Abstract
Background Modern-generation metal-on-metal articulations have a high wear resistance and may therefore be able to improve the long-term performance of artificial joints. However, the biological effects are still under debate. This study was undertaken to review the histopathological changes in neo-capsule tissues of different metal-on-metal hip arthroplasties.
Methods Neo-capsule tissue samples from 46 hips with modern second-generation metal-on-metal articulations (39 hip resurfacings and 7 non-cemented total hip replacements) with a variety of failure mechanisms were examined histopathologically and immunohistochemically.
Results A distinct lymphocytic infiltration was found in all cases with in situ times of more than 7 months, consisting of CD20-positive B-lymphocytes and CD3positive T-lymphocytes and sometimes thinly distributed CD138-positive plasma cells without dominant T-cell or plasma cell infiltrates.
Interpretation This distinct lymphocytic infiltration has not been reported in tissue analyses of metal- or ceramic-on-polyethylene hip replacements, and may therefore be considered to be a characteristic histological pattern of tissue reactions on metal particles and/or ions around metal-on-metal bearings.
Reduction of wear debris is an important issue for improvement of long-term results of total hip replacement, and has re-stimulated interest in alternative bearing materials (Willert Citation1977, Willert and Semlitsch Citation1996). Cobalt-chromium-molybdenum alloys and ceramics have a higher wear resistance than polyethylene. Studies of retrieved historical metal-on-metal prostheses have shown extremely low wear rates, even 10–20 years after implantation (Semlitsch et al. Citation1989, McKellop et al. Citation1996). Today, several designs of modern-generation metal-on-metal total hip replacement and hip resurfacing devices are available. Different research groups have reported satisfying short- to mid-term results of these articulations (Schmidt et al. Citation1996, Wagner and Wagner Citation1996, Citation2000, Dorr et al. Citation2000, Korovessis et al. Citation2003, McMinn Citation2003, Amstutz et al. Citation2004, Beaule et al. Citation2004, Daniel et al. Citation2004, Knecht et al. Citation2004, Witzleb et al. Citation2004, Treacy et al. Citation2005). Only Bachfischer et al. (Citation2000) reported a higher rate of heterotopic ossifications and considered the metal-on-metal bearing to be responsible. The amount of wear found on retrieved implants turned out to be as low as suggested by hip simulator tests (Schmidt et al. Citation1996, Streicher et al. Citation1996, Kremling et al. Citation2004).
Even so, the biological effects of metal alloys containing cobalt, chromium, and molybdenum are still under discussion (Jacobs et al. Citation2003). Immunological reactions to these metals are of great interest, since Co, Cr, and also Ni ions can induce contact allergy in humans. Several research groups have found lymphocyte infiltrations in periprosthetic tissue surrounding historic metal-on-metal articulations (Evans Citation1974, Willert Citation1977, Vernon-Roberts Citation1978). Similar changes were observed in the periprosthetic tissue of revised modern metal-on-metal hip replacement devices (Willert et al. Citation2000, Campbell et al. Citation2001, Willert et al. Citation2001, Lintner et al. Citation2003, Willert et al. Citation2005). Willert et al. (Citation2000, Citation2001) reported intense diffuse and perivascularly oriented lymphocytic infiltrates in all of the 14 cases investigated, and suggested a cell-mediated hypersensitivity reaction of type IV (delayed type hypersensitivity) caused by the release of metal particles from the articulation.
Here we report a semi-quantitative histological and immunochemical analysis of neo-capsule tissues retrieved from second-generation metal-on-metal hip replacement and hip resurfacing arthroplasties, performed with a view to determining the clinical relevance of the above findings.
Material and methods
Neo-capsules, which had been retrieved from 46 hips with modern second-generation metal-on-metal articulations, were donated by 11 surgeons from Australia and Europe from 2000 through 2004. The original devices were hip resurfacings in 39 cases and non-cemented total hip replacements in 7 cases. Tissue samples were obtained at the time of revision of the original components because of aseptic loosening (21 hips), femoral neck fracture (7 hips), infection (6 hips) and recurrent dislocation (2 hips), or at the time of exploration of the joint for removal of heterotopic bone (5 hips). 5 joints had been revised due to chronic pain related to chronic synovitis or recurrent joint effusion (1 case showed the histological pattern of a low-grade infection). All primary implantations were performed due to osteoarthritis; patients with inflammatory disorders such as rheumatoid arthritis were excluded. The 24 male and 22 female patients who were included had a mean age of 57 (39–79) years at the time of revision. Their implants had been in situ from 3 weeks to 8 years (median 14 months).
