Abstract
Background The role of glucocortiocid injection therapy in spontaneous tendon rupture is controversial. We hypothesized that glucocorticoids suppress proteoglycan production in tendon and studied the in vitro effects of dexamethasone and triamcinolone on proteoglycan production by cultured human tenocytes.
Material and methods We obtained primary cultures of human tenocytes from explants of healthy human patellar tendon. The human tenocytes were treated with 1 μM dexamethasone or 1 μM triamcinolone. The amount of proteoglycan production was measured by 35S-sulfate incorporation assay and compared with control cultures. The reversibility of the effect of dexamethasone by co-incubation with 10 ng platelet-derived growth factor (PDGFBB) was also tested.
Results Treatment with 1 μM triamcinolone reduced the amount of 35S-sulfate incorporation to 80% of control cultures (p = 0.007), whereas 1 μM dexamethasone reduced it to 72% (p = 0.01). Co-incubation of 10 ng/mL PDGFBB with 1 μM dexamethasone returned the 35S-sulfate incorporation to a level thatwas significantly higher than for dexamethasone treatment alone (108%; p = 0.01).
Interpretation Glucocorticoids suppressed proteoglycan production in cultured human tenocytes. The suppression by dexamethasone was reversed by simultaneous addition of PDGFBB. Suppressed proteoglycan production may affect the viscoelastic properties of tendon and increase the risk of spontaneous rupture.
▪
Glucocorticoid injection therapy is widely used in the treatment of many inflammatory conditions and musculoskeletal soft tissue disorders, especially tendonitis, paratendinitis, bursitis and other acute or chronic tendinopathies (Leadbetter Citation1990, Benson and Ptaszek Citation1992, Read and Motto Citation1992, Nelson et al. Citation1995). Glucocorticoids are effective anti-inflammatory agents and give rapid clinical response. However, there have been many case reports of spontaneous tendon rupture, both after local glucocorticoid injections (Chechick et al. Citation1982, Kleinman and Gross Citation1983, Stannard and Bucknell Citation1993, Smith et al. Citation1999) and with systemic glucocorticoid therapy (Kotnis et al. Citation1999). Previous studies on histological and mechanical changes after glucocorticoid injections using in vivo animal models have produced conflicting results (Oxlund et al. Citation1981, Kapetanos Citation1982, McWhorter et al. Citation1991, Wiggins et al. Citation1995, Campbell et al. Citation1996, Tatari et al. Citation2001). On the other hand, there is little information on the effect of glucocorticoids on tendon at the cellular level. The role of glucocorticoids in tendon rupture remains controversial.
It is well known that the effects of glucocorticoids differ significantly with cell type, and may vary with the growth state and other associated factors (Oikarinen et al. Citation1991). Glucocorticoids block the growth of hepatocytes (Ledda-Columbano et al. Citation1994), but induce cell growth and proliferation in fibroblasts (Delany and Brinckerhoff Citation1992). Changes observed may differ when different animal species are used. In order to obtain information relevant to tendon rupture in humans, it is imperative that the effect of glucocorticoids should be tested using human tendons. Unfortunately, there have been very few studies on human tendons.
We have previously investigated the effects of glucocorticoids on cultured human tenocytes. We found that both dexamethasone and triamcinolone significantly reduce cell viability, suppress cell proliferation, and reduce collagen synthesis in cultured human tenocytes (Wong et al. Citation2003, Citation2004). Simultaneous administration of platelet-derived growth factor (PDGFBB) reversed the effects of 1 μM dexamethasone on cultured human tenocytes (Wong et al. Citation2003). However, it was unclear whether glucocorticoids have any effect on proteoglycan production in human tenocytes. We hypothesized that glucocorticoids also suppress proteoglycan synthesis in human tenocytes. We studied the in vitro effects of dexamethasone and triamcinolone on proteoglycan synthesis in human tenocytes using 35S-sulfate labeling in a human tenocyte cell culture system. We also tested whether PDGFBB could reverse the effect of dexamethasone on proteoglycan synthesis.
