Abstract
Background A new bone preparation technique, compaction, has been shown to enhance initial implant fixation. However, short-term compaction has resulted in more non-vital bone being in contact with the implant. Also, compaction may result in inferior long-term implant fixation as the compacted non-vital bone at the bone-implant interface is resorbed.
Methods We tested the hypothesis that compaction would result in inferior implant fixation after 10 weeks of weight bearing. We compared compaction with the conventional bone removal technique (drilling) for (1) porous coated titanium (Ti) implants inserted exact-fit into medial femoral condyles, and for (2) hydroxy-apa-tite (HA) porous coated implants inserted press-fit into lateral femoral condyles. In each of 8 dogs, we prepared the implant cavities of one knee joint with drilling, and the other with compaction. Implants were tested mechanically to failure by push-out test, and histomor-phometry was done.
Results For all specimens, non-vital bone implant contact contributed very little to the total bone implant contact. Inferior mechanical or histological implant fixation with compaction was not found for either Ti implants or HA implants.
Interpretation Compaction does not appear to result in inferior implant fixation as the compacted bone at the bone implant interface is resorbed.
▪
Implant stability initially is crucial for long-term implant survival (Ryd et al. Citation1995, Kärrholm et al. Citation1997). A new bone preparation technique, compaction, has been shown to increase the initial stability of femoral stems in vitro as compared to conventional broaching (Chareancholvanich et al. Citation2002). The compaction technique sequentially expands cancellous bone using increasing sizes of smooth tamps (Chareancholvanich et al. Citation2002). This contrasts with conventional rasping/broaching techniques where cancellous bone is partially removed during preparation of the bone cavity. In vivo, enhanced implant fixation has been found with compaction at early time points (Green et al. Citation1999, Kold et al. Citation2005 b, Citationc). Even though these animal studies compared compaction with drilling (which may remove more bone than the conventional broaching/rasping techniques), we have found the shorter-term results of compaction to be encouraging. However, the longer-term biological response to weight-bearing implants that have been inserted with compaction is unknown. We have previously found that after 2 weeks of weight bearing, compaction results in more non-vital bone at the bone-implant interface (Kold et al. Citation2005c), and it may be that the longer-term stability of implants is jeopardized as this non-vital bone undergoes remodeling. However, in two non-weight-bearing implant studies involving compaction, no signs of inferior implant fixation were found for titanium implants after 9 weeks (Green et al. Citation1999), or for HA implants after 12 weeks (Vail et al. Citation2000). As both of these previous studies (Green et al. Citation1999, Vail et al. Citation2000) used non-weight-bearing models in which differences in implant fixation diminished with time (Kienapfel et al. Citation1992), it is diffcult to extrapolate the results from these studies to clinically inserted implants that do carry load. Further-more, it is important to evaluate new bone preparation techniques using weight-bearing implants since different implantation techniques have shown different responses during loaded and unloaded conditions (Mouzin et al. Citation2001, Wang et al. Citation2000).
As the longer-term effects of compaction on fixation of weight-bearing implants have yet to be investigated, we compared compaction with drilling after 10 weeks of weight bearing in the dog. We compared compaction with drilling both in a basic implant model using porous coated titanium (Ti) implants inserted exact-fit, and in a more optimal implant model using hydroxyapatite coated (HA) implants inserted press-fit. For both weight-bear-ing models, we tested the hypothesis that compaction would result in inferior mechanical and histological implant fixation as compared to drilling.
Animals and methods
Study design
We used 8 male skeletally mature mongrel dogs (Harlan, the Netherlands) with a mean weight of 30 (27–32) kg. The dogs were bred for scientific purposes and were handled according to the Danish law on animal experimentation. The sample size was calculated from a nomogram for continuous paired data (Altman Citation1999). The power of the experiment was set to 80%. The minimal clinically relevant difference was set to 55%, and the standard deviation of the expected changes was set to 50%. Based on these assumptions, at least 7 experimental subjects should be included.
Implants were inserted into both femoral condyles of each knee joint. Ti implants were inserted exact-fit into the medial femoral condyles, and HA-coated implants were inserted press-fit into the lateral femoral condyles (). Each dog served as its own control; thus, by randomization one knee was subjected to compaction, and the other to drilling.