Histological analysis
Capsule tissue samples were obtained intraoperatively and immediately preserved in buffered 4% formalin. A minimum of 3 different representative samples from each explant were embedded in paraffin wax, cut into 4-μm serial sections, and stained with hematoxylin and eosin (H&E), Prussian blue and giemsa. 10 high-power fields (1 HPF = 0.24 mm2) were examined from each tissue section and the results were presented as the mode. All histological analyses were performed using an Olympus BHS light microscope in the transmitted and polarized modes at final magnifications of 25×, 100× and 400×.
Grading of periprosthetic particle storage and histiocytic tissue reaction
The amount of metal particles in the tissue was estimated according to Willert and Semlitsch Citation1996 and Willert et al. Citation2005 (“none”: no or only isolated phagocytosed particles without major macrophage reaction; “few”: few particles phagocytosed in some spots and/or accumulated perivascularly; “many”: evident accumulation of particles phagocytosed in macrophages; “abundant”: tissue loaded with particles, including foreign-body granulomas; “excessive”: tissue overloaded with particles, with foreign-body granulomas predominating everywhere).
A semiquantitative rating scale described by Doorn et al. (Citation1996) was used to assess the extent of necrosis (“1+”: 1–2 mm of necrosis/slide, “2+”: 3–9 mm of necrosis/slide, “3+”: < 1 cm of necro-sis/slide), polymethylmethacrylate globules (“1+”: 1–3 globules/low power field (LPF = 3.8 mm2), “2+”: 4–6 globules/LPF, “3+”: < 7 globules/LPF), bone chips (“1+”: 1–5 bone chips/LPF, “2+”: 6–10 bone chips/LPF, “3+”: < 10 bone chips/LPF) and metal particles (“slate blue” histiocytes: few (< 10) light microscopic visible metal particles (indicative of predominantly sub-micron particles), “dusty” histiocytes: isolated black specks in the cytoplasm, “jet black” histiocytes: abundant debris).
Bacterial infection
To detect bacterial infection in the capsule tissue, histological criteria described by Athanasou et al. (Citation1995) were assessed (“septic loosening” characterized by 5 or more neutrophil polymorphs per high-power field, “low-grade infection” characterized by 1–4 neutrophil polymorphs).
Grading of lymphocytic infiltration
Neo-capsule tissues from infected hips or hips with a histological pattern of infection (7 cases) were excluded from the investigation. Lymphocytic infiltration was assessed by counting the individual cells of a diffuse infiltration (magnification 40×) and the number of perivascular agglomerations (magnification 4×) per field of view (“none”: ≤ 10 cells of diffuse infiltration and/or no perivascular agglomeration, “few”: 11–30 cells and/or 1–2 agglomerations, “many”: 31–50 cells and/or 3–6 agglomerations, “abundant”: 51–100 cells and/or 7–10 agglomerations, “excessive”: < 100 cells and/ or < 10 agglomerations) (Willert et al. Citation2005).
Immunohistochemical staining methods used for grading
Immunohistochemical analysis
To further characterize inflammatory cells involved in the tissue response, immunohistochemistry was performed. The specimens were stained automatically (Benchmark; Ventana Medical Systems Inc., Illkirch, France) using the Avidin-Biotin-Complex (ABC) method and antibodies listed in the Table (DakoCytomation, Glostrup, Denmark). Positive controls (lymphoma tissue, tonsillar tissue and histiocytic tumor tissue) and negative controls were stained using the same method. The immunohistological pattern of T-and B-lymphocytes and plasma cell distribution were examined.
Proliferation was classified using the Ki-67/MIB 1 index, i.e. nuclear staining of lymphocytes for the proliferation-associated Ki-67 antigen. A proliferation index of over 5% was taken to indicate “increased” proliferation (Gerdes et al. Citation1991).
Statistics
As indicated above, each histological and immunohistochemical grading was scaled in terms of a 3–5-stage classification rule (such as the classifications “none” to “excessive” defined to characterize lymphocytic infiltration. The resulting classification data were analyzed by means of absolute and appropriate relative (%) frequencies. For the sake of illustration, the original rating scales were then aggregated by combining some of the original classes into binary classifications (such as a “positive” vs. a “negative” finding).
Descriptive sub-sample comparisons concerning these ratings were performed by means of bivariate contingency tables (graphically on bar diagrams, accordingly); significance comparisons were based on Fisher’s exact test.
Agreement analysis between parallel ratings (i.e. between different histological findings) was based on pairwise kappa coefficients after appropriate re-scaling of the underlying gradings into a binary classification rule. Kappa coefficients were presented by means of point estimates and 95% confidence intervals; a kappa point estimate of 80% or more was considered to be an indication of good agreement between the respective gradings. Pairwise McNemar tests were used to test for grading bias between the respective (binary) gradings, i.e. to detect a systematic tendency of one grading towards positive findings. Results of significance tests were summarized in terms of p-values, and statistical significance was assumed for p < 0.05.