Material and methods
Reagents and culture medium
Dexamethasone sodium phosphate and triamcinolone acetonide were obtained from Sigma Chemical Co. (St. Louis, MO) for use in cell culture. Dulbecco's Modification of Eagle's Medium, penicillin-streptomycin, and fetal calf serum were obtained from Gibco Laboratories (Grand Island, NY). Recombinant human PDGFBB was purchased from R & D Systems (Minneapolis, MN).
Commercially available heat-inactivated fetal calf serum was treated with dextran-coated charcoal at 4°C for 12 h prior to use, in order to minimize the effects of endogenous steroids in the serum. Phenol red-free Dulbecco's Modification of Eagle's Medium supplemented with 10% charcoal-treated fetal calf serum was used in all experiments (abbreviated as DMEM).
Human tenocyte cell culture
The research protocol was approved by the Human Research Ethics Committee of our institution. With informed consent, we harvested tendon tissue blocks (2 × 2 × 3 mm) from healthy patellar tendon during anterior cruciate ligament reconstructions with bone-patellar tendon-bone autograft. These were used as explants for primary cell cultures. The culture method followed our previous modification of Chard's method (Chan et al. Citation1997, Chard et al. Citation1987). The tissue was rinsed twice in phosphate-buffered saline (PBS) containing 1% antibiotic mixture (100 U/mL penicillin and 100 μg/mL streptomycin), cut into 1 mm3 pieces under sterile conditions, and then digested with a mixture of 1 mM 0.25% trypsin (w/v) and EDTA for 5 min. The digested tissue blocks were transferred to a 35-mm culture dish with DMEM, and incubated in an atmosphere of 5% CO2, 95% air at 37°C. The culture medium was changed twice a week. Tendon fibroblasts from explants were trypsinized with trypsin-EDTA solution and subcultured onto 75-cm2 culture flasks at a seeding density of 105 cells per flask under the same culture conditions. Cells from the second and third passages were used for the experiments.
Proteoglycan production
The amount of proteoglycan produced by the human tenocytes was measured by 35S-sulfate labeling (Yanagishita et al. Citation1987, Vogel and Hernandez Citation1992). Cultured human tenocytes were seeded onto 24-well culture plates at a density of 2 × 104 cells per well and incubated for 6 days until a confluent monolayer was obtained. The cells were treated with either 1 μM triamcinolone, 1 μM dexamethasone, 10 ng PDGFBB, or 1 μM dexamethasone together with 10 ng PDGFBB for 48 h. DMEM alone was added to the control well. 35S-sulfate (10 μCi/mL) (Radiochemical Center, Amersham, UK) was added to each well in the last 24 h. The culture medium was then removed and the cells were lysed with 0.25N NaOH. Cell debris and extracellular matrix were scraped off with a cell scraper. The homogenate was then dialyzed in PBS using dialysis tubing (Arthur H. Thomas Co. PA). Radioactivity was measured with a liquid scintillation counter (Packard Instrument Company, Meriden, CT). For each set of data, three sets of cells were used for radioactivity measurements and three simultaneous sets were reserved for cell counting using a hemocytometer after trypsinization. All activities were normalized against cell number and expressed as counts per minute (cpm) per 1,000 cells.
Statistics
The sample size (n) refers to the number of human subjects tested for each assay. All tests were performed in triplicate. Results were normalized against cell number and calculated as percentage of the control, and presented as mean (SE). Comparison between treatments was performed with 2-tailed Wilcoxon signed-ranks test. All statistical tests were performed with SPSS version 11.0 (SPSS Inc., Chicago, IL). Differences were considered to be statistically significant at p-values less than 0.05.
