Abstract
Background The progress of radiolucent lines (RLs) around the stems of cementless hip endoprostheses and their implications for implant survival are unclear.
Patients and methods 150 consecutive total hip replacements (THRs) were performed between January and July 1993. The presence and development of RLs and osteolyses were monitored with a standardized special radiographic technique, and the results were compared at 6 and 10 years of follow-up in 95 patients.
Results At 6 years, RLs were detectable in 43 of 95 patients. RLs with an extension of 50% of the respective zone were present in 35 patients, almost all in Gruen zones 1 and 7. 2 patients presented with osteolysis. At 10 years, the RLs were almost identical—both in number and thickness. Only 1 additional osteolytic lesion had developed; no cup osteolyses were detected, and there was no significant polyethylene wear.
Interpretation The RLs detected at 6 years were virtually unchanged at 10 years. As they did not progress, they would not jeopardize implant stability. However, the osteolyses seen in 3 patients emphasize the importance of follow-up examinations.
The survival of hip implants largely depends on the quality of implant anchorage in the surrounding bone. Cemented implants derive their stability from the interdigitation of the cement at the bone surface, while cementless implants are stabilized by the direct contact of the implant with the surrounding bone—and later on also by the ongrowth of newly formed bone tissue to the implant surface (Albrektsson et al. Citation1981, Lintner et al. Citation1986, Citation1988, Zweymüller et al. Citation1988, Brånemark et al. Citation2001). Any abnormality, which elicits the growth of a connective tissue layer between the bone cement and the bone (Goldring et al. Citation1983, Jones and Hungerford Citation1987, Pacheco et al. Citation1988) or between the implant and the bone (Engh et al. Citation1990, Lintner et al. Citation1999) may interfere with the bone-implant unit, so that the implant may loosen.
Gaps between the bone cement and the femoral bone are reflected by radiolucent lines (RLs). These usually begin to develop around the proximal stem, are generally progressive, and confront the surgeon with a considerable challenge, if they are not diagnosed or long left alone. In contrast, around cementless tapered straight stems, welldemarcated RLs (usually small) are often seen and are considered irrelevant for implant survival (Zweymüller and Samek Citation1990, Pieringer et al. Citation2003, Schneider and Knahr Citation2004, Pospischill and Knahr Citation2005).
We routinely use a further developed straight tapered implant (SL-Plus stem) irrespective of the underlying anatomy, the age of the patient or the bone density (Lhotka and Zweymüller Citation2002, Zweymüller Citation2002). Around this implant, radiolucent lines tend to be seen—as around its predecessor, the Alloclassic stem (Dohle et al. Citation2001, Garcia-Cimbrelo et al. Citation2003, Grübl et al. Citation2003, Pieringer et al. Citation2003), which we implanted until 1993.
All studies of radiolucent lines conducted so far have focused on a specific point in time after implantation and thus give no clues as to their fur ther development. Consequently, they have been useless for predicting the prognosis of the implants (Wick und Lester Citation2004). This prompted us to analyze the outcome at 10 years and compare it with the 6-year data, to find out whether radiolucent lines progress and whether their progression predicts the long-term prognosis of the implant. The site of the radiolucent lines and their extension within the Gruen zones was another point of interest. The rate and radiographic appearance of osteolytic lesions were also evaluated.
Patients and methods
Between January and July 1993, 150 consecutive primary THRs were performed at our department using a cementless implant (Plus Orthopedics AG, Rotkreuz, Switzerland) with a tapered straight stem with a rectangular cross-section made of a titanium-aluminum-niobium alloy (Ti6Al7Nb), a self-tapping cementless double-cone (biconical) cup of pure titanium, and a ceramic ball head articulating with a polyethylene inlay (Zweymüller Citation2002). This system was developed by modifying a previous design (Zweymüller and Semlitsch Citation1982, Zweymüller et al. Citation1988) and has been used at our department since early 1993.