Figure 1. The weight-bearing implant model.The implants were inserted into cancellous bone of the distal femur.Ti implants (5.6 mm in diameter) were inserted medially and HA implants (6.0 mm in diameter) were inserted laterally.During weight-bearing, the load was transferred through the polyethylene plug from the tibial plateau to the test implant.Post-mortem, two sections were cut.The first section was used for push-out testing.The second section was used for histomorphometry as serially cut vertical sections were produced after initially random rotating the implant around its long axis.
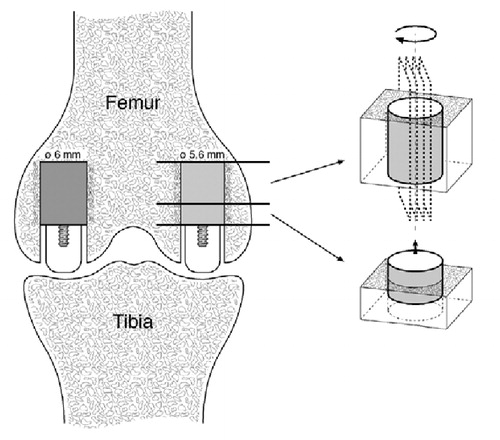
Implant characteristics
16 cylindrical Ti implants (height 10 mm, diameter 5.6 mm), and 16 cylindrical HA implants (height 10 mm, diameter 6.0 mm) were used. The Ti implants consisted of titanium alloy (Ti-6A1-4V) and had a porous-coated Ti-6A1-4V surface deposited by the plasma-spray technique. Analyses for roughness were performed at the Danish Technology Institute on 2 separate Ti implants and 2 separate HA implants. 4 longitudinal measurements were performed on each implant, with 90 degrees between the measurements. A stylus with a tip radius of 2 µm without skid was used. The cut-off filter was 0.8 mm and the evaluation length was 4.0 mm per measurement. The mean roughness (Ra) of the porous titanium surface was 29 (SD 9) µm, and 31 (SD 7) µm for the HA-coated surface. The HA implants consisted of a core of titanium alloy (Ti-6A1-4V) onto which a titanium alloy porous coated surface (Ti-6A1-4V) was coated with HA deposited by the plasma-spray technique. The thickness of the HA coating was 50 µm. For both Ti and HA implants, the implant part closest to the knee joint had a threaded extension onto which a polyethylene plug (height 6 mm, diameter 5.6 mm) was screwed.
Bone preparation technique
The implants were inserted intra-articularly into the weight-bearing part of the femoral condyle during general inhalatory anesthesia with isoflurane. Using sterile technique, the weight-bearing portion of the femoral condyles was exposed by the subvastus approach (Hofmann et al. Citation1991). The implantation site was selected in the central portion of the femoral condyles, which makes contact with the meniscus and tibia during the stance and walking phases (Adrian et al. Citation1966). Initially, a 2.1-mm guide wire was inserted perpendicular to the articular surface. All drilling procedures were done with a power-drill at low speed, and the bone was cooled by profuse irrigation to avoid thermal trauma.
Drilling procedure.
A cannulated trap-drill was used to expand the hole to a diameter of 5.6 mm at the 10 mm deep part and 6.0 mm at the 6 mm superficial part.
Compaction procedure.
A cannulated trap-drill was used to expand the hole to a diameter of 4.2 mm at the 10 mm deep part and 6.0 mm at the 6 mm superficial part. The surrounding cancellous bone in the deep part of the cavity was then gradually expanded radially by use of a specially designed compacter (Kold et al. Citation2005c). Compaction was performed until the deep part of the cavity had similar geometry and dimensions (diameter 5.6 mm) to those of a drilled cavity.
The prepared cavities were cleaned of loos-ened cancellous bone, and irrigated with physiological saline. The 5.6-mm diameter Ti implants were inserted with exact-fit, whereas the 6.0-mm diameter HA implants were hammered firmly into the deep part of the 0.4 mm undersized cavities, ensuring a press-fit with the surrounding cancellous bone. In the superficial part of the prepared cavity, a polyethylene plug was screwed on top of the implant. The plug protruded slightly above the femoral articular cartilage. Extension of the knee confirmed contact between the tibia and the polyethylene plug, so a load was transferred to the implant at each gait cycle () (Søballe et al. Citation1990). The polyethylene plug did not contribute to the initial implant fixation, as a gap of 0.2 mm existed between the polyethylene plug and the surrounding bone.
