In 1986, a 36-year-old woman presented with a 2-month history of progressive left shoulder pain. Radiographs revealed a large radiolucent lytic lesion involving the entire proximal humerus, with extraosseous extension. Open biopsy confirmed the diagnosis of giant cell tumor. A wide resection of the proximal humerus and reconstruction using a fresh frozen osteoarticular allograft was performed, secured with a plate and screws. To obtain adequate surgical margins, the deltoid muscle and axillary nerve were sacrificed. The surgical procedure and postoperative course were without complications.
The patient did well for the first 4 postoperative years, and was then lost to followup for almost 12 years. In 2001, the patient presented with painful glenohumeral joint instability and subluxation. In 2003, she underwent a revision procedure in which the proximal allograft was resected and reconstructed by combined allograft prosthesis.
Radiographic results
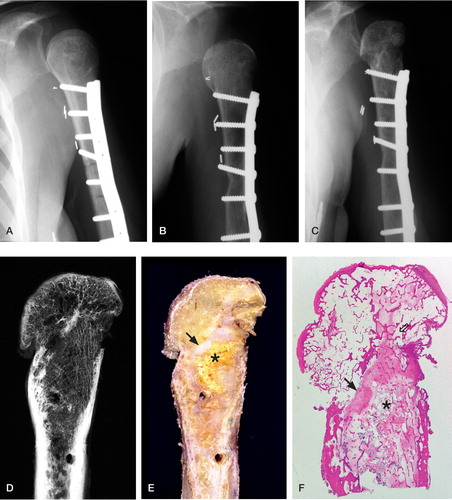
Radiographs taken 2 months after the first surgery in 1986 showed good fit of the osteoarticular allograft head in the glenoid fossa (). Radiographs at 2-year follow up showed healing of the allografthost bone interface (). Radiographs taken over the next 12 years showed gradually increasing deformity of the allograft humeral head with subchondral sclerosis and peripheral osteophyte formation, suggesting revascularization (). At the time of the revision surgery, the surrounding soft tissue was completely and firmly attached to the allograft. Plain radiographs of the resected specimen showed considerable deformity of the humeral head, which contained a prominent medial osteophyte and substantial subchondral bone density, juxtaposed laterally by an area of radiolucency ().
Figure 1. A. Left humerus, AP view, at 2 months postoperatively (Oct 14,1986) showing good fit of the osteoarticular allograft into the glenoid fossa. B. 19 months postoperatively (May 9, 1988) showing subluxation of the humeral head. C. 15 years postoperatively (December 12, 2001). D. Specimen radiograph.A large osteophyte on the inferiormedial aspect of the humeral, subchondral sclerosis and areas of resorption are evident. E. Photomacrograph of a sagittal section of the explanted specimen showing remnants of soft tissue attachment to the graft.The bright yellow area (asterisk) corresponds to residual nonviable graft abutting the fibrovascular tissue (black arrow).The holes represent the sites of screw insertion for plate fixation. F. Low-power view of hematoxylin and eosin stained histological section of humeral head and proximal diaphysis showing nonviable graft (asterisk) separated from the viable areas by dense fibrovascular tissue (black arrow), which corresponds to the white region in E. An additional area of necrotic bone can be seen in the humeral head at the right.
Pathology results
The resected osteoarticular specimen consisted of a left proximal humerus allograft measuring 9.5 cm in length, attached to 1 cm of host humeral diaphysis distal to the osteotomy site. The surface of the graft displayed imprints of a metallic plate and several holes for screws. Overall, there was no callus visible at the hostgraft junction, which contained normal viable bone and marrow. The proximal region of the medullary cavity contained gray-yellow bone marrow and a bright yellow area with serpiginous borders that measured 4 cm, corresponding to the residual nonviable graft material with reparative changes seen histologically ( and ).