Results
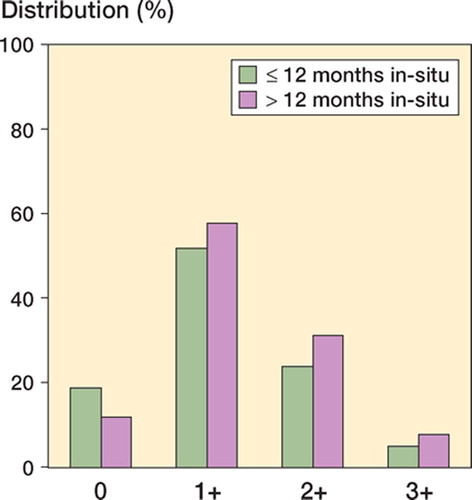
The neo-capsule tissues showed varying extents of ulceration and superficial necrosis. In median 1–2 mm of the superficial layer were necrotic per slide (grade 1+). 3 cases (revision caused by infection, femoral neck fracture and aseptic loosening, respectively) showed an extensive necrosis of more than 1 cm per slide. Cases with in situ times of more than one year showed a slightly (although not significantly) stronger degree of necrosis (Fisher’s exact test, p = 0.8) ().
In cases where revision was caused by loosening or femoral neck fracture, a detritic synovitis and polymethylacrylate globules (grade 1+ in 3 cases, grade 2+ in 7 cases, grade 3+ in 6 cases) and/or bone chips (grade 1+ in 8 cases, grade 2+ in 9 cases, grade 3+ in 2 cases) surrounded by giant cells or small foreign body granulomas were present. Furthermore, by sandwich technique, titanium and polyethylene particles were seen in 1 case involving a loose cementless THR with a metal-on-metal bearing.
All retrieved neo-capsule tissues showed a more or less strong fibrosis with some mast cells and siderophages. In 3 cases where revision was caused by aseptic loosening, a distinct sclerosis was found. All 3 cases had in situ times of more than 2 years (24, 74 and 93 months, respectively).
None of the cases showed any infiltration of eosinophilic granulocytes.
Particle storage and histiocytic tissue reaction
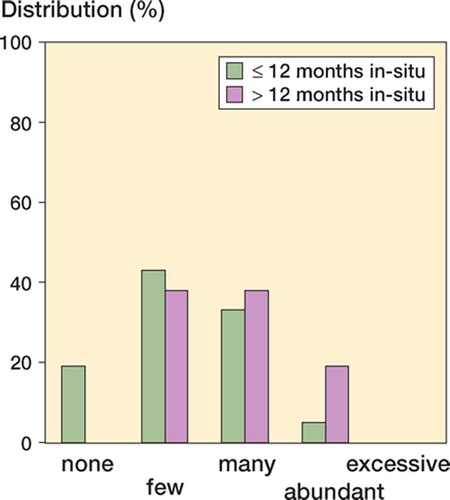
Particulate metallic debris was identified within CD68-positive macrophages in 42 of the 46 cases. In most of these, only small amounts of phagocytosed metallic particles were found. In 6 cases (revision caused by aseptic loosening in 3 cases, recurrent dislocation in 1, and chronic pain in 1 case) the neo-capsule tissue was loaded with metallic particles, including a few giant cells and isolated small foreign-body granulomas (“abundant” particle storage). These cases showed the grayish discoloration of a metallosis even macroscopically. None of the cases showed “excessive” particle storage. Cases with in situ times of more than one year had a slightly (although not significantly) stronger storage of metallic particulate debris and histiocytic reaction (Fisher’s exact test, p = 0.2) ().
Figure 2. Storage of metallic particulate debris and histiocytic tissue reaction in cases with in situ times of ≤ 12 months and < 12 months.
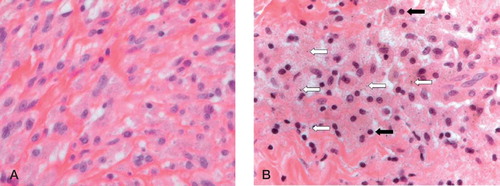
In 24 of the 46 cases, histiocytes showed a normal or “slate-blue” cytoplasmic appearance according to Doorn et al. (Citation1996), indicative of small (submicron) particle sizes (). 17 cases showed a few isolated black specks in the cytoplasm of the histiocytes (with a “dusty” appearance) (). Abundant debris (“jet-black” histiocytes) was present in only 5 neo-capsule samples.
Figure 3. Micrographs of hematoxylin and eosin (H&E) stained sections (magnification 400×). A. Clusters of macrophages with “slate blue” cytoplasmic appearance. B. “Dusty” macrophage appearance, which is characterized by isolated black specks in the cytoplasm (white arrows). The particle size is up to 1 μm, while the normal size of lymphocytes is 5–7 μm (black arrows).