Prophylactic ampicillin (Anhypen, Brocades Pharma, BV, Leiderkop, the Netherlands) was administered immediately before surgery. Analgesics (Fentanyl plaster) were applied continuously for the next 3 days. Unrestricted weight bearing was allowed postoperatively.
Specimen preparation
The dogs were killed after 10 weeks, and the distal femur was immediately cleaned of soft tissue and stored at –20°C. Two standardized sections orthogonal to the long axis of the implant were cut with a water-cooled diamond band saw (Exakt-Cutting Grinding Systems, Exakt Apparatebau, Norder-stedt, Germany) (). The section with the 3.5-mm implant part closest to the joint space was stored at –20°C and used for mechanical testing. The section with the remaining 6.5 mm of the implant was fixed in 70% ethanol for histomorphometry.
Mechanical testing
Implants were tested to failure by push-out test on an Instron Universal test machine (Instron Ltd., High Wycombe, Bucks, UK). The specimens were placed on a metal platform with a central circular opening supporting the bone 700 µm from the bone-implant interface, as recommended by Dhert et al. (Citation1992). A push-out direction equal to the load transfer direction was chosen. Preload of 2 N was applied to define the contact position for the start of the test. We used a displacement rate of 5 mm/min, and load displacement curves were obtained on a personal computer. Ultimate shear strength (in MPa) was determined from the maximum force applied until failure of the bone implant interface. Apparent shear stiffness (in MPa/mm) was obtained from the slope of the straight part of the load displacement curve. Energy absorption (in kJ/m2) was calculated as the area under the load-dis-placement curve until failure. All push-out parameters were normalized by the surface area of the implant specimen tested.
Histomorphometry
Specimens were dehydrated in graded ethanol (70–100%) containing basic fuchsin, and embedded in methyl methacrylate. Four serially vertical sections (Baddeley et al. Citation1986) were cut with a microtome (KDG-95, MeProTech, the Netherlands) through the center part of the implants. The sections were cut with a distance of 400 µm between, after initially randomly rotating the implant around its long axis. The sections were approximately 25 µm thick, and were counterstained with 4% light green (Gotfredsen et al. Citation1989). Blinded histomorphometry was performed by use of a stereological software program (CAST-Grid, Olympus Denmark A/S, Denmark). The software applies a user-specified grid on microscopic fields captured on a monitor (attached to a light microscope with 10 × objective; total magnification 100 ×). Vertical sections and the applied grid made it possible to calculate unbiased stereological estimates, even though anisotropy of cancellous bone exists (Gundersen et al. Citation1988, Overgaard et al. Citation2000).
We defined bone implant contact to be the surface of the implant covered by bone, and it was determined using the linear intercept technique. An average of 490 intersections were counted on 4 sections per implant, thus reducing the variance at the section level to a minimum as compared to the biological variation between individuals (Over-gaard et al. 2000).
We determined bone density in a 200-µm zone immediately adjacent to the implant as percentage of bone by a point-counting technique. 72 random fields (an average of 288 counts) per implant were evaluated.
Statistics
Data are presented as medians with interquartile ranges in brackets. The Wilcoxon signed ranks test was used to test differences between compaction and drilling within Ti implants and HA implants, respectively. Two-tailed p-values below 0.05 were considered significant.
Results
All 8 dogs were fully weight-bearing within 3 days of surgery. 1 dog was excluded, as it was preterm killed after 6 weeks due to sudden onset of limping. Post-mortem, all implants with polyethylene plugs were well-fixed in situ, and there was no apparent reason for the limping in the dog that was excluded. In addition, no clinical signs of infection were present at autopsy.
Mechanical testing
All specimens failed at the bone-implant interface during push-out test. We found no significant differences in energy absorption, ultimate shear strength, or apparent shear stiffness between compaction and drilling, either for Ti implants inserted exact-fit ( and ) or for HA implants inserted press-fit ( and ).
Figure 2. Energy absorption of Ti implants inserted with compaction versus drilling.Paired data from each dog are connected by dashed lines.Solid horizontal lines represent median values.There was no significant difference between compaction and drilling (p = 0.6).