In 1998/1999, i.e. at least 5 years after the primary THR, we tried to contact the 150 patients for clinical and radiographic follow-up examinations. Data from 118 patients could be evaluated (). The mean age of the patients available for follow-up was 65 (40–83) years and the mean follow-up time was 6 (5–7) years. The pre-THR diagnoses are listed in . In 2003, the patients were again recalled. 95 individuals from the original 6-year group were available for followup ().
Table 1. Patients at 6-year follow-up (FU) (n = 150) and 10-year follow-up (n = 118)
Table 2. Preoperative diagnoses (n = 118)
The patients underwent both clinical and radiographic follow-up examinations. Harris Hip Score was determined preoperatively and at follow-up. Radiographic follow-up consisted of anterior-poste-rior radiographs of both hips and of anterior-poste-rior and axial radiographs of the replaced hip. Care was taken to image the implants in strictly orthograde views. On anterior-posterior radiographs, the implant holes in the trochanteric region had to be round (). On axial films, the implants were imaged precisely in the frontal plane. Frog-leg positions (Lauenstein position) were omitted because they would not provide more information.
The patients underwent first postoperative radio graphy about 1 week after surgery in all projections except for axial views of the femur, which were first obtained after 6 weeks. These constituted the zero control with which all 6- and 10-year films were compared.
Radiolucent lines (RLs), radiodense lines (RDs) and osteolysis were defined as follows.
RL: A radiolucent zone between the implant and the surrounding bone, usually parallel to the implant surface (). RLs mostly remain constant, without any tendency to progress and reflect a connective tissue layer. They are therefore considered to be a sign of absence of osseointegration (Lintner et al. Citation1986, Citation1988, Engh et al. Citation1990, Brånemark et al. Citation2001).
Figure 1. A. Illustration showing definition of radiolucent line (RL) and radiodense line (RD) around proximal stem. B. Illustration showing definition of osteolysis (longitudinal and focal) around proximal stem.
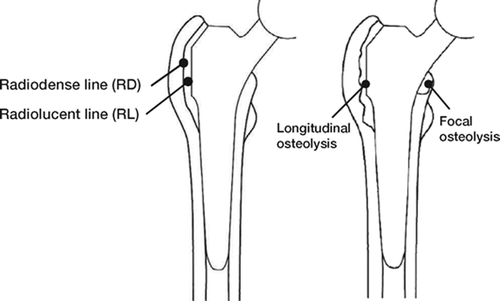
RD: Bony demarcation of RLs on the bone side (), also known as inner corticalis (Mittelmeier Citation1976) or secondary bone ring (Lintner et al. Citation1986). RDs are clearly defined, homogeneous scle rotic zones without irregularities or bone resorption. Like RLs, they usually remain constant without any tendency to progress.
B. Illustration showing definition of osteolysis (longitudinal and focal) around proximal stem.
Osteolysis: Irregularly shaped radiolucent zone along the implant-to-bone interface, irregularly demarcated from the surrounding bone, which may show breaks or resorption (). Osteolyses tend to progress.
RLs were rated twice based on their extension relative to the long axis of the femur: (a) all visible RLs detected were considered, and (b) in accordance with Wick and Lester (Citation2004), only RLs involving 50% or more of the zone affected were considered.
Results
RLs and RDs were mainly present in Gruen zones 1 and 7 at 6 years. In most cases they had a width of no more than 1–2 mm ( and ). At the 10-year follow-up, there was a minimal increase in the number of double contours compared to the 6-year follow-up ().
Figure 2. Radiolucent lines and osteolyses by Gruen zones. Comparison of results at 6–year and 10–year followup (FU).