The extent of necrosis (none and “1+” as opposed to “2+” or “3+”) showed only moderate agreement with the observed metallic particle burden of the neo-capsule probes of “none”/“few” vs. “many”/ “abundant”. However, in 12/46 cases a notable particle burden was observed without necrosis, whereas in only 4 cases the extent of necrosis was rated “2+”/“3+” and the corresponding extent of particle burden was “many/abundant” (McNemar test, p = 0.08).
Bacterial infection
6 cases with microbiologically proven infection (4 early infections, 2 late infections/septic loosenings) showed 5 or more neutrophil polymorphs per high-power field and were also classified histologically as infections. 1 additional case with revision 40 months postoperatively due to chronic pain and joint effusion showed the histological pattern of a low grade infection (1 to 5 neutrophil polymorphs per high-power field). These 7 cases were excluded from the investigation of lymphocytic infiltration.
Lymphocytic infiltration
In 3/39 cases who underwent early revision 4–7 months after implantation (revision was caused by femoral neck fracture in two hip resurfacing cases, and by heterotopic bone formation in 1 case), not more than 10 diffuse distributed lymphocytes per field of view and no perivascular lymphocytic infiltration was detectable. All of the other 36 cases showed a distinct degree of lymphocytic infiltration (“few” to “excessive”), consisting of CD20-positive B-lymphocytes and CD3-positive T-lymphocytes, and sometimes thinly distributed CD138-positive plasma cells. The inner layer of the neo-capsule samples showed predominantly diffusely distributed lymphocytes (diffuse infiltration). In the intermediate vascular layer, the infiltrates were mostly found to be surrounding postcapillary venules (perivascular infiltration; Figures 4 and ). Dominant T-lymphocytic or plasma cell infiltrates were not found in any of the 46 cases.
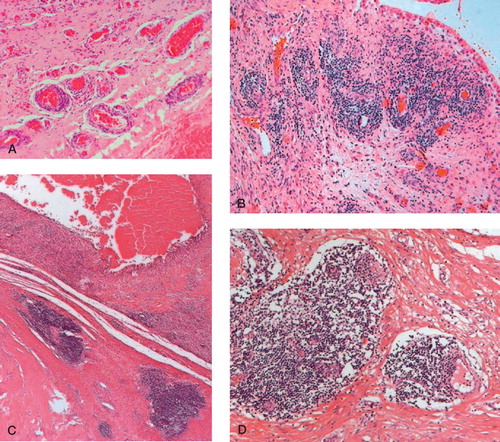
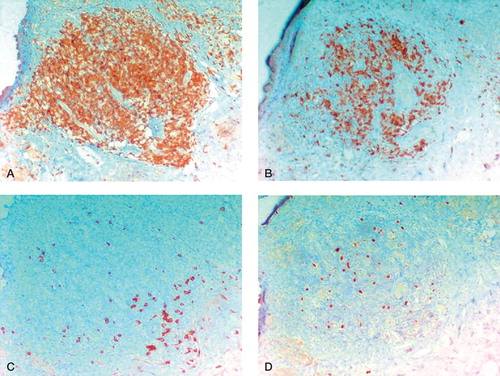
Figure 5. Perivasal accumulated lymphocytic infiltrates (H&E-stained sections). A. “Many” perivascular lymphocytic infiltrates (magnification 100×). B. “Abundant” perivascular lymphocytic infiltrates; few lymphocytic follicles without germinal centers (magnification 100×). C. and D. “Excessive” lymphoplasma cellular infiltration; lymphocytic follicles with formation of germinal centers in the intermediate vascular layer (magnification 25× (C) and 100× (D)).
Diffuse lymphocytic infiltrates
11 of the 39 cases showed stronger (“many”/ “abundant”) diffuse infiltrations of the inner neocapsular layer without any significant correlation to the diagnosis leading to revision. No “excessive” diffuse lymphocytic infiltrations were present in any of the cases. The extent of diffuse lymphocytic infiltration (“none”/“few” as opposed to “many”/“excessive”) was no different between cases with in situ times of more or less than 1 year (59% vs. 47%). Furthermore, there was no correlation between the extent of diffuse lymphocytic infiltration and the extent of particle storage and histiocytic reaction of the neo-capsule probes (McNemar test; p = 0.04, κ = 1%) and the extent of necrosis (p = 0.8), respectively.
Perivascular lymphocytic infiltration
14 cases (7 cases with aseptic loosening, 2 cases after femoral neck fracture, 3 cases revised due to heterotopic ossification, 2 cases with chronic synovitis) showed a stronger (“many”/“abundant”) perivascular lymphocytic infiltration. In 2 cases (1 with aseptic loosening and 1 with recurrent dislocation), an “excessive” perivascular infiltration was present ().