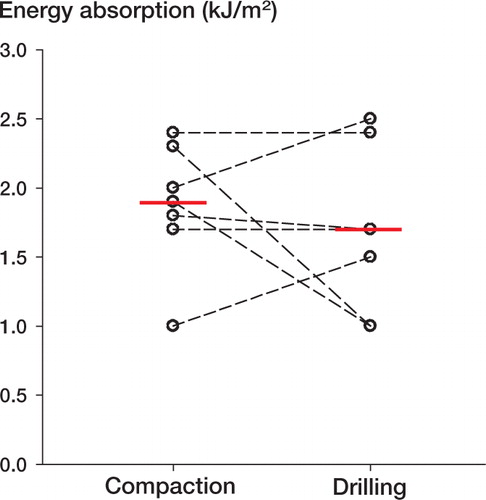
Figure 3. Energy absorption of HA implants inserted with compaction versus drilling.Paired data from each dog are connected by dashed lines.Solid horizontal lines represent median values.There was no significant difference between compaction and drilling (p = 0.8).
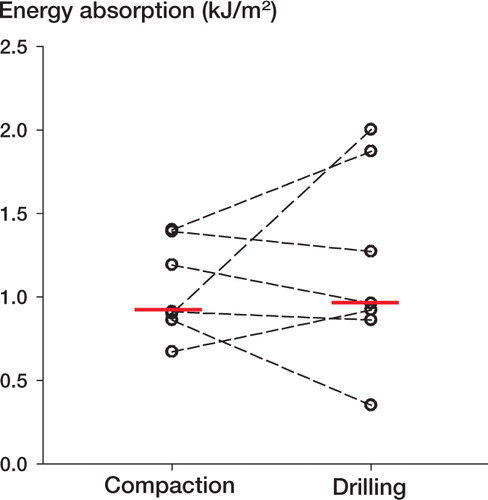
Table 1. Ultimate shear strength (MPa) and apparent shear stiffness (MPa/mm) for Ti implants inserted exactfit into medial condyles, and for HA implants inserted press-fit into lateral condyles. Median values with interquartile ranges are given
Histology
The peri-implant bony tissue in both compacted and drilled specimens consisted of a mixture of non-vital bone, vital woven bone and vital lamellar bone. The non-vital bone consisted of small bony chips or larger pieces of bone with cracking of the lamellar structure and loss of osteocytes. Fibrous tissue was only present around Ti implants (2 of 7) inserted exact-fit with drilling.
Histomorphometry
For Ti implants inserted exact-fit, compacted specimens had significantly more non-vital bone in contact with the implant than drilled specimens. However,for all other tissues in contact with the Tiimplants, there were nosignificant differences between compaction and drilling (). For HA implants, there were no significant differences between compaction and drilling for any of the tissue s in contact with the implants (). For all specimens,the amount of non-vital bone in contact with the implants contributed little to the total amount of bone in contact with the implants.
Table 2. Tissue implant contact expressed as percentage of the implant surface. Ti implants were inserted exact-fit into medial condyles, and HA implants were inserted press-fit into lateral condyles. Median values with interquartile ranges are given
For both Ti implants and HA implants (), there were no differences between compacted and drilled specimens for the peri-implant density of any of the tissues examined.
Table 3. Peri-implant tissue density in a 200-μm periimplant zone. Ti implants were inserted exact-fit into medial condyles, and HA implants were inserted pressfit into lateral condyles. Median values with interquartile ranges are given
Discussion
In this study, we compared the effects of compaction with drilling in two different weight-bearing models. We used the basic implant model with exact-fit insertion of Ti-implants to investigate the isolated effects of compaction versus drilling in conditions mimicking the clinical situation, where areas of an inserted porous coated Ti implant are in close proximity to cancellous bone. After 10 weeks, there were no signs of compromized mechanical implant fixation with compaction, as there were no differences in push-out data between compacted and drilled specimens. This is in agreement with a previous non-weight-bearing study where no difference in fixation of titanium implants was found between compaction and drilling after 9 weeks (Green et al. Citation1999).
Implants inserted press-fit have shown superior fixation in vivo (Søballe et al. Citation1990, Ramamurti et al. Citation1997) and HA-coated cementless joint replacements give superior implant fixation as compared to implants without HA coating (Søballe et al. Citation1993, Kärrholm et al. Citation1994, Nelissen et al. Citation1998, Önsten et al. Citation1998, Regner et al. Citation2000, Toksvig-Larsen et al. Citation2000). Thus, we also compared the effects of compaction versus drilling using an optimal implant model with press-fit insertion of HA-coated implants. For HA implants inserted press-fit, there were no signs of compromized implant fixation after 10 weeks with compaction—as there were no differences in push-out data or histomorphometric data between compacted and drilled specimens. This is in agreement with a previous non-weight-bearing study where no difference in implant fixation between compaction and drilling was found for HA implants after 12 weeks (Vail et al. Citation2000).
