Abstract
Background The choice of optimal implant fixation in total hip replacement (THR)—fixation with or without cement—has been the subject of much debate.
Methods We performed a systematic review and meta-analysis of the published literature comparing cemented and uncemented fixation in THR.
Results No advantage was found for either procedure when failure was defined as either: (A) revision of either or both components, or (B) revision of a specific component. No difference was seen between estimates from registry and single-center studies, or between randomized and non-randomized studies. Subgroup analysis of type A studies showed superior survival with cemented fixation in studies including patients of all ages as compared to those that only studied patients 55 years of age or younger. Among type B studies, cemented titanium stems and threaded cups were associated with poor survival. An association was found between difference in survival and year of publication, with uncemented fixation showing relative superiority over time.
Interpretation While the recent literature suggests that the performance of uncemented implants is improving, cemented fixation continues to outperform uncemented fixation in large subsets of study populations. Our findings summarize the best available evidence qualitatively and quantitatively and provide important information for future research.
The success of total hip replacement (THR) and the frequency in which it is performed are largely due to the development of the cemented low-fric-tion arthroplasty (Charnley Citation1960); its survival rate of 80% at 25 years (Berry et al. Citation2002, Callaghan et al. Citation2004) remains unsurpassed. The improved survival of circumferentially coated uncemented cups and stems that allow bone to grow into or onto the prosthesis (Zicat et al. Citation1995, Kim et al. Citation1999, Della Valle et al. Citation2004, Sinha et al. Citation2004,) has supported their growing use in the United States, despite the higher costs (Agins et al. Citation1988, Barber and Healy Citation1993, Clark Citation1994, Mendenhall Citation2004). In 2003, an estimated two-thirds of all primary THRs were performed with uncemented fixation (Mendenhall Citation2004). This contrasts with some European countries such as Sweden, which have adopted these newer uncemented technologies more cautiously and have much lower revision rates (Malchau et al. Citation2002, Kurtz et al. Citation2005).
Both cemented and uncemented implants are heterogeneous groups with many factors that can influence survivorship, such as geometry, materials, surface finishes, and bearings. Moreover, study-specific factors including surgical approach, expertise of the surgeon, and study design may add to baseline differences between studies. In order to summarize the best available evidence on the relative success of cemented and uncemented fixation in THR from comparative studies, we conducted a systematic review of the literature and a meta-analysis. We concentrated specifically on the impact of cemented versus uncemented fixation on revision rates.
Material and methods
Search strategy and selection criteria
We performed a comprehensive search of Medline (1966–2005), BIOSYS (1990–2005), Embase (1993–2005), Web of Science (1990–2005), and the Cochrane Library (2005, issue 5) for articles published in English and those published in other languages. The reference list of each comparative study was manually examined to find additional relevant studies. Finally, additional studies were identified by contacting experts in the field and manufacturers of implants.
Inclusion criteria were established a priori to minimize any possible selection bias. The objective was to identify all studies including information on: (1) THR performed for any reason other than acute fracture, (2) controlled comparison of cemented vs. uncemented fixation, and (3) outcome as measured by survival to time of revision surgery for any reason. All randomized controlled trials and comparative observational studies with a control group were included. The following were excluded: (1) studies that included revision cases, (2) studies including cancer or tumor cases, (3) animal studies, (4) studies containing previously published data, (5) studies that did not report any revision events, and (6) case reports. Initial screening of articles was performed by one of us (SM). Two reviewers (SM and KJB) then independently assessed each of the studies for eligibility for inclusion. If the title or the abstract was judged by either reviewer to be potentially eligible, the full article was examined. Any disagreements were resolved by consensus.
Data extraction and synthesis
Data were extracted by one of us (SM) and checked for accuracy by a second investigator (KJB). Information retrieved from each study included survivorship estimates, study design, participants, implants and methods of fixation employed, definition of outcome measures, study setting, number of surgeons, statistical methods employed, factors that were used to match or stratify patients, patient characteristics, sample size and follow-up duration, withdrawal or censorship data, and potential sources of conflict of interest. Failure events were described as any revision surgery for removal or exchange of (A) either cup, stem or both, or (B) one specific component. We performed stratified analysis on key components of study design (i.e. randomized vs. non-randomized studies, age range, and definition of failure event) and regression analysis (meta-regression) on aggregate measures of patient characteristics within studies, in assessing whether study outcomes varied systematically with these features (Colditz et al. Citation1995). Reporting was carried out in line with QUOROM (Moher et al. Citation1999) and MOOSE (Stroup et al. Citation2000) guidelines.
