Abstract
Background The identity of the vital active ingredient within synovial fluid (SF)—to which we owe the near frictionless performance of diarthrodial joints—has been the quest of researchers for many years. Initially, hyaluronic acid (HA) was thought to be the lubricant, but it has been shown not to possess the load-bearing ability required within the physiological joint. The glycoprotein fraction of synovial fluid (lubricin) has been shown to have the same lubricating ability as synovial fluid. All or part of this is thought to be due to the sur-face-active phospholipids (SAPLs) present in lubricin. We characterized the SAPLs adsorbed on the surface of retrieved prostheses which have been implicated as the boundary lubricant.
Material and methods Rinsing fluids collected from the bearing surfaces of 40 prostheses removed from hip and knee revision operations were analyzed using highperformance liquid chromatography (HPLC).
Results SAPLs were detected on all retrieved implants. During the study, 8 different species of phosphatidylcholines were identified. We also determined the relative concentration of each species, which suggested that the unsaturated SAPL species predominate.
Interpretation It is of value to know the identity of the lubricating constituents of SF, not only for the future development of artificial joints, but also in developing cures for several disease processes in which lubrication plays a role.
Synovial joints are perhaps the most sophisticated and complex tribological system ever to exist. Much research over the past 75 years has generated several competing theories as to how the joint is lubricated. In recent years, boundary lubrication has gained much support (Hills Citation1989, Saikko and Ahlroos Citation1997, Jay and Cha Citation1999, Liao et al. Citation1999, Hills Citation2000, Sarma et al. Citation2001, Gale et al. Citation2004, Mazzucco and Spector Citation2004, Ozturk et al. Citation2004, Gale et al. Citation2007) and the focus has shifted towards identifying the actual component of the synovial fluid that has this remarkable role. Briefly, the apposing bearing surfaces are protected at the molecular level by boundary (solid-to-solid) lubrication involving some unknown substance. Several different substances have been proposed as possible boundary lubricants. However, the nature of the surfactants (i.e. surface-active agents) has not been fully resolved (Swann and Radin Citation1972, Davis et al. Citation1978, Jay and Cha Citation1999, Sarma et al. Citation2001, Hills and Jay Citation2002). Earlier models considered HA as the predominant boundary lubricant, but later work (Linn and Radin Citation1968, Swann et al. Citation1974, Hills and Monds Citation1998b, Jay et al. Citation1998) showed that HA only affected the viscosity of the joint fluid and served no purpose as a load-bearing boundary lubricant. Subsequently, a highly purified glycoprotein fraction of synovial fluid named lubricin was claimed to be the boundary lubricant after it was observed to impart the lubricating properties of joint fluid (Swann et al. Citation1981). Lubricin consists mainly of protein and carbohydrate (Swann and Mintz Citation1979), with a small fraction (∼12%) identified as phospholipid in nature (Schwarz and Hills Citation1998). Schwarz et al. were able to show that this sub-frac-tion showed excellent lubricating properties akin to that found in the physiological joint. Proteolipids in synovial fluid have been implicated as the source of the surface-active phospholipid (SAPL) in the joint (Swann and Mintz Citation1979, Schwarz and Hills Citation1996).
Much research has been directed toward the lipid content of both SF and articular cartilage (AC) (Wright Citation1969, Prete et al. Citation1995, Pickard et al. Citation1998, Sarma et al. Citation2001, Ballantine and Stachowiak Citation2002). There is strong evidence of lipids in the normal joint (Jay Citation2004, Graindorge et al. Citation2006), but little attention has been given to the role of SAPLs in the artificial joint. Purbach et al. (Citation2002) were the first to report detection of SAPLs on retrieved hip prosthesis surfaces; however, their work did not reveal the identity of the SAPLs present.
There are two types of SAPLs in synovial fluid, (1) phosphatidylcholines (PCs) which predominate, and (2) non-phosphatidylcholines (e.g. phosphatidyl glycerol) which are present in smaller quantities. There are saturated PCs (SPCs) and unsaturated species (USPCs) (determined by the presence of double bonds in their fatty acid chains). Saturated PCs are the dominant class of PC in the lung, and, of these, dipalmitoyl phosphatidylcholine (DPPC) is the most surface-active (Hills Citation1988). To date, most research in boundary lubrication (Hills Citation1989, Williams III et al. Citation1993, Saikko and Ahlroos Citation1997, Jones et al. Citation2002, Gale et al. Citation2007) has employed DPPC, a saturated PC, as the lubricant. While this has been due in part to the fact that DPPC is the only PC commercially available for medical applications, it was also due to the fact that the role of unsaturated PCs has been investigated only recently (Chen and Hills Citation2004). The focus on saturated PCs may also be due to an assumption that PCs found outside the lung would be the same as those in the lung, which are mainly saturated PCs with DPPC being dominant. This assumption could not be further from the truth, as recent studies have shown an unequivocal dominance of unsaturated PCs in many other non-lung locations in the body (Hills Citation1991, Bernhard et al. Citation2001, Paananen et al. Citation2002, Mills et al. Citation2005a, Citationb). However, no studies have been conducted to completely characterize the molecular species of different classes of phospholipids bound to the joint surfaces. One study by Sarma et al. (Citation2001) included an attempt to identify some of the classes indirectly by analyzing the fatty acid chains attached to phosphatidylcholine backbones.