Our animal model partly mimicked the clinical conditions of joint replacements since the implants were weight-bearing, and since they were inserted intraarticularly into cancellous bone in the dog, which resembles human cancellous bone (Aerssens et al. Citation1998). However, as for most animal models, we inserted implants of smaller size than those used for total joint replacements in humans. Accordingly, only 1.4 mm of bone, out of a total cavity diameter of 5.6 mm, was enlarged by radial compaction. Even though this degree of bone compaction may seem minor, in previous studies we have demonstrated an initial effect of this compaction procedure (as compared to conventional drilling) on mechanical implant fixation, bone implant contact and peri-implant bone density (Kold et al. Citation2005 b, Citationc). The present study was designed to evaluate whether the compaction technique might jeopardize fixation of weight-bearing implants after remodeling of the compacted bone at the bone implant interface. However, there are methodological problems when using an animal model to examine potentially negative effects of a bone preparation technique on longer-term implant fixation. The dog has great healing capacity compared to humans (Kimmel and Jee Citation1982), and all biocompatible implants that are inserted with exact- or press-fit into the dog femoral condyle are expected to achieve rigid bony fixation after longer-term observation periods. Thus, we had to choose an observation period that was short enough to ensure that a potentially inferior implant fixation would still be present at the time of evaluation. At the same time, however, the observation period should be long enough to allow non-vital bone at the bone implant interface to be resorbed. As the bone remodeling rate in dogs is 2–3 times faster than in humans (Kimmel and Jee Citation1982), and signs of bone remodeling at the bone implant interface have been observed in animals after only 4 weeks (Dhert et al. Citation1998), we chose to compare the effects of compaction versus drilling after 10 weeks in vivo. When comparing histomorphometric data of Ti implants inserted with compaction in the current study with a previous compaction study (Kold et al. Citation2005 c) using a similar weight-bearing model with exact-fit insertion of Ti implants for 4 weeks, it seems that bone resorption occurred within our 10-week observation period: from 4 to 10 weeks the amount of non-vital bone in contact with the implant had declined by a factor of 3, and the amount of woven bone in contact with the implant had doubled. The absence of a statistically significant difference between compaction and drilling in the current study using paired data from 7 dogs does not exclude the possibility that there might be a negative effect of compaction. The study was designed to have a power of 80%, and thus there is a 20% risk that the current study would falsely conclude that no differences exist between compaction and drilling after 10 weeks. However, when considering the distribution of paired data as presented in and , it does not seem likely that there is a relevant negative effect of compaction in the current study.
For both compacted and drilled specimens, the titanium implants were more strongly fixated mechanically, but had less bone implant contact than the HA implants. A mechanically stronger fixation of titanium implants compared with HA implants has been demonstrated previously after 4 weeks of tight press-fit conditions (Søballe et al. Citation1991). However, the current study was not designed to test differences between titanium and HA implants, and therefore statistical tests were not applied to compare the two different implant types. The titanium implants were systematically inserted into the medial femoral condyles, and the HA implants into the lateral femoral condyles. The mechanically stronger fixation of titanium implants may be a result of better initial peri-implant bone quality and quantity, or of more optimal weight-bearing conditions in the medial femoral condyle than in the lateral condyle.
