Abstract
Background Periosteal transplantation is commonly used for the treatment of articular cartilage defects. However, the cellular origin of the regenerated tissue after periosteal transplantation has not been well defined. The objective of this study was to investigate the cellular origin of the regenerated tissue after periosteal transplantation.
Method Free periosteum was harvested from the tibia of 10-week-old adolescent enhanced green fluorescent protein (GFP-) expressing transgenic Sprague Dawley (SD) rats and was transplanted to full-thickness articular cartilage defects of the patellar groove in normal 10-week-old adolescent SD rats. The periosteum was sutured to the defect with the cambium layer facing the joint cavity. 8 SD rats were killed at 4 weeks and 8 SD rats were killed at 8 weeks after surgery. The repaired tissue was assessed histologically and histochemically. GFP-positive cells derived from the donor periosteum could easily be detected in the repaired tissue by use of a fluorescent microscope.
Results At both 4 and 8 weeks after transplantation, the entire area of the defects had been repaired, with the regenerated tissue being well stained histologically with safranin-O. Most cells in the whole area of the regenerated tissue were GFP-positive, indicating that very few of the cells were GFP-negative cells originating from the recipient rats.
Interpretation This experiment demonstrates that most cells in regenerated tissue after periosteal transplantation using adolescent animals do not originate from recipient cells but from the periosteal cells of the donor.
▪
In recent years, transplantation of the periosteum has been performed for the treatment of cartilage injury (Niedermann et al. Citation1985, Hoikka et al. Citation1990, Lorentzon et al. Citation1998, Alfredsson and Lorentzon Citation1999, Alfredson et al. Citation1999). Salter et al. (Citation1980), Rubak et al. (Citation1982a, b) and O'Driscoll et al. (Citation1988) reported that autogenous periosteal grafts have the potential to resurface full-thickness cartilage defects with cartilaginous tissue, whereas Brittberg et al. (Citation1996) and Driesang and Hunziker (Citation2000) have reported poor results from transplantation of the periosteum in a rabbit model and a goat model, respectively. Thus, the efficacy of periosteal transplantation for cartilage defects is controversial.
Despite the existence of many clinical reports and more basic reports on periosteal transplantation, the cellular origin of the regenerated tissue after periosteal transplantation has not been well defined. The objective of this study was to investigate the cellular origin of regenerated cartilage-like tissue after transplantation of allogeneic periosteal grafts using rats expressing green fluorescent protein (GFP).
Animals and methods
Experimental rats were kept in the research facilities for laboratory animal science at our university. The research protocol for this experiment was approved by the ethical committee of Hiroshima University.
We used 8 10-week-old GFP rats and 16 normal Sprague Dawley (SD) rats. The GFP rats were purchased from Japan SLC Co. (Hamamatsu, Japan). The surgical procedures for rats were carried out under general anesthesia induced by an intraperitoneal injection of sodium pentobarbital. The patella was dislocated laterally through a medial parapatellar incision, to expose the patellar groove. A full-thickness transverse osteochondral defect was created, which measured 2 mm from the proximal side to the distal side and traversed the entire width of the patella groove. We chose the patellar groove as the repair site because it was technically easy to make an experimental osteochondral defect uniformly. The depth of this defect was extended to about 1 mm below the surface of the adjacent normal cartilage in the middle of the patellar groove with a rongeur. The thickness of articular cartilage in this area at surgery was about 0.3 mm. Free periosteum (2.5 × 5 mm) was harvested from the medial aspects of the proximal tibia of the GFP rats. When harvesting the periosteum, extreme care was taken not to damage the cambium layer of the periosteum (). The harvested periosteum was transplanted to the full-thickness articular cartilage defect of the patellar groove in the SD rats. Each corner of the periosteum was sutured with 8-0 nylon (Bear Surgical Sutures, Chiba, Japan) to the defect, with the cambium layer facing the joint cavity.
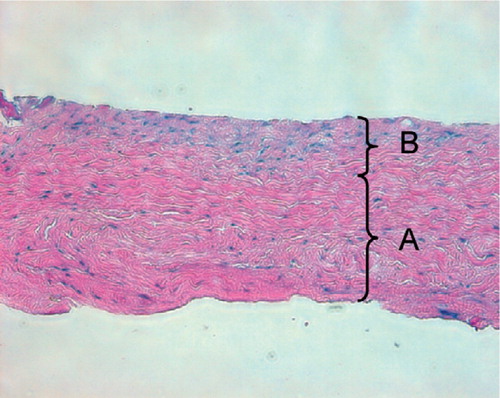
Figure 1. Typical microscopic appearance of the harvested periosteum. The periosteum contains two distinct layers: (A) a thick, outer fibrous layer, and (B) a thin, inner cambium layer which is adjacent to the bone (hematoxylin and eosin, × 200).