Chen et al. (Citation2005) were able to show that USPCs from peritoneal dialysate performed better as a boundary lubricant than DPPC, a saturated PC. The question has been raised as to what species are predominant in the joint and what implications this has for joint lubrication
The aim of the present study, the first of its kind, was to analyze the SAPLs found on the surface of retrieved artificial implants, and to generate a profile of the various constituents. In particular, we felt it important to classify all species of phosphatidylcholines and to identify individual saturated and unsaturated phosphatidylcholines using highperformance liquid chromatography (HPLC).
Material and methods
Retrieved implants, removed from patients at the time of revision surgery by one of the authors, were used. Initially, we studied hip implants only but later we included knee implants. 38 hip and 2 knee prostheses were assessed. Patient data were not disclosed. The implants were of typical construction, a metallic component coupled to an ultra-high molecular weight polyethylene (UHMWPE) component.
Implants removed from patients were analyzed immediately (after transportation in a suitable cool box), or in some cases they were frozen for various lengths of time. Previous studies (Gale et al. Citation2004, Citation2007) have shown that washing with saline has little effect on the retention of lipids; therefore, where necessary saline was used to wash the bearing surfaces free of cellular debris. Lipids were removed by holding the component over a beaker and washing the bearing surface with a strong lipid solvent (Folch solution; 2:1 chloroform/methanol). Special attention was paid to ensure that only the bearing surfaces were washed. As there were many different types and sizes of implants, the amount of lipid removed varied. The aim of the study was to determine the various species of PC present. This was achieved by comparing the amounts of each PC species recovered against the total amount of PCs removed from the bearing surface of each component. Both components, metallic and UHMWPE, were analyzed. One-way ANOVA was used to compare the PC profiles of the metallic and polymer components of the implant.
The rinsings were then analyzed using HPLC. An 1100-series HPLC system (Agilent Technologies, Forest Hill, Victoria, Australia) was used in combination with an RF-10AXL fluorescence detector (Shimadzu, Kyoto, Japan). Separations were screened on a Phenosphere-NEXT C18 column (250 ± 2 mm internal diameter; 5‐µm particles) from Phenomenex Pty Ltd. (Pennant Hills, NSW, Australia). The chromatographic conditions were based on those used in a published study (Bernhard et al. Citation1994). Briefly, the mobile phase was methanol (92.5% v/v) and water (7.5% v/v) with 40 mM choline chloride. The flow rate was 0.6 mL/min. The eluent was monitored by a fluorescence detector at 340/460 nm (excitation/emission) after postcolumn derivatization of mixed micelles with DPH (diphenyl hexatriene) using a 100-cm reaction coil at 50°C (Bernhard et al. Citation1994).
Results
All samples exhibited substantial amounts of sur-face-active phospholipids. Because of the various types and sizes of implants analyzed and the nature of the rinsing process, the actual amounts measured in µg will not be published here, but rather the profiles of the SAPLs detected. As a result of the study, 8 different species of phosphatidylcholines were identified: DLPC dilinoleoyl phosphatidylcholine (USPC) PLPC palmitoyl linoleoyl phosphatidylcholine (USPC)
DPPC dipalmitoyl phosphatidylcholine (SPC) POPC palmitoyl oleoyl phosphatidylcholine (USPC)
DOPC dioleoyl phosphatidylcholine (USPC) SLPC stearoyl linoleoyl phosphatidylcholine (USPC)
PSPC palmitoyl stearoyl phosphatidylcholine (SPC)
OSPC oleoyl stearoyl phosphatidylcholine(USPC)
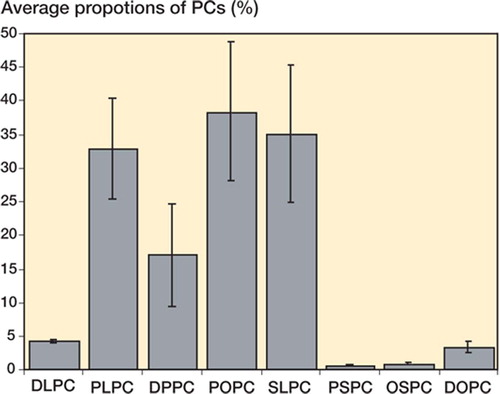
Average proportion of PC species (%)
The relative proportion of each PC was determined (Table). The Figure represents the total profile of all the implants, including both the metallic and polymer components. The predominant SAPL components identified were POPC, SLPC, and PLPC, all of which are unsaturated PCs. DPPC predominated in the saturated species detected. USPCs were more common than SPCs by a factor of 4.