Based on gross and microscopic appearance, it was apparent that more than 90% of the cortical surface of the diaphysis and metaphysis formed attachments to viable host fibroadipose tissue and skeletal muscle. Frequently, along the shaft blood vessels arising from the surface tissue attachments penetrated the haversian canals of the graft. How ever, the extent of bone and marrow revitalization and repair of the allograft varied greatly along the shaft. In particular, at the host-graft junction we could see that the bone marrow was fully viable and that nearly 100% of the lacunae of the cortex and trabeculae were repopulated by osteocytes, implying full remodeling of the bone. Similarly, in the distal one-third of the graft, 80–90% of the cortex and bone marrow was viable. However, in the middle one-third of the graft, tissue viability was considerably lower, ranging from totally nonviable to partially viable.
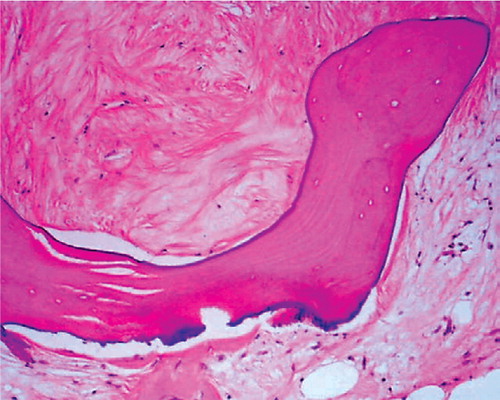
Locally, the process of revitalization and repair of the allograft showed a random pattern: osteons devoid of osteocytes were proximal to fully viable osteons. The nonviable medullary bone was frequently surrounded by hyalinized, poorly cellular tissue with calcification, as well as fibrous and proteinaceous amorphous bundles of collagen tissue (). This proteinaceous material usually contained fibrovascular tissue and small blood vessels.
Figure 2. High-power (100 ×) view of fibrovascular tissue in marrow surrounding an allograft trabecula.The dense collagenous tissue is almost acellular, but contains numerous small blood vessel components.
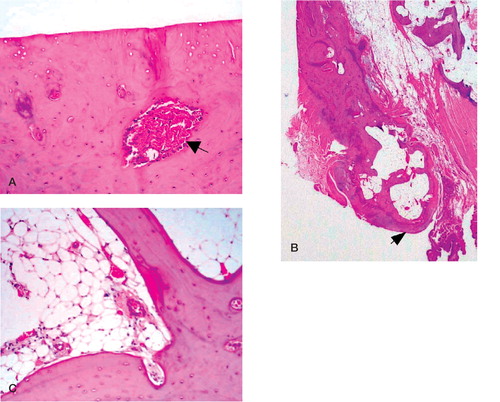
The humeral head was fiattened and partly covered with pearly gray-brown cartilage. Microscopically, about one-third of the medial portion of the humeral head was viable and contained a 2 × 1.5 cm area of eburnation. Subchondral sclerosis was also apparent macroscopically on cut sections of the graft, particularly below the eburnated surface of the medial aspect (). The inferomedial aspect of the humeral head contained a large viable osteophyte (). The bone marrow was also viable (). In contrast, the bone in the lateral portion of the humeral head remained largely nonviable, and the articular surface was covered by hyaline cartilage and viable fibrocartilage. Some viable synovial tissue was apparent at the periphery of the humeral head, but no infiammatory pannus was present.
Figure 3. A. High-power (100×) view of the medial aspect of the proximal portion of the humeral head joint surface showing considerable eburnation.The subchondral bone is revascularized (black arrow) and contains numerous viable osteocytes. B. Low-power view depicting the viable osteophyte (black arrow). C. High-power (100×) of viable cortical and trabecular bone and mature bone marrow that is rich in vascular elements.