Statistics
Differences in survival and standard error were derived from reported survival analysis estimates or from reported differences in the proportion of revised THRs. We performed meta-analysis using inverse-variance weighting (Sharp and Sterne Citation1998) to calculate fixed and random effects summary estimates. The convention in reporting results here is that summary estimates greater than zero favor uncemented fixation and those less than zero favor cemented fixation. Between-study heterogeneity was assessed using a Chi-square statistic (Lau et al. Citation1997) and the more conservative random effects estimate was reported. Studies performing multiple comparisons on the same treatment group or not specifying whether there was patient overlap between such repeated comparisons could result in a potential loss of independence. In such cases, adjustments were made to the weighting of studies using a previously described method for conservatively inflating variance estimates (Jordan et al. Citation2002, Enanoria et al. Citation2004).
We used subgroup analysis to explore heterogeneity potentially caused by discrete factors identified a priori. These included study design (randomized vs. non-randomized), study site (registry vs. single institution), component followed (cup versus stem), and patient age range (≤ 55 versus < 55 years of age). We also tested the hypothesis that certain groups of implants that have performed poorly in observational studies could influence summary estimates, such as titanium stems and screw-fit or macro-ingrowth cups (Robinson et al. Citation1989, Tompkins et al. Citation1994, Rorabeck et al. Citation1996b, Kubo et al. Citation2001, Aldinger et al. Citation2004, Fink et al. Citation2004, Grant and Nordsletten Citation2004). Sensitivity analysis was performed to assess the contribution of each individual comparison to the summary estimate. Meta-regression was used to evaluate the association between study results and year of publication, duration of follow-up, and characteristics of the study sample including sample sex ratios and average age. A p-value of less than 0.05 was considered significant. Potential for publication bias was evaluated with the use of Egger's test for funnel plot asymmetry (Egger et al. Citation1997). All analyses were performed using STATA 8.2 (Stata Corporation, College Station, TX).
Results
Of the 747 citations identified after literature searches, 20 studies (reporting 24 comparisons) met our inclusion criteria (). Study characteristics and survival estimates are summarized in and . When all 24 comparisons were pooled (), no significant benefit to either fixation method was found among subgroups defined by study setting (registry- or multiple center-based vs. those from single institutions), study design (randomized and non-randomized studies), or failure definition (type A: either component or both, vs. type B: specific component failure). All subsequent analyses were performed within subgroups defined by failure definition.
Figure 1. Flow diagram showing details of the literature search, including articles excluded at each stage of the review.
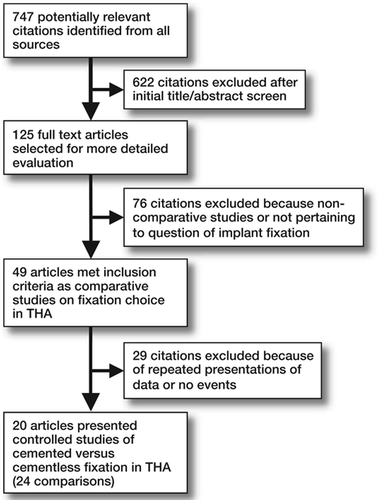
Table 1. Characteristics of the studies included (n = 20)
Table 2. Comparisons of fixation strategies and comparative survivorship results
Table 3. Results of meta-analysis
Type A failure definition: revision of cup or stem, or both
The forest plot () represents the pooled estimate showing no significant overall advantage of one fixation method over the other. The seven comparisons that did not restrict analysis to patients less than or equal to 55 years of age favored cemented fixation by 4% and differed significantly from the group of two studies that did (). Sensitivity analysis did not show a significant result with omission of any single study. Meta-regression did not show any significant associations between duration of follow-up, year of publication, age, or sex ratio and the outcome estimate. The Egger test for funnel plot asymmetry did not reveal any evidence of publication bias (p = 0.2).
Figure 2. Forest plot of cemented vs. uncemented fixation survivorship difference for type A studies (where failure is defined as revision of either or both components). Shaded boxes represent study-specific estimates with area proportional to sample size and attached horizontal lines representing 95% CIs. The diamond at the bottom represents combined random effects estimate. Positive numbers (< 0) favor uncemented implant fixation and negative numbers (< 0) favor cemented implant fixation. The Danish registry reported on by Lucht et al. (Citation2000) is entered twice because of stratification of results of patients into age groups of ≤ 55 years and < 55 years, but does not require adjustment of weights because the comparisons are independent.