Discussion
Synovial fluid has been studied extensively in an attempt to identify the components that provide effective boundary lubrication under severe loading conditions (Swann and Mintz Citation1979, Hills and Butler Citation1984, Schwarz and Hills Citation1996, Jay Citation2004). Phospholipids have been implicated in the boundary lubrication of joints (Hills and Monds Citation1998a, Citationb, Schwarz and Hills Citation1998), lungs (Hills Citation2000), and the peritoneum (Chen and Hills Citation2000). We note, however, that these conclusions differ from those of others, who have proposed lubricin as the boundary lubricant. This debate has been discussed elsewhere (Hills and Jay Citation2002), the conclusion being that further investigations are required.
It is important to note here that the main aim of our study was to define the species present on retrieved implant surfaces, and to determine the relative amounts of these species rather than the actual amounts. The quantity of these lubricating molecules may play an important role in the longevity of the joint, be it natural or artificial. Quantifying and comparing the amount of lipids present between each retrieved implant was beyond the scope of this study, but should be considered in future studies. Because of the difficulty in obtaining healthy joints, this study dealt with what may be argued as being “abnormal” joints—those removed at the time of revision surgery. Osteoarthritis (OA), the main reason for total joint replacement, is still not fully understood, but it is known that there is a change in the synovial fluid. SF from OA joints has shown a reduction in viscosity (Swann et al. Citation1974) but only a loss of lubricating ability in 8% of patients (Swann et al. Citation1984). Even so, Purbach et al. (Citation2002) have shown the presence of lipids on retrieved implants in nearly all cases. OA may involve depletion in the amount of lubricating molecules, but it seems that traces of SAPLs are always present. This study has characterised the SAPLs present in human joints. Even if SAPLs are only present in reduced quantity, choosing a material for artificial joints that can utilise this type of boundary lubricant to its full potential will dramatically increase the life of the prosthesis.
Due to the small number of knee implants analyzed, no statistical conclusions can be drawn at this stage except to say that the same profile was seen for both hips and knee implants.
We observed no trends (using ANOVA; p < 0.05) in the proportion of PCs found in the two different components of the implant. Both the metallic and plastic components were found to have approximately the same proportions of PC species adsorbed to their surfaces (Table). It is intriguing that there was no difference between the 2 surfaces in this respect, given the very different nature of the materials.
From a tribological point of view, boundary lubricants are analyzed with regard to several parameters such as the chain length, orientation of the head group, packing, charge and adsorbance (tenacity) to the surface. The present study focused on the composition of the PCs, and addressed the degree of saturation.
The major SAPL components identified were POPC, SLPC, and PLPC (each representing about 30% of the total)—all 3 of which are unsaturated PCs. This constitutes a larger percentage of unsaturated species (∼80%) than saturated species. This finding is different to the PC profile found in the lung, which is predominantly saturated in nature (Mills et al. Citation2005a). The reason for the difference in SAPL profiles between lung tissue and non-lung tissues has been proposed to be due to differences in the ability of saturated and unsaturated PCs to reduce surface tension (Bernhard et al. Citation2001). Furthermore, DPPC has a gel-liquid crystal transition temperature of 41.5°C, which makes the molecule effectively rigid at body temperature and thus better able to reduce surface tension. However, unsaturated PCs have phase transition temperatures far below body temperature, which enables them to adsorb to surfaces more easily. In order to explain the phospholipid profile of the joint surface in more detail, the function of a boundary lubricant must be examined. Boundary lubrication occurs when a molecular layer separates the two bearing surfaces. During boundary lubrication, friction and wear between the two surfaces are determined by the properties of the surfaces and the properties of the lubricant, other than viscosity (Fein Citation1984). It has been shown in several boundary lubrication studies that longer hydrocarbon chain lengths and the degree of saturation affect the coefficient of friction (Fuller Citation1956, Hills and Monds Citation1998b). Our recent research (Chen et al. Citation2005), in which we compared the lubricating ability of PLPC and POPC (unsaturated) with that of DPPC (saturated), showed that PLPC and POPC were able to reduce the coefficient of friction more than DPPC. It can be concluded that the unsaturated species of PCs reduce the coefficient of friction more than saturated species. Previous work suggested that DPPC is the boundary lubricant, and while frictional performance was impressive, our study offers the promise of even better frictional performance using the more predominant unsaturated species. The data obtained in this study shows that POPC, SLPC, and PLPC are the major constituents of the SAPLs adsorbed to joint surfaces, and the main component is not just DPPC as previously assumed. It is clear that a combination of SAPLs rather than a single SAPL constitutes the boundary lubricant of diarthrodial joints. Thus, we intend to formulate a lubricant with a composition that closely resembles the proportion of unsaturated and saturated PCs found in the joint. This would represent an “artificial joint fluid” suitable for future friction and wear studies.