Discussion
Although massive bone allografts have been used after tumor resection for over 30 years, our literature survey yielded only 4 articles that included histopathological descriptions of 6 massive humerus osteoarticular grafts. Furthermore, we have not encountered any publications containing histological descriptions of radius or ulna grafts. Among the articles that provided the age of individual grafts, the time ranged from 7 months to 4.5 years until explantation (Ottolenghi Citation1966, Enneking and Mindell Citation1991, Caldora et al. Citation1995) and biopsy at 7 years (Aho et al. Citation1998). By comparing our case to reports of relatively short-term non-weight-bearing osteoarticular allografts and also long-term weight-bearing grafts, we hoped to establish a more comprehensive view of the evolution of such grafts over a followup period covering almost two decades.
The literature that has described the histomorphology of juxtaarticular humerus allografts mainly focuses on aspects of articular surface changes, internal repair, and softtissue attachment. For example, Caldora et al. (Citation1995) described two frozen humerus osteoarticular allografts—one distal and one proximal—both of which were explanted due to fracture after 7 and 8 months. The proximal humerus graft showed more than 90% softtissue attachment, while the distal humerus graft achieved 50–90% soft tissue adhesion. With few exceptions, osteoarticular allografts of the upper or lower extremity achieved more than 80% soft tissue attachment by 1 year of implantation (Caldora et al. Citation1995, Enneking and Campanacci Citation2001). Thus, the finding that our graft achieved greater than 90% of host soft tissue surface attachment is consistent with other histological reports concerning this process. Tissue adhesion allows incursion of the host fibrovascular buds into the allografts, which in turn serves as ingress for osteoclasts that form cutting cones. This enables subsequent entry of osteoprogenitor cells and additional vascular elements into the necrotic grafts, thus contributing to bone apposition and remodeling within the graft (Enneking and Mindell Citation1991, Caldora et al. Citation1995, Enneking and Campanacci Citation2001).
In contrast to the ubiquitous formation of hosttissue adhesions, it has been observed that the extent of graft revitalization and repair is highly variable after 2 years and beyond (Kandel et al. Citation1984, Enneking and Campanacci Citation2001). It has also been noted that revascularization and internal repair show a random distribution, with progression at rates of up to 2 mm per year—such that often by two years of implantation, vascular elements only reach a depth of 5 mm and less than 20% of the graft surface area has been repaired (Enneking and Mindell Citation1991, Enneking and Campanacci Citation2001). Based on the histological features of our allograft case at 18 years of implantation time, we estimated that longitudinal revascularization proceeded at a minimum rate of about 2.2 mm per year, which is similar to the figures published for other osteoarticular allografts. We arrived at these figures with the assumption that the difference in magnitude of bone viability between the proximal (4 cm) and more distal portions (6 cm) of the graft was due to the supplemental longitudinal revascularization that originated from the host and spread proximally through the haversian canals and medullary cavity of the graft. However, this activity was superimposed on the surface approach of revascularization, which was essentially present throughout the graft.
In addition to obtaining substantial revascularization, the graft in our patient acquired mature bone marrow with hematopoietic elements. We have encountered one study that identified a 7-year-old massive knee osteoarticular allograft containing normal living bone marrow (Alho et al. Citation1989). It appears that viable bone marrow represents an advanced stage in the revitalization process.
Although the allograft in our study developed viable bone marrow and subchondral bone, the remaining hyaline cartilage was completely nonviable. Certainly, without preservatives, the freezing process used in the preparation of the grafts results in loss of chondrocyte and osteocyte viability (Salenius et al. Citation1982). Nevertheless, there have been reports of deepfrozen knee osteoarticular allograft, ranging in age from 1.5 to over 7 years, that contained living hyaline cartilage (Salenius et al. Citation1982, Alho et al. Citation1989).