The extent of perivascular lymphocytic infiltration (“none”/“few” as opposed to “many”/ “excessive”) was no different between cases with in situ times of more or less than 1 year (41% vs. 42%). Furthermore, the extent of perivascular lymphocytic infiltration did not show any agreement with the metallic particle burden of the neo-capsule probes (McNemar test; p = 0.3, κ = 14%) and the extent of necrosis (p = 0.6, κ = 4%), respectively. On the other hand, higher grade perivascular infiltration (“many”/“excessive”) tended to coincide with stronger diffusely distributed infiltrates in the inner layer of the neo-capsule (McNemar test; p = 0.1, 95% CI: 42–80%).
Cases with excessive lymphocytic infiltration
2 cases showed “excessive” perivascular lymphocytic infiltrates in the intermediate vascular layer, associated with abundant diffuse infiltrates in the inner layer of the neo-capsule. In both specimens lymphatic follicles were detectable, with formation of germinal centers (). The follicles consisted of T- and B-lymphocytes. The follicle centers showed increased proliferative activity (Ki 67/MIB 1 index < 5% positive cells) and in the periphery a plasma-cell line was recognizable ().
Figure 6. Different immunohistological stainings for a prominent synovial perivascular lymphatic follicle (magnification 100×). A. Follicular pattern of CD3-positive T-lymphocytes (brown-colored). B. Follicular pattern of CD20-positive B-lymphocytes. C. In the periphery, a small line of CD138-positive plasma cells. D. Increased number of MIB-1-positive lymphocytes showing proliferation.
In both cases, the revision surgeons reported milky-caseous exudates. Fibrinous exudates and a stronger degree of necrosis (grade 2+ and 3+) were also detectable histologically. 1 case (a 56-year-old woman) was revised due to aseptic loosening accompanied by a massive pelvic osteolysis 21 months after primary implantation. The second case (a 62-year-old woman) was revised due to recurrent dislocations 35 months after primary implantation. Implants were stable and there was no osteolysis present, but the joint was filled with milky-caseous exudates, which are considered untypical for cases of dislocation.
Discussion
Metal-or ceramic-on-polyethylene total hip replacements have been shown to generate wear particles in high numbers. Sub-micron-sized polyethylene particles are phagocytosed by histiocytes in periprosthetic tissues, larger particles are phagocytosed by giant cells, leading to the release of inflammatory and osteolytic mediators—result-ing in periprosthetic osteolysis, and often aseptic loosening (Howie et al. Citation1988, Maloney et al. Citation1990, Murray and Rushton Citation1990, Amstutz et al. Citation1992, Athanasou et al. Citation1992, Schmalzried et al. Citation1992, Glant et al. Citation1993, Campbell et al. Citation1994, Margevicius et al. Citation1994, Shanbhag et al. Citation1994, Willert and Semlitsch Citation1996, Massin et al. Citation2004, Ingham and Fisher Citation2005). Lymphocytic reactions do not appear to play an important role in polyethylene wear particle-related osteolysis (Jiranek et al. Citation1995, Doorn et al. Citation1996, Davies et al. Citation2005, Willert et al. Citation2005).
The hypothetical advantage of metal-on-metal total hip replacements and hip resurfacings is based on the observation that the wear rate is much lower than in metal- or ceramic-on-polyethylene prostheses (Sieber et al. Citation1999, McMinn Citation2003, Kremling et al. Citation2004). The particles produced are, however, orders of magnitude smaller and many more particles are produced (Goldsmith et al. Citation2000, Ingham and Fisher Citation2000, Firkins et al. Citation2001). Such very small particles may provoke biological reactions that have been unrecognized previously.
Most neo-capsule tissue samples in our series contained only small amounts of metallic wear particles that could be seen in the light microscope. “Slate blue” histiocytes were mostly present, indicative of predominantly sub-micron particle sizes. Accordingly, the histiocytic foreign-body reaction was relatively mild. Only 6/46 cases showed larger numbers of phagocytosing macrophages, but there was no substantial granuloma formation.
The characteristic histological pattern of the neo-capsules examined consisted of diffuse and perivascularly-oriented infiltration of T-and Blymphocytes accompanied by plasma cells. This histological phenomenon has not been reported in tissue analyses of metal- or ceramic-on-polyethyl-ene hip replacements (Heilmann et al. Citation1975, Mirra et al. Citation1976, Willert Citation1977, Howie Citation1990, DiCarlo and Bullough Citation1992, Willert and Semlitsch Citation1996, Davies et al. Citation2005, Willert et al. Citation2005).