Postoperatively, the SD rats were allowed to move freely within the cage. The immunosuppressive agent tacrolimus (Fujisawa Phamaceutical Co., Osaka, Japan) was administered subcutaneously at a dose of 1mg/kg/day for 14 days to minimize any immune reaction. 16 SD rats were killed at either 4 weeks (8 rats) or 8 weeks after surgery by administering an overdose of thiopental. The operated knee was excised and all neighboring tissue was carefully cleaned up. The distal femur was cut and the repaired tissue in the defect of the patellar groove was assessed macroscopically. The specimens were fixed in 4% paraformaldehyde and decalcified in EDTA. They were embedded in plastic legin (Technovit 8100, Okenshoji Co., Tokyo, Japan), cut into 5-μm sections and examined histologically using safranin-O fast green staining. A fluorescent microscope (Leica Microsystems, Germany) was used to detect the GFP-positive cells derived from the donor periosteum in the repaired tissues (i.e. the fluorescence of enhanced GFP (wavelength 508 nm) was evaluated on unstained slices using a 489 nm wavelength excitation ray).
Results
2 rats died from anesthetic complications. At 4 weeks and at 8 weeks after surgery, 4 animals (2 at each time point) sustained a dislocation of the patella while moving freely in the cage. These 6 rats were excluded from the experiment. Thus, we could evaluate 5 rats at 4 weeks and 5 rats at 8 weeks after surgery.
Macroscopic examination ()
There were no contractures and no free bodies, and there was no limited range of motion and no osteophyte formation in the operated knee joints at either 4 or 8 weeks after surgery.
Figure 2. Typical macroscopic appearance of the regenerated tissue in the patellar grooves at 4 weeks (A) and 8 weeks (B) after surgery. A. The regenerated tissue appears slightly thicker than the surrounding normal articular cartilage.The regenerated cartilage-like tissue is opaque-white. B. The regenerated cartilage-like tissue appears translucent.The defect heals with smooth tissue, which is well integrated into the surrounding normal cartilage.
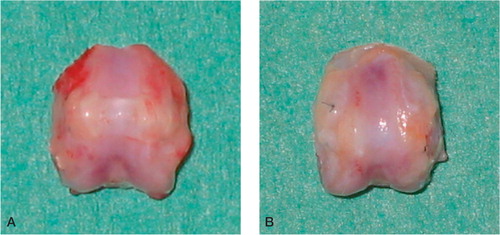
At 4 weeks after surgery, the regenerated tissue in the defects of all five rats was opaque-white, and was slightly more hypertrophic than neighboring normal cartilage in all knee joints. The newly formed tissue of all five rats had filled the defects completely. The borderlines between the new regenerated tissues and surrounding original cartilage were apparent. At 8 weeks, the newly formed tissue had filled the defects completely in 4 rats and partially in one rat. The defects which were filled completely were repaired with the translucent and smooth regenerated tissue, which resembled normal articular cartilage in all knee joints. The margins of the regenerated tissues were well integrated into the normal surrounding articular cartilage.
Histological examination ( and )
At 4 weeks after surgery, we could not detect the fibrous layer and cambium layer of the periosteum in any specimen. All of the defects were filled with regenerated smooth tissue, which was thicker than the surrounding articular cartilage. The regenerated tissue slightly protruded to the level of the surrounding articular cartilage. In the deep layer of the regenerated tissue, many round or oval chondrocyte-like cells were surrounded by a matrix well stained by safranin-O, indicating rich production of glycosaminoglycans. In the super-ficial layer of the regenerated tissue, however, many fibroblast-like cells were found. At 8 weeks after surgery, the defects which were filled completely by the regenerated tissue were well stained by safranin-O. This was of the same thickness as the tissue at 4 weeks, manifesting good integration with surrounding articular cartilage. The level of the regenerated tissue was almost the same as that of the surrounding articular cartilage. Chondrocyte-like cells surrounded by a matrix that was well stained by safranin-O were detected in the deep layer of the regenerated tissue, like those at 4 weeks.