Results
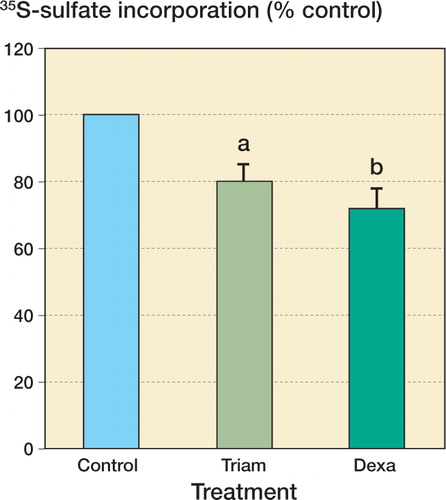
Treatment of confluent monolayers of human tenocytes with 1 μM triamcinolone acetonide for 48 h resulted in a reduction in the amount of 35S-sulfate incorporation to 80% (5%) of control values (n = 10; p = 0.007), while 1 μM dexamethasone reduced the 35S-sulfate incorporation to 72% (7%) of control values (n = 10; p = 0.01). There was no difference between the response to triamcinolone and the response to dexamethasone ().
Figure 1. Treatment with 1 μM dexamethasone (Dexa) and 1 μM triamcinolone (Triam) both suppressed proteoglycan synthesis by cultured human tenocytes, as measured by 35S-sulfate incorporation.Values represent mean (SE) percentage of control 35S-sulfate incorporation (10 subjects, with 3 replicates per subject). a p = 0.007 and b p = 0.012 compared with control (DMEM), 2-tailed Wilcoxon signed-ranks test.
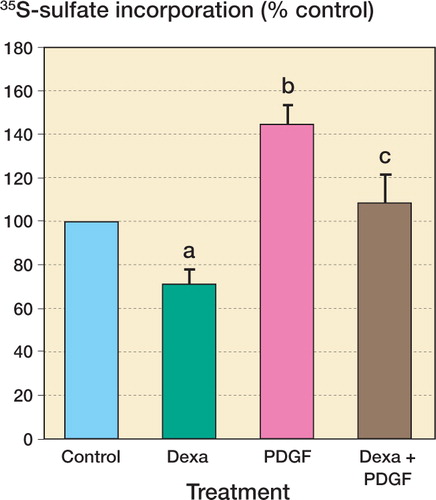
Treatment of confluent monolayers of human tenocytes with 10 ng/mL PDGFBB increased the amount of 35S-sulfate incorporated to 144% (9%) (n = 10; p = 0.012). Co-incubation of cells with 10 ng/mL PDGFBB and 1 μM dexamethasone increased 35S-sulfate incorporation to 108% of control, a significant increase compared with dexamethasone treatment alone (n = 10; p = 0.01) ().
Figure 2. The effect of dexamethasone on proteoglycan synthesis by human tenocytes and reversibility of the effect by PDGFBB.The results represent 35S-sulfate incorporation with DMEM alone (DMEM), treatment with 1 μM dexamethasone (Dexa), with 10 ng/mL PDGFBB (PDGF), and co-incubation with 1 μM dexamethasone and 10 ng/mL PDGFBB (Dexa+PDGF).Values represent mean (SE) percentage of control 35S-sulfate incorporation (10 subjects, with 3 replicates per subject). a p = 0.01 and b p = 0.01 compared with control. c p = 0.01 compared with 1 μM dexamethasone treatment, 2-tailed Wilcoxon signed-ranks test.
Discussion
Our findings confirmed our hypothesis that glucocorticoids suppress proteoglycan synthesis by human tenocytes. 1 μM is within the range of physiological serum concentration after oral ingestion of glucocorticoids in humans (Loew et al. Citation1986). The dosage of 1 μM dexamethasone and triamcinolone has already been shown to effectively suppress cellular metabolism and collagen synthesis in cultured human tenocytes, and the effect of dexamethasone is reversed by 10 ng/mL PDGFBB (Wong et al. Citation2003, Citation2004). We therefore chose the same glucocorticoids and same dosages in the current study to examine their effects on proteoglycan synthesis. Both dexamethasone and triamcinolone treatment at 1 μM significantly suppressed proteoglycan synthesis in cultured human tenocytes.
The biosynthesis of proteoglycans involves the formation of repeating disaccharide chains linked to a core protein. Radiolabeling provides a convenient way of following proteoglycan synthesis. The 35S-sulfate incorporation method was used to measure the amount of proteoglycan synthesis (Yanagishita et al. Citation1987, Vogel and Hernandez Citation1992), as more than 90% of the activity incorporated with this precursor will be in proteoglycan (Yanagishita et al. Citation1987).