We are very grateful to Sarah Whitehouse for the statistical analysis.
Contributions of authors
LG performed most of the study assisted by YC, under the supervision of BH (recently deceased) and RC.
- Ballantine G C, Stachowiak G W. The effects of lipid depletion on osteoarthritic wear. Wear 2002; 253(3-4)385–93
- Bernhard W, Linck M, Creutzburg H, Postle A, Arning A, Martin-Carrera I. High-performance liquid chromatographic analysis of phospholipids from different sources with combined fluorescence and ultraviolet detection. Anal Biochem 1994; 1(220)172–80
- Bernhard W, Postle A, Rau G, Freihorst J. Pulmonary and gastric surfactants. A comparison of the effect of surface requirements on function and phospholipid composition. Comp Biochem Physiol A Mol Integr Physiol 2001; 129: 173–82
- Chen Y, Hills B A. Surgical Adhesions: Evidence for Adsorption of Surfactant to Peritoneal Mesothelium. ANZ J Surg 2000; 70(6)443–7
- Chen Y, Hills B A. Unsaturated phosphatidylcholines and uses thereof. International Patent Application - Australia. 2004
- Chen Y, Hills B A, Hills Y C. Unsaturated phosphatidylcholine and its application in surgical adhesion. ANZ J Surg 2005; 75(12)1111–4
- Davis W H, Jr., Lee S L, Sokoloff L. Boundary lubricating ability of synovial fluid in degenerative joint disease. Arthritis Rheum 1978; 21(7)754–6
- Fein R S. Boundary Lubrication. CRC Handbook of Lubrication (Theory and practice of tribology), E R Booser, R Boca. CRC Press, , Florida 1984; 49–68
- Fuller D D. Boundary Lubrication. Theory and practice of lubrication for engineers1st. Chapman & Hall, New York 1956; 342–72
- Gale L R, Coller R, Hargreaves D J, Hills B A, Crawford R W (2004) The role of SAPL as a boundary lubricant in Prosthetic Joints. Nordtrib. 11th Nordic Symposium on Tribology. June, 1-42004. Norway
- Gale L R, Coller R, Hargreaves D J, Hills B A, Crawford R. The role of SAPL as a boundary lubricant in prosthetic joints. Tribology Int 2007; 40(4)601–6
- Graindorge S, Ferrandez W, Ingham E, Jin Z, Twigg P, Fisher J. The Role of the surface amorphous layer of articular cartilage in joint lubrication. Part H. J Engin Med. Proceedings of the Institution of Mechanical Engineers. 2006; 220: 597–607, (5)
- Hills B A. The biology of surfactant. Cambridge University Press, Cambridge 1988
- Hills B A. Oligolamellar lubrication of joints by surface active phospholipid. J Rheumatol 1989; 16(1)82–91
- Hills B A. A common physical basis for the gastric mucosal barrier and the action of sucralfate. Am J Med 1991; 91: 43–9
- Hills B A. Boundary lubrication in vivo. Part H. J Engin Med. Proceedings of the Institution of Mechanical Engineers. 2000; 214: 83–94, (1)
- Hills B A, Butler B D. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann Rheum Dis 1984; 43(4)641–8
- Hills B A, Jay G D. Identity of the joint lubricant. J Rheumatol 2002; 29(1)200–1
- Hills B A, Monds M K. Deficiency of lubricating surfactant lining the articular surfaces of replaced hips and knees. Br J Rheumatol 1998a; 37: 143–7
- Hills B A, Monds M K. Enzymatic identification of the loadbearing boundary lubricant in the joint. Br J Rheumatol 1998b; 37(2)137–42
- Jay G D. Lubricin surfacing of articular joints. Curr Opin Orthop 2004; 15(5)355–9
- Jay G D, Cha C J. The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. J Rheumatol 1999; 26(11)2454–7
- Jay G D, Haberstroh K, Cha C J. Comparison of the bound-ary-lubricating ability of bovine synovial fluid lubricin Healon. J Biomed Mater Res 1998; 40(3)414–8