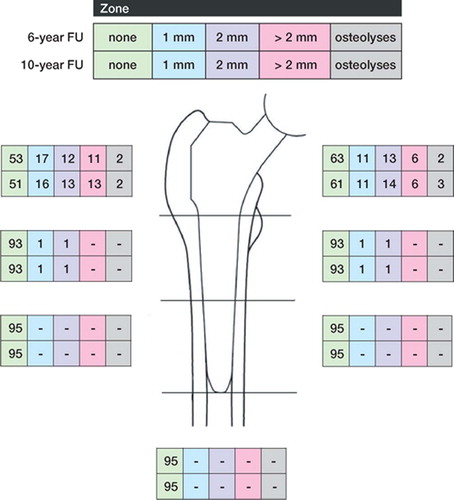
Figure 3. A) 50 year–old woman. Postoperatively. B) 1.5 years postoperatively. There was an RL of nearly 2 mm in zones 1 and 7. The boundary of the RL is a regular formed dense line (RD) corresponding to the preexisting bone stock, and running parallel to the implant surface. Atrophy of the medial cortical bone at zone 7. C) 4.5 years postoperatively. No change of RL and RD compared to B. At the distal end of the lines there is a small area of sclerosis. D) 10 years postoperatively. No change except a slight increase in the sclerosis in zone 7, thus reducing the extent of the RL and RD.
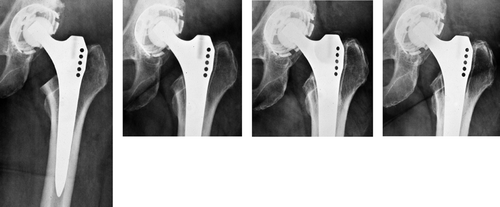
Table 3. Radiolucent lines and radiodense lines at 6-year and 10-year follow-up. Data in parentheses relate to radiolucent lines involving 50% or more of the zones affected (n = 95)
The mean Harris Hip Score (HHS) was 54 (26–88, SD 13) preoperatively, 97 (73–100, SD 5.4) at the 6-year follow-up, and 95 (32–100, SD 9.2) at 10-year follow-up. There was no correlation between the HHS and the presence or extent of RLs.
2 patients presented with alterations at the 6year follow-up, which were clearly distinct from RLs and RDs. Located in zones 1 and 7, they were irregularly demarcated and showed a tendency to affect the peri-implant bone in terms of osteolysis (). One of them was longitudinal in appearance, and the other focal.
Figure 4. A) 65 year–old woman. Postoperatively. B) 3 years postoperatively: RL showing different diameters in zone 1; RL of 2 mm in zone 7. Inhomogeneous structure of RD in zone 1. C) 6 years postoperatively: increasing RL, in both width and distal extension, in zones 1 and 7. D) 10 years postoperatively: significantly increased dia meter of RL and widening also towards distally in zones 1 and 7. The corresponding RD showed an irregular density and distance to the implant surface.
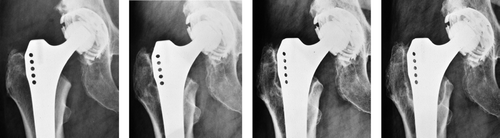
At 10 years, these 2 osteolyses were still present and another longitudinal osteolytic lesion had developed (). They had not yet caused any instability of the stems. Patients with osteolysis did not show any signs suggestive of radiographic changes clinically and in terms of the HHS (the score being 100 for all of them). The corresponding cups were normal. There were no osteolytic lesions in the peri-cup bone. The amount of wear from the polyethylene inlays was practically nil in 1 case, less than 1 mm in another case and 1.5 mm in the third case.
Early postoperative complications within the first 8 weeks included 3 dislocations. 2 patients were revised because of trauma during the follow-up period (). There were no revisions because of infection or aseptic loosening.
Discussion
This study was intended to allow us to establish the rate of radiolucent lines around the stepless (SL) stems we use, and to show whether these remain constant or progress. The extension of RLs and the zones affected by them, as well as their differentiation from osteolytic lesions, were other points of interest. Based on these data, we wanted to find out whether a predictable long-term outcome can be achieved with these implants.
The consistency of RLs and RDs between the 6-year and the 10-year follow-ups can be interpreted as signaling a positive prognosis for implant survival. It argues against a pathological process but simply reflects the absence of bone-to-implant contacts in the areas affected. In all other Gruen zones, the implants were firmly anchored in terms of osseointegration as defined by Albrektsson et al. (Citation1981) and by Brånemark et al. (Citation2001). Apparently, RLs and double contours do not jeopardize stem stability and are unlikely to do so in later years, provided PE wear does not reach significant levels over time, which exceed the patient's tolerance limit and thus initiate an osteolytic process (Dumbleton et al. Citation2002).