Described as aseptic lymphocyte-dominated vas-culitis-associated lesion (ALVAL) or as lympho-cyte-dominated immunological answer (LYDIA), lymphocytic infiltration has also been observed in periprosthetic tissues obtained during revision of first-and second-generation metal-on-metal implants by other study groups (Willert and Semlitsch Citation1996, Willert et al. Citation2000, Citation2001, Citation2005, Campbell et al. Citation2001, Al-Saffar Citation2002, Bohler et al. Citation2002). It is important to note that these histological patterns were seen in association with both total hip replacement and hip resurfacing designs of metal-on-metal prostheses. As in our investigation, too, devices of various different manufacturers showed similar histological changes. Davies et al. (Citation2005) concluded that perivascular lymphocytic cuffs and surface ulceration were associated with metal-on-metal prostheses. Our findings support the notion that prominent lymphocytic infiltration is the characteristic histological pattern of this bearing type. In contrast to the findings of Davis et al., the extent of superficial necrosis was neither associated with the extent of diffuse lymphocytic infiltration of the inner neo-capsule layer nor with the extent of peri-vascularly-oriented lymphocytic infiltrates. Our findings do not support the hypothesis of lymphocytic infiltration leading to necrosis, ulceration and implant failure per se.
The lymphocytic infiltrates consisted of CD3-pos-itive T-cells and CD20-positive B-cells. Occasional, but sparsely distributed CD138-positive plasma cells were found. None of our cases showed a dominant infiltration of T-lymphocytes, plasma cells or eosinophilic granulocytes. Considering the immunohistological results, the cases without “excessive” lymphocytic infiltration may represent the changes of an adaptive immune response. This histological pattern was also found to be present in joints revised for heterotopic bone formation, and has been reported in periprosthetic tissues from well-fixed metal-on-metal implants (Davies et al. Citation2005).
2 of our 46 cases showed “excessive” perivascular lymphocytic infiltrates without clinical and histological signs of a bacterial infection. In both specimens, the histological pattern of an activation and maturation of B-cells into plasma cells was found. This observation seems to have been the consequence of an adaptive specific immune response and was associated with necrosis of the neo-capsule in both cases, and pelvic osteolysis and implant loosening in one. It may be hypothesized that this pathological immune reaction might cause premature failure of metal-on-metal implants. The histological pattern of contact allergy to metals and reported cases of peri-implantar T-cell rich inflammatory infiltrations, interpreted as allergic reactions, differ from the pattern found in these two cases (Hallab et al. Citation2001, Davies et al. Citation2005). But a type-IV (delayed type) hypersensitivity reaction on a soluble antigen cannot be ruled out, and is in fact more probable given the abundant infiltrates shown. The diagnostic relevance of patch tests— reported in studies on total knee replacements and other metallic implants—is therefore unclear (Thomas et al. Citation2000, Schoberl et al. Citation2004, Niki et al. Citation2005, Citation2006).
From a methodological point of view, the above conclusions must be carefully reconsidered. Since several sources of heterogeneity in the underlying data must be kept in mind, the overall sample size of 46 cases imposes obvious limitations on the generalizability of the above results.
In summary, distinct lymphocytic infiltration may be considered to be a characteristic histological pattern of tissue reactions on metal particles and/ or ions around metal-on-metal bearings, not necessarily leading to implant failure. The prevalence of specific immune reactions, which may cause premature failure of metal-on-metal implants, is probably low given the reported success of these bearings; nevertheless, it is still unknown at present. Thus, further investigations on the prevalence, association with clinical failure patterns, and diagnostic tools for detection of these pathological immune reactions are recommended.
The authors wish to thank Dr Patrick Hunt for his accurate revision of the article and Midland Medical Technologies Ltd., Birmingham, UK, for an unrestricted grant.
Contributions of authors
WCW and UH: designed the study and gathered the data. UH, NK, FK and WCW: analyzed the data. WCW: wrote the initial drafts. WCW and KPG: ensured the accuracy of the data and analysis. WCW and UH: contributed equally to the publication.