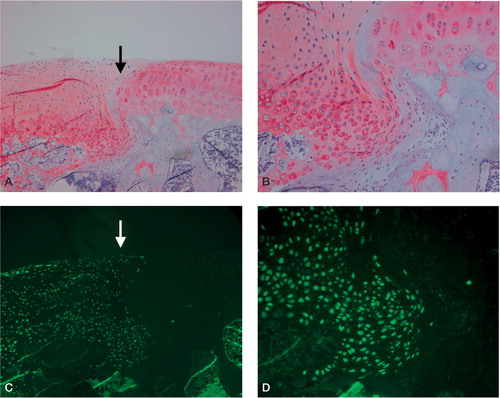
Figure 3. Typical light microscopic appearance (A, B) and fluorescent microscopic appearance (C, D) at 4 weeks after surgery.The arrow indicates the borderline between the repaired tissue and normal cartilage. The left side is repaired tissue. A. The defect is filled with the regenerated tissue that has been well stained by safranin-O.The regenerated cartilage-like tissue reaches over the level of the surrounding articular cartilage (safranin-O, × 100). B. The deep layer of the regenerated tissue has chondrocyte-like cells that are surrounded by a well-stained matrix, and the superficial layer of the regenerated tissue contains fibroblast-like cells.The regenerated cartilage-like tissue integrates well with the surrounding tissue (safranin-O, × 200). C. and D.Most cells in the regenerated tissue are GFP-positive cells derived from the donor periosteum in the superficial, middle, and deep region of the defect (C: × 100, D: × 200).
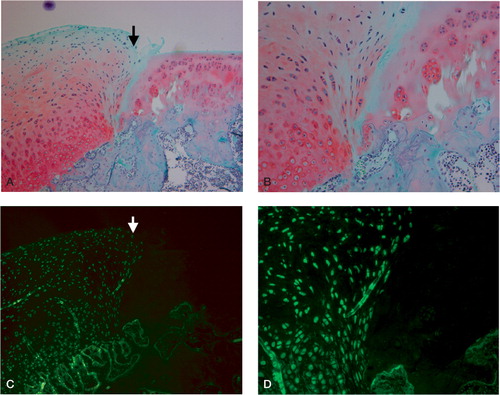
Figure 4. Typical light microscopic appearance (A, B) and fluorescent microscopic appearance (C, D) at 8 weeks after surgery.The arrow indicates the borderline between the repaired tissue and normal cartilage.The left side is repaired cartilage. A. The defect is repaired with regenerated cartilage-like tissue which integrates well with the surrounding normal cartilage (safranin-O, × 100). B. The deep layer of the regenerated tissue also has chondrocyte-like cells that are surrounded by a well-stained matrix (safranin-O, × 200). C. and D.Most cells in the regenerated tissue are GFP-positive cells derived from the donor periosteum in the superficial, middle, and deep region of the defect (C: × 100, D: × 200).
No tidemark was observed in the newly formed cartilage in any specimen, either at 4 weeks or 8 weeks after surgery. At both time points after surgery, the regenerated tissue showed good integration with the subchondral bone-plate. The level of the subchondral bone-plate underneath the regenerated cartilage was seen below the level of the surrounding subchondral bone-plate in all specimens, at both 4 weeks and 8 weeks after surgery. No specimen showed any immunological reaction at either time point.
Fluorescent microscopic examination ()
At both 4 and 8 weeks after transplantation, most cells in the whole area of the regenerated tissue in all specimens were GFP-positive, including chondrocyte-like cells and fibroblast-like cells, indicating that they were derived from the donor periosteal cells. However, we could not detect any GFPpositive cells in the subchondral bone area below the regenerated tissue.
Discussion
In this study, free periosteum was harvested from the tibia of GFP rats and was transplanted to fullthickness articular cartilage defects of the patellar groove in normal SD rats. We found that most of the cells in the whole area of the regenerated tissue which were well stained by safranin-O after both 4 and 8 weeks after surgery were the GFP-positive cells, indicating that very few cells originated from the recipient rats.
Zarnett et al. (Citation1987) reported on the cellular origin of regenerated hyaline-like cartilage after periosteal transplantation. They labeled periosteal grafts with 3H-thymidine before autotransplantation and assessed the cellular origin of the regenerated tissue using autoradiography. Thymidine-labeled cells were seen throughout the entire regenerated tissue, suggesting that the regenerated hyaline-like cartilage in the defects originated from the periosteum. However, these authors evaluated the regenerated tissue for only 3 weeks after periosteal transplantation. Zarnett et al. (Citation1989) reported a second experiment with periosteal allografts transplanted from males into females, and karyotyping of the regenerated tissue. They concluded that the cellular origin of the regenerated tissue was both from the periosteal allograft and the pluripotential mesenchymal cells in the subchondral bone tissue. However, once again, they evaluated the cellular origin of the regenerated tissue for only 3 weeks after the periosteal graft. Also, only a limited number of cells in the regenerated tissue could be evaluated, and the procedure for evaluating the karyotype is complicated and technically demanding, although their technique was very skilled.
One advantage of our study is that our experiment is simpler than past experiments which used autoradiography and karyotyping. GFP-positive cells can be detected in the regenerated tissue without any staining or other procedures, which makes it possible to reduce false-positive or false-negative phenomena when detecting the transplanted periosteal cells. Another advantage of our study is the possibility of assessing the cellular origin for longer time periods after periosteal transplantation, and we have been able to demonstrate that even at 8 weeks after surgery, the origin of cells in the regenerated tissue is the periosteal cells of the donor. This is new information.