Proteoglycans are complex macromolecules consisting of polyanionic glycosaminoglycan side-chains attached to a core protein. They are strongly hydrophilic. Proteoglycans increase the turgor of tendon, and enable rapid diffusion of water-soluble molecules and cell migration. Proteoglycans are involved in the regulation of extracellular matrix organization and collagen fibrillogensis (Scott et al. Citation1981, Scott and Hughes Citation1986). They play an important role in the viscoelastic properties of tendon (Elliott et al. Citation2003). The production of an appropriate amount of proteoglycans is therefore very important for the normal organization and function of tendons. The ability of human tenocytes to increase proteoglycan synthesis and extra-cellular matrix production is particularly important at times of tendon healing and repair. The suppression of proteoglycan synthesis by dexamethasone and triamcinolone may disturb the organization of the extracellular matrix and collagen fibrillogensis during healing, subsequently affecting the visco-elastic properties of the tendon and predisposing it to spontaneous rupture.
The co-incubation of 1 μM dexamethasone with 10 ng/mL PDGFBB reversed the effect of 1 μM dexamethasone on human tenocyte proteoglycan production. The amount of proteoglycan production with co-incubation was comparable to that of the control. A different effect might be obtained if other dosages are chosen. The current balancing dosages of dexamethasone and PDGFBB were determined in previous well-controlled in vitro experiments (Wong et al. Citation2003). The actual combating dose for clinical applications can be expected to be different, and would have to be determined separately (Chan et al. Citation1997).
The effects of PDGF have been investigated in other types of tissue (Heldin and Westermark Citation1999, Adcock and Caramori Citation2001). The cellular effects of PDGFBB in rabbit were found to differ in different tendons (Yoshikawa and Abrahamsson Citation2001). In contrast to their balancing effects seen on co-incubation demonstrated in this study, the fibroproliferative effect of PDGF on skin fibroblasts was not apparently affected by dexamethasone (Peterson et al. Citation1994). These findings again illustrate the tissue specificity of dexamethasone and PDGF, and the need for extreme care in extrapolating results from other studies. The importance of selecting the appropriate cells or tissues for studies cannot be over-emphasized.
We used an in vitro cell culture system to study the effect of glucocorticoids and PDGFBB on proteoglycan synthesis by human tenocytes. The presence of tendinopathy, inflammatory reactions, circulation, other cytokines and growth factors in the human body would much complicate the actual in vivo response. Our findings should thus be interpreted in perspective.
This study was supported by the Research Grant Council (Direct Grant for Research), the Chinese University of Hong Kong.
- Adcock I M, Caramori G. Crosstalk between pro-inflammatory transcription factors and glucocorticoids. Immunol Cell Biol 2001; 79: 376–84
- Benson L S, Ptaszek A J. Injection versus surgery in the treatment of trigger finger. J Hand Surg Am 1992; 22: 138–44
- Campbell R B, Wiggins M E, Cannistra L M, Fadale P D, Akelman E. Influence of steroid injection on ligamnent healing in the rat. Clin Orthop 1996, 332: 242–53
- Chan B P, Chan K M, Maffulli N, Webb S, Lee K K.H. Effect of basic fibroblastic growth factor. An in vitro study of tendon healing. Clin Orthop 1997, 342: 239–47
- Chard M D, Wright J K, Hazleman B L. Isolation and growth characteristics of adult tendon fibroblasts. Ann Rheum Dis 1987; 46: 385–90
- Chechick A, Amit Y, Israeli A, Horoszowski H. Recurrent rupture of the Achilles tendon induced by corticosteroid injection. Br J Sports Med 1982; 16: 89–90