- Al-Saffar N. Early clinical failure of total joint replacement in association with follicular proliferation of B-lympho-cytes: a report of two cases. J Bone Joint Surg (Am) 2002; 84: 2270–3
- Amstutz H C, Campbell P, Kossovsky N, Clarke I C. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop 1992, 276: 7–18
- Amstutz H C, Beaule P E, Dorey F J, Le Duff M J, Campbell P A, Gruen T A. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg (Am) 2004; 86: 28–39
- Athanasou N A, Quinn J, Bulstrode C J. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J Bone Joint Surg (Br) 1992; 74: 57–62
- Athanasou N A, Pandey R, de Steiger R, Crook D, Smith P. M. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg (Br) 1995; 77: 28–33
- Bachfischer K, Karpf P M, Schwarz R. An unusual observation of total hip replacement with meta on metal gliding bearings. Z Orthop Ihre Grenzgeb 2000; 138: 230–4
- Beaule P E, Le Duff M, Campbell P, Dorey F J, Park S H, Amstutz H C. Metal-on-metal surface arthroplasty with a cemented femoral component: a 7–10 year follow-up study. J Arthroplasty 2004; 19: 17–22
- Bohler M, Kanz F, Schwarz B, Steffan I, Walter A, Plenk H, Knahr K. Adverse tissue reactions to wear particles from Co-alloy articulations, increased by alumina-blasting particle contamination from cementless Ti-based total hip implants. J Bone Joint Surg (Br) 2002; 84: 128–36, A report of seven revisions with early failure
- Campbell P, Ma S, Schmalzried T, Amstutz H C. Tissue digestion for wear debris particle isolation. J Biomed Mater Res 1994; 28: 523–6
- Campbell P, Mirra J, Doorn P F. Histopathology of metal-on-metal hip joint tissues. C B. S Rieker, U Wyss. Hans Huber Verlag, Bern 2001; 167–80, World tribology forum in arthroplasty
- Daniel J, Pynsent P B, McMinn D J. W. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg (Br) 2004; 86: 177–84
- Davies A P, Willert H G, Campbell P A, Learmonth I D, Case C P. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg (Am) 2005; 87: 18–27
- DiCarlo E F, Bullough P G. The biologic responses to orthopedic implants and their wear debris. Clin Mater 1992; 9: 235–60
- Doorn P F, Mirra J M, Campbell P A, Amstutz H C. Tissue reaction to metal on metal total hip prostheses. Clin Orthop 1996, 329 S: S187–205
- Dorr L D, Wan Z, Longjohn D B, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. Four to seven-year results. J Bone Joint Surg (Am) 2000; 82: 789–98
- Evans E M. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Joint Surg (Br) 1974; 56: 626–42
- Firkins P J, Tipper J L, Saadatzadeh M R, Ingham E, Stone M H, Farrar R, Fisher J. Quantitative analysis of wear and wear debris from metal-on-metal hip prostheses tested in a physiological hip joint simulator. Biomed Mater Eng 2001; 11: 143–57
- Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad H D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 1991; 138: 867–73
- Glant T T, Jacobs J J, Molnar G, Shanbhag A S, Valyon M, Galante J O. Bone resorption activity of particulate-stimu-lated macrophages. J Bone Miner Res 1993; 8: 1071–9
- Goldsmith A A, Dowson D, Isaac G H, Lancaster J G. A comparative joint simulator study of the wear of metal-on-metal and alternative material combinations in hip replacements. Proc Inst Mech Eng [H] 2000; 214: 39–47
- Hallab N, Merritt K, Jacobs J J. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg (Am) 2001; 83: 428–36
- Heilmann K, Diezel P B, Rossner J A, Brinkman K A. Morphological studies in tissues surrounding alloarthroplastic joints. Virchows Arch A Pathol Anat Histol 1975; 366: 93–106
- Howie D W. Tissue response in relation to type of wear particles around failed hip arthroplasties. J Arthroplasty 1990; 5: 337–48
- Howie D W, Vernon-Roberts B, Oakeshott R, Manthey B. A rat model of resorption of bone at the cement-bone interface in the presence of polyethylene wear particles. J Bone Joint Surg (Am) 1988; 70: 257–63
- Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng [H] 2000; 214: 21–37
- Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005; 26: 1271–86
- Jacobs J J, Hallab N J, Skipor A K, Urban R M. Metal degradation products: a cause for concern in metal-metal bearings?. Clin Orthop 2003, 417: 139–47
- Jiranek W, Jasty M, Wang J T, Bragdon C, Wolfe H, Goldberg M, Harris W. Tissue response to particulate polymethylmethacrylate in mice with various immune deficiencies. J Bone Joint Surg (Am) 1995; 77: 1650–61
- Knecht A, Witzleb W C, Beichler T, Guenther K P. Functional results after surface replacement of the hip: comparison between dysplasia and idiopathic osteoarthritis. Z Orthop Ihre Grenzgeb 2004; 142: 279–85
- Korovessis P, Petsinis G, Repanti M. Zweymueller with metal-on-metal articulation: clinical, radiological and histological analysis of short-term results. Arch Orthop Trauma Surg 2003; 123: 5–11
