: 3–9
- Deramond H, Depriester C, Galibert P, Le Gars D. Percu-taneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am 1998; 36(3)533–46
- Duncan J A. Intra-operative collapse or death related to the use of acrylic cement in hip surgery. Anaesthesia 1989; 44(2)149–53
- Dustmann H O, Schulitz K P, Koch H. Fat embolism follow-ing the use of bone cement in hip joint prosthesis. Animal experiments. Arch Orthop Unfallchir 1972; 72(2)114–21
- Ereth M H, Weber J G, Abel M D, Lennon R L, Lewallen D G, Ilstrup D M, Rehder K. Cemented versus noncemented total hip arthroplasty--embolism, hemodynamics, and intrapulmonary shunting. Mayo Clin Proc 1992; 67(11)1066–74
- Fahmy N R, Chandler H P, Danylchuk K, Matta E B, Sunder N, Siliski J M. Blood-gas and circulatory changes during total knee replacement. Role of the intramedullary align-ment rod. J Bone Joint Surg (Am) 1990; 72(1)19–26
- Fairman R P, Morrow C, Glauser F L. Methylmethacrylate induces pulmonary hypertension and increases lung vas-cular permeability in sheep. Am Rev Respir Dis 1984; 130(1)92–5
- Fallon K M, Fuller J G, Morley-Forster P. Fat embolization and fatal cardiac arrest during hip arthroplasty with meth-ylmethacrylate. Can J Anaesth 2001; 48(7)626–9
- Hallin G, Modig J, Nordgren L, Olerud S. The intramedul-lary pressure during the bone marrow trauma of total hip replacement surgery. Ups J Med Sci 1974; 79(1)51–4
- Heini P F, Walchli B, Berlemann U. Percutaneous transpe-dicular vertebroplasty with PMMA: operative technique and early results. A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J 2000; 9(5)445–50
- Herndon J H, Bechtol C O, Crickenberger D P. Fat embolism during total hip replacement. A prospective study. J Bone Joint Surg (Am) 1974; 56(7)1350–62
- Hofmann S, Hopf R, Mayr G, Schlag G, Salzer M. In vivo femoral intramedullary pressure during uncemented hip arthroplasty. Clin Orthop 1999, 360: 136–46
- Homsy C A, Tullos H S, Anderson M S, Diferrante N M, King J W. Some physiological aspects of prosthesis sta-bilization with acrylic polymer. Clin Orthop 1972, 83: 317–28
- Johansen I, Benumof J. Methylmethacrylate and the cardio-vascular system. Anaesth Rev 1977; 4: 27–32
- Jones R H. Physiologic emboli changes observed during total hip replacement arthroplasty. A clinical prospective study. Clin Orthop 1975, 112: 192–200
- Malik A B. Pulmonary microembolism. Physiol Rev 1983; 63(3)1114–207
- Malik A B, van der Z H. Time course of pulmonary vascular response to microembolization. J Appl Physiol 1977; 43(1)51–8
- McLain R F, Yerby S A, Moseley T A. Comparative mor-phometry of L4 vertebrae: comparison of large animal models for the human lumbar spine. Spine 2002; 27(8)E200–E206
- Morawa L G, Manley M T, Edidin A A, Reilly D T. Trans-esophageal echocardiographic monitored events during total knee arthroplasty. Clin Orthop 1996; 331: 192–8
- Ohkuda K, Nakahara K, Weidner W J, Binder A, Staub N C. Lung fluid exchange after uneven pulmonary artery obstruction in sheep. Circ Res 1978; 43(2)152–61
- Orsini E C, Byrick R J, Mullen J B, Kay J C, Waddell J P. Cardiopulmonary function and pulmonary microem-boli during arthroplasty using cemented or non-cemented components. The role of intramedullary pressure. J Bone Joint Surg (Am) 1987; 69(6)822–32
- Parmet J L, Berman A T, Horrow J C, Harding S, Rosen-berg H. Thromboembolism coincident with tourniquet deflation during total knee arthroplasty. Lancet 1993; 341(8852)1057–8
- Parvizi J, Holiday A D, Ereth M H, Lewallen D G. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop 1999, 369: 39–48
- Patterson B M, Healey J H, Cornell C N, Sharrock N E. Car-diac arrest during hip arthroplasty with a cemented long-stem component. A report of seven cases. J Bone Joint Surg (Am) 1991; 73(2)271–7
- Peebles D J, Ellis R H, Stride S D, Simpson B R. Cardio-vascular effects of methylmethacrylate cement. Br Med J 1972; 1(796)349–51
- Pell A C, Christie J, Keating J F, Sutherland G R. The detec-tion of fat embolism by transoesophageal echocardiog-raphy during reamed intramedullary nailing. A study of 24 patients with femoral and tibial fractures. J Bone Joint Surg (Br) 1993; 75(6)921–5
- Pitto R P, Koessler M, Draenert K. The John Charnley Award. Prophylaxis of fat and bone marrow embolism in cemented total hip arthroplasty. Clin Orthop 1998, 355: 23–34
- Rudigier J F, Ritter G. Pathogenesis of circulatory reactions triggered by nervous reflexes during the implantation of bone cements. Res Exp Med (Berl) 1983; 183(2)77–94
- Samii K, Elmelik E, Goutalier D, Viars P. Hemodynamic effects of prosthesis insertion during knee replacement without tourniquet. Anesthesiology 1980; 52(3)271–3
- Schemitsch E H, Turchin D C, Anderson G I, Byrick R J, Mullen J B, Richards R R. Pulmonary and systemic fat embolization after medullary canal pressurization: a hemodynamic and histologic investigation in the dog. J Trauma 1998; 45(4)738–42
- Sherman R M, Byrick R J, Kay J C, Sullivan T R, Waddell J P. The role of lavage in preventing hemodynamic and blood-gas changes during cemented arthroplasty. J Bone Joint Surg (Am) 1983; 65(4)500–6
- Talucci R C, Manning J, Lampard S, Bach A, Carrico C J. Early intramedullary nailing of femoral shaft fractures: a cause of fat embolism syndrome. Am J Surg 1983; 146(1)107–11
- Tryba M, Linde I, Voshage G, Zenz M. Histamine release and cardiovascular reactions to implantation of bone cement during total hip replacement. Anaesthesist 1991; 40(1)25–32
- Wheelwright E F, Byrick R J, Wigglesworth D F, Kay J C, Wong P Y, Mullen J B, Waddell J P. Hypotension during cemented arthroplasty. Relationship to cardiac output and fat embolism. J Bone Joint Surg (Br) 1993; 75(5)715–23
- Woo R, Minster G J, Fitzgerald R H, Jr., Mason L D, Lucas D R, Smith F E. The Frank Stinchfield Award. Pulmonary fat embolism in revision hip arthroplasty. Clin Orthop 1995, 319: 41–53