In vitro studies on tibia and femurs from human cadavers have demonstrated that compaction increases the stability of cementless tibial and femoral stems compared to conventional press-fit (Channer et al. Citation1996, Chareancholvanich et al. Citation2002). In vivo, a spring-back effect of compacted bone has been shown (Kold et al. Citation2003a), and compaction has improved early fixation of both non-weight-bearing and weight-bearing titanium porous coated implants inserted with exact-fit (Green et al. Citation1999, Kold et al. Citation2005 c). Furthermore, compaction has improved early in vivo fixation of weight-bearing HA coated implants inserted press-fit (Kold et al. Citation2005 b), and of implants inserted with an initial gap to surrounding cancellous bone (Kold et al. Citation2005πa, Citationd). Thus, substantial experimental research has shown that compaction provides superior implant fixation at early time points when compared with conventional bone removing techniques. An important finding of the current study is that the initial superior implant fixation with compaction does not seem to occur at the expense of the longer-term implant fixation after the non-vital compacted bone at the bone-implant interface has been resorped under weight-bearing conditions. However, the results of this study represent the use of compaction in a clinically relevant, but experimental animal model. Further research in the compaction technique has been advocated, as the smooth tamp instrumentation used for compaction in total hip replacement may increase the risk of an intraoperative femoral fracture (Breusch et al. Citation2001, Kold et al. Citation2003b, Citation2005e). Furthermore, neither a negative nor a positive effect of compaction was found after 10 weeks, and it may be that the compaction technique will not increase longer-term femoral stem survival as compared to the conventional broaching/rasping techniques. Thus, the compaction technique must be compared with conventional bone removal techniques in prospective, randomized clinical trials before the compaction technique can be recommended for general use in total joint replacement.
In conclusion, compared to drilling, our study has not shown inferior implant fixation with compaction after 10 weeks of weight-bearing—either for Ti implants inserted exact-fit or for HA implants inserted press-fit. These results suggest that longer-term implant fixation is not jeopardized as the compacted non-vital bone at the bone implant interface is resorbed.
The authors wish to thank Anette Milton and Jane Pauli for their technical expertise. Biomet Inc. kindly provided the implants. The study was supported financially by the Danish Rheumatism Association and Institute of Experimental Clinical Research, University of Aarhus, Denmark.
- Adrian M J, Roy W E, Karpovich P V. Normal gait of the dog: an electrogoniometric study. Am J Vet Res 1966; 27: 90-5
- Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 1998; 139: 663-70
- Altman D G. Practical statistics for medical research. 8 ed. Chapman and Hall/CRC. 1999; 126
- Baddeley A J, Gundersen H J, Cruz-Orive L M. Estimation of surface area from vertical sections. J Microsc 1986; 142: 259-76
- Breusch S J, Norman T L, Revie I C, Lehner B, Caillouette J T, Schneider U, Blaha J D, Lukoschek M. Cement penetration in the proximal femur does not depend on broach surface finish. Acta Orthop Scand 2001; 72: 29-35
- Channer M A, Glisson R R, Seaber A V, Vail T P. Use of bone compaction in total knee arthroplasty. J Arthroplasty 1996; 11: 743-9
- Chareancholvanich K, Bourgeault C, Schmidt A H, Gustilo R, Lew W. In Vitro Stability of Cemented and Cementless Femoral Stems With Compaction. Clin Orthop 2002, 394: 290-302
- Dhert W J, Verheyen C C, Braak L H, de Wijn J R, Klein C P, de Groot K, Rozing P M. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res 1992; 26: 119-30
- Dhert W J, Thomsen P, Blomgren A K, Esposito M, Ericson L E, Verbout A J. Integration of press-fit implants in cortical bone: a study on interface kinetics. J Biomed Mater Res 1998; 41: 574-83
- Gotfredsen K, Budtz-Jorgensen E, Jensen L N. A method for preparing and staining histological sections containing titanium implants for light microscopy. Stain Technol 1989; 64: 121-7
- Green J R, Nemzek J A, Arnoczky S P, Johnson L L, Balas M S. The effect of bone compaction on early fixation of porous-coated implants. J Arthroplasty 1999; 14: 91-7