- Kremling U, Franke F, Witzleb W-C. Tribological investigations on metal-on-metal bearings during wear tests in a hip simulator according ISO 14 242. Mat.-wiss. u. Werkstofftech 2004; 35: 1–8
- Lintner F, Boehm G, Huber M, Zweymueller K. Synovial tissue following revision total hip replacement with metal-on-metal joints. Histologic, immunohistologic, morphometric and bacteriologic evaluation. Osteologie 2003; 12: 233–46
- Maloney W J, Jasty M, Harris W H, Galante J O, Callaghan J J. Endosteal erosion in association with stable uncemented femoral components. J Bone Joint Surg (Am) 1990a; 72: 1025–34
- Maloney W J, Jasty M, Rosenberg A, Harris W H. Bone lysis in well-fixed cemented femoral components. J Bone Joint Surg (Br) 1990b; 72: 966–70
- Margevicius K J, Bauer T W, McMahon J T, Brown S A, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg (Am) 1994; 76: 1664–75
- Massin P, Chappard D, Flautre B, Hardouin P. Migration of polyethylene particles around nonloosened cemented femoral components from a total hip arthroplasty-an autopsy study. J Biomed Mater Res B Appl Biomater 2004; 69: 205–15
- McKellop H, Park S H, Chiesa R, Doorn P, Lu. B, Normand P, Grigoris P, Amstutz H C. In vivo wear of three types of metal on metal hip prostheses during two decades of use. Clin Orthop 1996, 329S: S128–40
- McMinn D J W. Development of metal/metal hip resurfacing. Hip International 2003; 13: 41–53
- Mirra J M, Amstutz H C, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop 1976, 117: 221–40
- Murray D W, Rushton N. Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg (Br) 1990; 72: 988–92
- Niki Y, Matsumoto H, Otani T, Yatabe T, Kondo M, Yoshimine F, Toyama Y. Screening for symptomatic metal sensitivity: a prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials 2005; 26: 1019–26
- Niki Y, Matsumoto H, Otani T, Yatabe T, Funayama A, Maeno S, Tomatsu T, Toyama Y. Phenotypic characteristics of joint fluid cells from patients with continuous joint effusion after total knee arthroplasty. Biomaterials 2006; 27: 1558–65
- Schmalzried T P, Jasty M, Harris W H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg (Am) 1992; 74: 849–63
- Schmidt M, Weber H, Schon R. Cobalt chromium molybdenum metal combination for modular hip prostheses. Clin Orthop 1996, 329S: S35–47
- Schoberl A, Summer B, Jakob K, Barnstorf S, Thomas P. Periimplantar cobalt-specific DTH reaction in a patch test negative patient with failure of hip arthroplasty. J Allergy Clin Immunol 2004; 113: S250
- Semlitsch M, Streicher R M, Weber H. The wear behavior of capsules and heads of CoCrMo casts in long-term implanted all-metal hip prostheses. Orthopade 1989; 18: 377–81
- Shanbhag A S, Jacobs J J, Black J, Galante J O, Glant T T. Macrophage/particle interactions: effect of size, composition and surface area. J Biomed Mater Res 1994; 28: 81–90
- Sieber H P, Rieker C B, Kottig P. Analysis of 118 secondgeneration metal-on-metal retrieved hip implants. J Bone Joint Surg (Br) 1999; 81: 46–50
- Streicher R M, Semlitsch M, Schon R, Weber H, Rieker C. Metal-on-metal articulation for artificial hip joints: laboratory study and clinical results. Proc Inst Mech Eng [H] 1996; 210: 223–32
- Thomas P, Summer B, Sander C A, Przybilla B, Thomas M, Naumann T. Intolerance of osteosynthesis material: evidence of dichromate contact allergy with concomitant oligoclonal T-cell infiltrate and TH1-type cytokine expression in the peri-implantar tissue. Allergy 2000; 55: 969–72
- Treacy R B, McBryde C W, Pynsent P B. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg (Br) 2005; 87: 167–70
- Vernon-Roberts B. Prosthetic implant reactions. Aust N Z J Med (Suppl 1) 1978; 8: 159–62
- Wagner M, Wagner H. Preliminary results of uncemented metal on metal stemmed and resurfacing hip replacement arthroplasty. Clin Orthop 1996, 329S: S78–88
- Wagner M, Wagner H. Medium-term results of a modern metal-on-metal system in total hip replacement. Clin Orthop 2000, 379: 123–33
- Willert H G. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res 1977; 11: 157–64
- Willert H G, Semlitsch M. Tissue reactions to plastic and metallic wear products of joint endoprostheses. Clin Orthop 1996, 333: 4–14
- Willert H G, Buchhorn G H, Fayyazi A, Lohmann C. H. Histopathological changes around metal/metal joints indicate delayed type hypersensitivity. Osteologie 2000; 9: 2–16, Preliminary results of 14 cases
- Willert H G, Buchhorn G H, Fayyazi A, Lohmann C H. Histopathological changes in tissues surrounding metal/metal joints -Signs of delayed type hypersensitivity?. C Rieker, S Onerholzer, U Wyss. Hans Huber Verlag, Bern 2001; 147–66, World tribology forum in arthroplasty
- Willert H G, Buchhorn G H, Fayyazi A, Flury R, Windler M, Koster G, Lohmann C H. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg (Am) 2005; 87: 28–36
- Witzleb W-C, Knecht A, Beichler T, Koehler T, Guenther K P. Hip resurfacing arthroplasty. Orthopade 2004; 33: 1236–42