Based on 10- and 15-year follow-up data, others have also reported RLs in about the same proportion of cases (Garcia-Cimbrelo et al. Citation2003, Pieringer et al. Citation2003, Pospischil and Knahr Citation2005). However, their data were derived from retrospective single-date follow-up studies, which at best give instantaneous information, but do not provide any clues as to the time at which the alterations occur, and as to their progression or consistency.
The comparative study of Wick and Lester (Citation2004) of the Alloclassic stem and the SL-Plus stem, in which they reported varying percentages of RLs, has a similar problem. Although explicitly described as a follow-up study, the authors were unable to provide any information on the nature of the RLs, i.e. whether or not they were progressive, but they insisted that the presence of RLs was predictive of the outcome of only the SL-Plus stem generation. In contrast, our study has shown that RLs represent a steady state in the overwhelming majority of cases, without any tendency of progression so that they do not compromise the outcome. In an earlier publication, the frequency, position and width of RLs was evaluated around Alloclassic stems at a mean follow-up time of 2 years (Zweymüller and Samek Citation1990). The percentages found with the earlier stem design were almost identical to those seen with the stem used today.
The mechanisms underlying the development of peri-implant RLs and RDs, particularly in zones 1 and 7, in what is in fact a relatively large percentage of cases, are still poorly understood. One likely explanation is that more cancellous bone is removed by rasping the bone at the level of the neck resection line and just distal to it than is replaced by the implant. As a result, a gap is present between the implant and the bone from the time of implant placement. This gap is apparently not obliterated by newly formed bone in the zones affected, although the peri-implant bone is still adequately vascularized by endosteal vessels because of the sparing preparation of the medullary canal using rectangular rasps (Lintner et al. Citation1988, Lester and Campbell Citation1996). This assumption is supported by the relatively early radiographic detectability of the alterations after THR, which are manifest at about 1 year. Köster et al. (Citation1998) confirmed this time course.
Micromotions of the end of the proximal stem can safely be ruled out as a factor in the development of RLs, in view of the thickness of the metal and the small extension of the RLs distally to zones 1 and 7. By contrast, relative motions of the femur on the rigid metal at the level of the trochanter caused by the muscle pull on the rigid metal may well be a factor underlying the development of RLs in zone 1. This would not explain RLs in zone 7, however.
Alterations compatible with our definition of osteolysis are a different matter. Their hallmarks are an irregular demarcation and a tendency to expand in width and distally in the peri-implant bone (). The cortical bone may also undergo lysis. An analysis of the cups of affected patients showed full stability without any signs of osteolysis. The PE wear amounted to just under 1 mm in one patient and to over 1 mm in another, however. That there may have been a specific individual response to particulate PE wear in these cases is, however, speculative.
Our comparative radiographic follow-up study underscores the need for a differential approach to peri-implant lines and critical interpretation of them. Radiolucent lines which do not progress over time carry a good long-term prognosis.
No competing interests declared.
Contributions of authors
KZ: performed many of the operations and wrote the paper. MS: also involved in operations, analyzed the radiographs and reviewed the patients’ charts. US: analyzed radiographs and reviewed the patients’ charts.