- Gundersen H J, Bendtsen T F, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, Sorensen F B, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988; 96: 379-94
- Hofmann A A, Plaster R L, Murdock L E. Subvastus (Southern) approach for primary total knee arthroplasty. Clin Orthop 1991, 269: 70-7
- Kärrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemented, hydroxyapatite-coated, and porous-coated stems with roentgen stereophotogrammetric analysis. J Bone Joint Surg (Am) 1994; 76(11)1692-705
- Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J Radiostereometry of hip prostheses. Review of methodology and clinical results. Clin Orthop 1997, 344: 94-110
- Kienapfel H, Sumner D R, Turner T M, Urban R M, Galante J O. Efficacy of autograft and freeze-dried allograft to enhance fixation of porous coated implants in the presence of interface gaps. J Orthop Res 1992; 10: 423-33
- Kimmel D B, Jee W S. A quantitative histologic study of bone turnover in young adult beagles. Anat Rec 1982; 203: 31-45
- Kold S, Bechtold J E, Ding M, Chareancholvanich K, Rahbek O, Søballe K. Compacted cancellous bone has a spring-back effect. Acta Orthop Scand 2003a; 74: 591-5
- Kold S, Mouzin O, Bourgeault C, Søballe K, Bechtold J E. Femoral fracture risk in hip arthroplasty: smooth versus toothed instruments. Clin Orthop 2003b, 408: 180-8
- Kold S, Rahbek O, Toft M, Ding M, Overgaard S, Søballe K. Bone compaction enhances implant fixation in a canine gap model. J Orthop Res 2005a; 23: 824-30
- Kold S, Rahbek O, Toft M, Overgaard S, Søballe K. Bone compaction enhances fixation of weight-bearing hydroxy-apatite-coated implants. J Arthroplasty accepted 2005b
- Kold S, Rahbek O, Toft M, Overgaard S, Søballe K. Bone compaction enhances fixation of weight-bearing titanium implants. Clin Orthop 2005c, 431: 138-44
- Kold S, Rahbek O, Zippor B, Bechtold J E, Søballe K. Bone compaction enhances fixation of hydroxyapatite coated implants in a canine gap model. J Biomed Mat Res: B 2005d;; 75: 49-55
- Kold S, Bechtold J E, Mouzin O, Bourgeault C, Søballe K. Importance of pre-clinical testing exemplified by femoral fractures in vitro with new bone preparation technique. Clin Biomech 2005e; 20: 77-82
- Mouzin O, Søballe K, Bechtold J E. Loading improves anchorage of hydroxyapatite implants more than titanium implants. J Biomed Mater Res 2001; 58: 61-8
- Nelissen R G, Valstar E R, Rozing P M. The effect of hydroxyapatite on the micromotion of total knee prostheses. A prospective, randomized, double-blind study. J Bone Joint Surg (Am) 1998; 80(11)1665-72
- Önsten I, Nordqvist A, Carlsson A S, Besjakov J, Shott S. Hydroxyapatite augmentation of the porous coating improves fixation of tibial components. A randomised RSA study in 116 patients. J Bone Joint Surg (Br) 1998; 80(3)417-25
- Overgaard S, Søballe K, Gundersen H J. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res 2000; 18: 313-21
- Ramamurti B S, Orr T E, Bragdon C R, Lowenstein J D, Jasty M, Harris W H. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res 1997; 36(2)274-80
- Regner L, Carlsson L, Karrholm J, Herberts P. Tibial component fixation in porous- and hydroxyapatite-coated total knee arthroplasty: a radiostereometric evaluation of migration and inducible displacement after 5 years. J Arthroplasty 2000; 15(6)681-9
- Ryd L, Albrektsson B E, Carlsson L, Dansgard F, Herberts P, Lindstrand A, Regner L, Toksvig-Larsen S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg (Br) 1995; 77(3)377-83
- Søballe K, Hansen E S, Brockstedt-Rasmussen H, Pedersen C M, Bunger C. Hydroxyapatite coating enhances fixation of porous coated implants. A comparison in dogs between press fit and noninterference fit. Acta Orthop Scand 1990; 61(4)299-306
- Søballe K, Hansen E S, Brockstedt-Rasmussen H, Hjortdal V E, Juhl G I, Pedersen C M, Hvid I, Bunger C. Fixation of titanium- and hydroxyapatite coated implants in arthritic osteopenic bone. J Arthroplasty 1991; 6(4)307-16
- Søballe K, Toksvig-Larsen S, Gelineck J, Fruensgaard S, Hansen E S, Ryd L, Lucht U, Bunger C. Migration of hydroxyapatite coated femoral prostheses. A Roentgen Stereophotogrammetric study. J Bone Joint Surg (Br) 1993; 75(5)681-7
- Toksvig-Larsen S, Jorn L P, Ryd L, Lindstrand A. Hydroxy-apatite-enhanced tibial prosthetic fixation. Clin Orthop 2000, 370: 192-200
- Vail T, Channer M, Glisson R. The effect of bone cavity preparation method on implant fixation. Trans Orthop Res 2000; 25: 530
- Wang J S, Tägil M, Aspenberg P. Load-bearing increases new bone formation in impacted and morselized allografts. Clin Orthop 2000, 378: 274-81