Of course, our experimental procedure has some limitations. We could not assess the cellular origin in the regenerated tissues quantitatively. A quantitative assessment would be necessary to evaluate accurately what percentage of cells in the regenerated tissue is derived from the donor periosteum. We believe that using GFP rats as the recipient animal will be necessary to evaluate the contribution of the recipient cells in the subchondral bone to the regenerated tissue. Another limitation is that we could only evaluate the cellular origin of the repaired tissue after periosteal transplantation for up to 8 weeks. Up to 8 weeks, the fluorescence of GFP rats was strong enough to permit detection of the GFP-positive cells without any false-negative phenomenon. After this, the GFP fluorescence gradually weakened, and our pilot study revealed that no GFP fluorescence was visible 6 months after transplantation.
Brittberg et al. (Citation1996) and Driesang and Hunziker (Citation2000) reported poor results from transplantation of the periosteum in a rabbit model and a goat model, respectively. Brittberg et al. (Citation1996) reported that in most successful experiments with periosteal grafting, the animals were adolescent and the periosteum formed significantly less regenerated cartilage-like tissue when adult animals were used, because the number of mesenchymal cells which have chondrogenic potential in the periosteum and bone marrow decreases steadily with age. In our experiment, because we evaluated the cellular origin of the regenerated tissue using only adolescent rats, there is a possibility that the repaired tissue in young animals is potentially better than in older animals. Further studies comparing the results between adolescent and adult animals will be necessary to determine whether this is the case.
Driesang and Hunziker (Citation2000) reported that the patellar groove was most likely to experience delamination or slippage of the periosteal grafts. In our work, however, we could not find any delamination or slippage of the grafts.
In conclusion, we have demonstrated that, even at 8 weeks after transplantation, the origin of most cells in the regenerated tissue after periosteal transplantation using adolescent animals is not the recipient cells, but the donor periosteal cells. Thus, creation of the most favorable conditions for the grafted periosteum is important in order to facilitate successful cartilage repair.
No competing interests declared.
- Alfredson H, Lorentzon R. Superior results with continuous passive motion compared to active motion after periosteal transplantation: A retrospective study of human patella cartilage defect treatment. Knee Surg Sports Traumatol Arthrosc 1999; 7: 232–8
- Alfredson H, Thorsen K, Lorentzon R. Treatment of tear of the anterior cruciate ligament combined with localized deep cartilage defects in the knee with ligament recon-struction and autologous periosteum transplantation. Knee Surg Sports Traumatol Arthrosc 1999; 7: 69–74
- Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop 1996; 326: 270–83
- Driesang I, Hunziker E. Delamination rates of tissue flaps used in articular cartilage repair. J Orthop Res 2000; 18: 909–11
- Hoikka V E J, Jaroma H J, Ristila V A. Reconstruction of the patellar articulation with periosteal grafts: 4-year followup of 13 cases. Acta Orthop Scand 1990; 61: 36–9
- Lorentzon R, Alfredson H, Hildingsson C. Treatment of deep cartilage defects of the patella with periosteal transplantation. Knee Surg Sports Traumatol Arthrosc 1998; 6: 202–8
- Niedermann B, Boe S, Lauritz J. Glued periosteal grafts in the knee. Acta Orthop Scand 1985; 56: 457–60
- O’Driscoll S W, Keely F W, Salter R B. Durability of regenerated articular cartilage produced by free autogenous periopsteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive amotion: A followup report at one year. J Bone Joint Surg (Am) 1988; 70: 595–606
- Rubak J M. Reconstruction of articular cartilage defects with free periosteal grafts: An experimental study. Acta Orthop Scand 1982; 53: 175–80
- Rubak J M, Poussa M, Ristila V. Chondrogenesis in repair of articular cartilage defects by free periosteal grafts in rabbits. Acta Ortop Scand 1982a; 53: 181–6
- Rubak J M, Poussa M, Ristila V. Effects of joint motion on repair articular cartilage with free periosteal grafts. Acta Orthop Scand 1982b; 53: 187–91
- Salter R B, Simmonds D F, Malcolm B W. The biological effect of continuous passive motion on the healing of full-thickness defects in the articular cartilage. J Bone Joint Surg (Am) 1980; 62: 1232–51
- Zarnett R, Salter R B. Periosteal neochondrogenesis for biologically resurfacing joints: Its cellular origin. Can J Surg 1989; 32: 171–4
- Zarnett R, Delaney J P. O’Driscoll S W. The cellular origin of neochondrogenesis in majopr full thickness defects of a joint surface treated by autogeneous periosteal grafts and subjected to continuous passive motion. Clin Orthop 1987; 222: 267–74