- Delany A M, Brinckerhoff C E. Post-transcriptional regulation of collagenase and stromelysin gene expression by epidermal growth factor and dexamethasone in cultured human fibroblasts. J Cell Biochem 1992; 50: 400–10
- Elliott D M, Robinson P S, Gimbel J A, Sarver J J, Abboud J A, Ioxxo R V, Soslowsky L J. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Engineering 2003; 31: 599–605
- Heldin C H, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999; 79: 1283–316
- Kapetanos G. The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop 1982, 163: 170–9
- Kleinman M, Gross A E. Achilles tendon rupture following steroid injection. Report of three cases. J Bone Joint Surg (Am) 1983; 65: 1345–7
- Kotnis R A, Halstead J C, Hormbrey P J. Atraumatic bilateral Achilles tendon rupture: an association of systemic steroid treatment. J Accid Emerg Med 1999; 16: 378–9
- Leadbetter W B. Glucocorticoids injection therapy in sports injuries. Sports-induced inflammation, W B Leadbetter, J A Buckwalter, S L Gordon, AAOS Park Ridge, IL 1990; 527–46
- Ledda-Columbano G M, Columbano A, Cannas A, Simbula G, Okita K, Kayano K, Kubo Y, Katyal S L, Shinozuka H. Dexamethasone inhibits induction of liver tumor necrosis factor-alpha mRNA and liver growth induced by lead nitrate and ethylene dibromide. Am J Pathol 1994; 145: 951–8
- Loew D, Schuster O, Graul E H. Dose dependent pharmacokinetics of dexamethasone. Eur J Clin Pharmacol 1986; 30: 225–30
- McWhorter J W, Francis R S, Heckmann R A. Influence of local steroid injections on traumatized tendon properties. A biomechanical and histological study. Am J Sports Med 1991; 19: 435–9
- Nelson K H, Briner W, Jr, Cummins J. Corticosteroid injection therapy for overuse injuries. Am Fam Physician 1995; 52: 1811–6
- Oikarinen A, Makela J, Vuorio T, Vuorio E. Comparison on collagen gene expression in the developing chick embryo tendon and heart. Tissue and development time-dependent action of dexamethasone. Biochim Biophys Acta 1991; 1089: 40–6
- Oxlund H, Manthorpe R, Viidik A. The biochemical properties of connective tissue in rabbits as influenced by short-term glucocorticoid treatment. J Biomech 1981; 14: 129–33
- Peterson T C, Isbrucker R A, Hooper M L. In vitro effect of platelet-derived growth factor on fibroproliferation and effect of cytokine antagonists. Immunopharm 1994; 28: 259–70
- Read M T, Motto S G. Tendo Achillis pain: steroids and outcome. Brit J Sports Med 1992; 26: 15–21
- Scott J E, Hughes E W. Proteoglycan-collagen relationships in developing chick and bovine tendons. Influence of the physiological environment. Conn Tiss Res 1986; 14: 267–78
- Scott J E, Orford C R, Hughes E W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J 1981; 195: 573–81
- Smith A G, Kosygan K, Williams H, Newsman R J. Common extensor tendon rupture following corticosteroid injection for lateral tendinosis of the elbow. Br J Sports Med 1999; 33: 423–5
- Stannard J P, Bucknell A L. Rupture of the triceps tendon associated with steroid injections. Am J Sports Med 1993; 21: 482–5
- Tatari H, Kosay C, Baran O, Ozcan O, Ozer E. Deleterious effects of local corticosteroid injections on the Achilles tendon of rats. Arch Orth Trauma Surg 2001; 121: 333–7
- Vogel K G, Hernandez D J. The effects of transforming growth factor-beta and serum on proteoglycan synthesis by tendon fibrocartilage. Eur J Cell Biol 1992; 59(2)304–13
- Wiggins M E, Fadale P D, Ehrlich M G, Walsh W R. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg (Am) 1995; 77: 1682–91
- Wong M W.N, Tang Y N, Lee K M, Fu S C, Chan B P, Chan K M. Effect of Dexamethasone on Human Tenocytes and its Reversibility by PDGFBB. J Bone Joint Surg (Am) 2003; 85: 1914–20
- Wong M W N, Tang Y N, Lee K M, Fu S C, Chan K M. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop 2004, 421: 277–81
- Yanagishita M, Midura R J, Hascall V C. Proteoglycans: isolation and purification from tissue cultures. Method Enzymol 1987; 138: 279–89
- Yoshikawa Y, Abrahamsson S O. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand 2001; 72(3)287–92