- Jones C F, Ozturk H E, Stoffel K K, Stachowiak G W. The Effect of Surface Active Phospholipids on the Lubrication of the Osteoarthritic Sheep Knee Joints: Wear. Tribology Letters 2002; 16(4)291–6
- Liao Y S, Benya P D, McKellop H A. Effect of protein lubrication on the wear properties of materials for prosthetic joints. J Biomed Mater Res 1999; 48(4)465–73
- Linn F C, Radin E L. Lubrication of animal joints III. The effect of certain chemical alterations of the cartilage and lubricant. Arthritis Rheum 1968; 11(5)674–82
- Mazzucco D, Spector M. The John Charnley Award Paper. The role of joint fluid in the tribology of total joint arthroplasty. Clin Orthop 2004, 429: 17–32
- Mills P, Chen Y, Hills Y, Hills B. Differences in surfactant lipids collected from pleural and pulmonary lining fluids. Pharm Res 2005a; 22(11)1926–30
- Mills P C, Hills Y, Hills B A. Surface-active phospholipid (surfactant) in equine tendon and tendon sheath fluid. N Z Vet J 2005b; 53(2)154–6
- Ozturk H E, Stoffel K K, Jones C F, Stachowiak G W, Ozturk H E, Stoffel K K. The Effect of Surface-Active Phospholipids on the Lubrication of Osteoarthritic Sheep Knee Joints: Friction. Tribology Letters 2004; 16(4)283–9
- Paananen R, Postle A, Clark G, Glumoff V, Hallman M. Eustachian tube surfactant is different from alveolar surfactant: determination of phospholipid composition of porcine eustachian tube lavage fluid. J Lipid Res 2002; 43: 99–106
- Pickard J E, Fisher J, Ingham E, Egan J. Investigation into the effects of proteins and lipids on the frictional properties of articular cartilage. Biomaterials 1998; 19(19)1807–12
- Prete P E, Gurakar-Osborne A, Kashyap M L. Synovial fluid lipids and apolipoproteins: a contemporary perspective. Biorheology 1995; 32(1)1–16
- Purbach B, Hills B A, Wroblewski B M. Surface-active phospholipid in total hip arthroplasty. Clin Orthop 2002, 396: 115–8
- Saikko V, Ahlroos T. Phospholipids as boundary lubricants in wear tests of prosthetic joint materials. Wear 1997; 207(1-2)86–91
- Sarma A V, Powell G L, LaBerge M. Phospholipid composition of articular cartilage boundary lubricant. J Orthop Res 2001; 19(4)671–6
- Schwarz I M, Hills B A. Synovial surfactant: lamellar bodies in type B synoviocytes and proteolipid in synovial fluid and the articular lining. Br J Rheumatol 1996; 35(9)821–7
- Schwarz I, Hills B. Surface-active phospholipid as the lubricating component of lubricin. Rheumatology 1998; 37(1)21–6
- Swann D, Mintz G. The isolation and properties of a second glycoprotein (LGP-II) from the articular lubricating fraction from bovine synovial fluid. Biochem J 1979; 179(3)465–71
- Swann D A, Radin E L. The Molecular basis of articular lubrication. I. Purification and properties of a lubricating fraction from bovine synovial fluid. J Biol Chem 1972; 247(24)8069–73
- Swann D A, Radin E L, Nazimiec M, Weisser P A, Curran N, Lewinnek G. Role of hyaluronic acid in joint lubrication. Ann Rheum Dis 1974; 33(4)318–26
- Swann D, Slayter H, Silver F. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem 1981; 256(11)5921–5
- Swann D A, Bloch K J, Swindell D, Shore E. The lubricating activity of human synovial fluids. Arthritis Rheum 1984; 27(5)552–6
- Williams P F, III, Powell G L, LaBerge M. Sliding friction analysis of phosphatidylcholine as a boundary lubricant for articular cartilage. Proc Inst Mech Eng [H] 1993; 207(1)59–66
- Wright V. Lubrication and wear in joints. V Wright. Sector Publishing Ltd, Leeds 1969