Perhaps the lack of viable chondrocytes predisposes the patient to joint degeneration and subsequent subchondral sclerosis. Osteoarthrosis is certainly a common phenomenon, and affects most osteoarticular grafts. In a study dealing with massive humerus and knee osteoarticular allografts, 20 of 29 weight-bearing and non-weight-bearing grafts displayed degenerative changes of the joint surface, generally by 2–4 years postimplantation (Aho et al. Citation1994). Enneking and Campanacci (Citation2001) suggested that such a process can be attributed to irregular mechanical distribution of forces, particularly in grafts that fitted poorly into the host articular surface—which may represent the same mechanism that led to the changes observed in the subluxated humerus allograft we have presented. Although grafts may show radiographic or histological signs of joint damage, function is generally preserved, possibly due to articular resurfacing by host fibrocartilage (Caldora et al. Citation1995). Indeed, one has article reported a frozen distal humerus osteoarticular allograft that was removed after 5 years and which subluxed at the graftradial head portion of the joint (Enneking and Mindell Citation1991). This produced severe erosions that were partially reconstructed with host fibrocartilage, and joint function remained intact. The function of knee grafts has been superior to that of the proximal humerus graft in at least one study (Aho et al. Citation1994). Similarly, in our case, the function of the graft specimen became compromised as joint deformation occurred in the last few years of implantation.
In older allografts, joint degeneration is often more extensive and involves more active bone repair. For example, one series of case reports included a 27-year-old femoral osteoarticular graft; osteoarthrosis involved the femoral head, which contained viable osteocytes and revascularized marrow (Muscolo et al. Citation1992). Similarly, while only radiographic evidence was presented, the osteophyte that formed in a 32-year-old osteoarticular allograft of the proximal femur would indicate revitalization in the femoral head (Bohm et al. Citation1996). These findings are similar to what we observed in the 18-year-old graft in this report.
Our case adds further evidence for the potential benefit of the combined allograftprostheses that have been developed in the last 15 years, with the intent of minimizing the complications associated with osteoarticular allografts. Certainly, further clinicopathological investigations are warranted in order to determine the long-term courses of both types of grafts—which should eventually enhance their longevity and quality. Indeed, as Caldora et al. (Citation1995) stated, histological study of explanted grafts offers an optimal means of gaining information about host-graft interactions.
No competing interests declared.
- Aho A J, Ekfors T, Dean P B, Aro H T, Ahonen A, Nikkanen V. Incorporation and clinical results of large allografts of the extremities and pelvis. Clin Orthop 1994; 307: 200–13
- Aho A J, Eskola J, Ekfors T, Manner I, Kouri T, Hollmen T. Immune responses and clinical outcome of massive human osteoarticular allografts. Clin Orthop 1998; 346: 196–206
- Alho A, Karaharju E O, Korkala O, Laasonen E M, Holmstrom T, Muller C. Allogeneic grafts for bone tumor. 21 cases of osteoarticular and segmental grafts. Acta Orthop Scand 1989; 60(2)143–53
- Bohm P, Renner E, Rossak K. Massive proximal femoral osteoarticular allograft. Arch Orthop Trauma Surg. 1996; 115(2)100–3
- Caldora P, Donati D, Capanna R, Di Liddo M, Benassi M S, Campanacci D A, Sangiorgi L, Zavatta M, Picci P. A histomorphologic study of explants of massive allografts: preliminary results. Chir Organi Mov 1995; 80(2)191–205
- Enneking W F, Campanacci D A. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg (Am) 2001; 83(7)971–86
- Enneking W F, Mindell E R. Observations on massive retrieved human allografts. J Bone Joint Surg (Am) 1991; 73(8)1123–42
- Kandel R A, Pritzker K P, Langer F, Gross A E. The pathologic features of massive osseous grafts. Hum Pathol 1984; 15(2)141–6
- Muscolo D L, Petracchi L J, Ayerza M A, Calabrese M E. Massive femoral allografts followed for 22 to 36 years. Report of six cases. J Bone Joint Surg (Br) 1992; 74(6)887–92
- Ottolenghi C E. Massive osteoarticular bone grafts. Transplant of the whole femur. J Bone Joint Surg (Br) 1966; 48(4)646–59
- Salenius P, Holmstrom T, Koskinen E V, Alho A. Histological changes in clinical halfjoint allograft replacements. Acta Orthop Scand 1982; 53(2)295–9