- Albrektsson T, Bränemark P I, Hansson H A, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 1981; 52(2)155–70
- Bränemark R, Bränemark P-I, Rydevik B, Myers R R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev 2001; 38(2)175–81
- Dohle J, Becker W, Braun M. Radiological analysis of osseointegration after implantation of the Zweymüller-Alloclassic total hip system. Z Orthop Ihre Grenzgeb 2001; 139(6)517–24
- Dumbleton J H, Manley M T, Edidin A A. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 2002; 17(5)649–61
- Engh C A, Massin P, Suthers K E. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop 1990, 257: 107–28
- Garcia-Cimbrelo E, Cruz-Pardos A, Madero R, Ortega-Andreu M. Total hip arthroplasty with use of the cementless Zweymüller Alloclassic system. A ten to thirteenyear follow-up study. J Bone Joint Surg (Am) 2003; 85(2)296–303
- Goldring S R, Schiller A L, Roelke M, Rourke C M, ÓNeill D A, Harris W H. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg (Am) 1983; 65(5)575–84
- Grübl A, Csepan R, Delaunay C, Gördes W, Kaider A, Parzer R, Zenz P, Gottsauner-Wolf F. Six to ten year results of use of the Alloclassic hip prosthesis. A multicentre survival analysis. Z Orthop Ihre Grenzgeb 2003; 141: 303–8
- Jones L C, Hungerford D S. Cement disease. Clin Orthop 1987, 225: 192–206
- Köster G, Leib S, Willert H G. Noncemented hip replacement using a conical screw-in cup and a straight press-fit stem. A six to eight-year clinical and radiological followup study. Hip International 1998; 8: 208–18
- Lester D K, Campbell P. 100-year-old patient with pressfit prosthesis: a postmortem retrieval study. Am J Orthop 1996; 25(1)30–4
- Lhotka Ch, Zweymüller K. Cementless hip prostheses in the elderly. K A Zweymüller. Huber, Bern 2002; 89–97, In: 20 years of Zweymüller hip endoprosthesis
- Lintner F, Zweymüller K, Brand G. Tissue reactions to titanium endoprostheses. Autopsy studies in four cases. J Arthroplasty 1986; 1(3)183–95
- Lintner F, Zweymüller K, Böhm G, Brand G. Reactions of surrounding tissue to the cementless hip implant Ti-6Al-4V after an implantation period of several years. Autopsy studies in three cases. Arch Orthop Trauma Surg 1988; 107(6)357–63
- Lintner F, Huber M, Böhm G, Attems J, Lhotka C. The significance of the human body's response to particles released from prosthetic implants. K Zweymüller. Huber, Bern. 1999: 55–72, In: Die Metall-Metall-Paarung SIKOMET(r), Band 2
- Mittelmeier H. Anchoring hip endoprosthesis without bone cement. M Schaldach, H Hohmann. Springer, Berlin. 1976: 387–402, In: Advances in artificial hip and knee joint technology
- Pacheco V, Shelley P, Wroblewski B M. Mechanical loosening of the stem in Charnley arthroplasties. Identification of the “at risk” factors. J Bone Joint Surg (Br) 1988; 70(4)596–9
- Pieringer H, Auersperg V, Grieβler W, Böhler N. Longterm results with the cementless Alloclassic brand hip arthroplasty system. J Arthroplasty 2003; 18(3)321–8
- Pospischill M, Knahr K. Cementless total hip arthroplasty using a threaded cup and a rectangular tapered stem. Follow-up for ten to 17 years. J Bone Joint Surg (Br) 2005; 87(9)1210–5
- Schneider W, Knahr K. Total hip replacement in younger patients. Survival rate after avascular necrosis of the femoral head. Acta Orthop Scand 2004; 75(2)142–6
- Wick M, Lester D K. Radiological changes in second and third-generation Zweymüller stems. J Bone Joint Surg (Br) 2004; 86(8)1108–14
- Zweymüller K. 20 years of cementless hip prostheses. K A Zweymüller. Huber, Bern 2002; 11–23, In: 20 years of Zweymüller hip endoprosthesis
- Zweymüller K, Samek V. Radiologische Grundphänomene des Titanium-Geradschaftes. K Zweymüller. Huber, Bern. 1990: 23–34, In: 10 Jahre Zweymül-ler-Hüftendoprothese
- Zweymüller K, Semlitsch M. Concept and material properties of a cementless hip prosthesis system with Al2O3 ceramic ball heads and wrought Ti-6Al-4V stems. Arch Orthop Trauma Surg 1982; 100: 229–36
- Zweymüller K A, Lintner F K, Semlitsch M F. Biologic fixation of a press-fit titanium hip joint endoprosthesis. Clin Orthop 1988; 235: 195–206