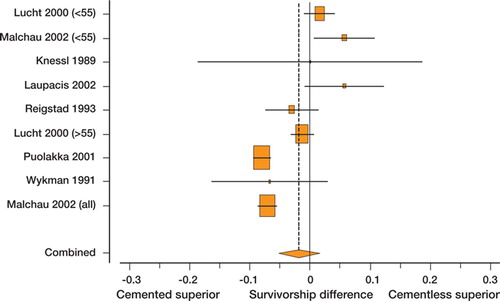
Table 4. Meta-analysis by selected subgroups (95% CI)
Type B failure definition: revision of cup or stem specifically
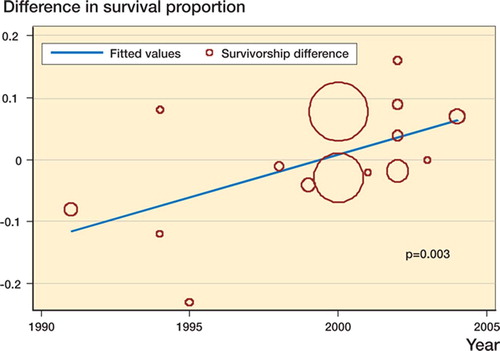
10 studies compared cemented and uncemented stems, and 5 compared cemented and uncemented cups; all were non-randomized. From the Norwegian registry (Havelin et al. Citation2000), uncemented stem and cup survivorship estimates were calculated by combining data on both hydroxyapatitecoated and porous-coated designs. There was significant heterogeneity present and the pooled estimate shown in shows a difference in survival probability that does not significantly favor either fixation method. In the analysis of subgroups (), several important sources of heterogeneity were discovered. Subgroup analysis differentiating studies using a titanium stem in the cemented group from those reporting use of a stainless steel or cobalt chrome cemented stem demonstrated that the former favored uncemented fixation whereas the latter favored cemented fixation, and the difference between the two was statistically significant. For comparisons of cups using a threaded or macro-ingrowth implant with those using a microingrowth or on-growth uncemented design, the former favored cemented fixation whereas the latter did not, and the difference between subgroups was significant. Sensitivity analysis revealed that omission from the pooled analysis of the study of cup survival by Gaffey et al. (Citation2004) () resulted in a shifting of the pooled estimate towards favoring cemented fixation. Meta-regression showed year of publication to be associated with improved survival of uncemented implants relative to cemented implants (). The Egger test for funnel plot asymmetry did not reveal any evidence of publication bias (p = 0.5).
Figure 3. Forest plot of cemented vs. uncemented fixation survivorship difference for type B studies (where failure is defined as revision of a specific component—cup or stem). Shaded boxes represent study-specific estimates with area proportional to study size and attached horizontal lines representing 95% CIs. The diamond at the bottom represents combined random effects estimate. Positive numbers (< 0) favor uncemented implant fixation and negative numbers (< 0) favor cemented implant fixation.
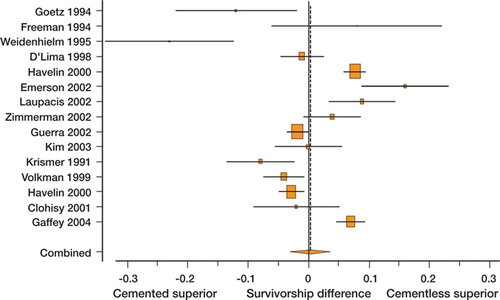
Figure 4. Sensitivity analysis of pooled estimate of type B studies (where failure is defined as revision of a specific component—the cup) to omission of each individual study. Positive numbers (< 0) favor uncemented implant fixation and negative numbers (< 0) favor cemented implant fixation.
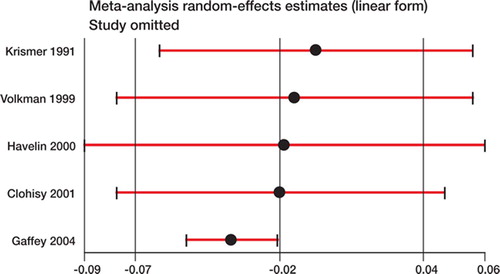
Figure 5. Scatter plot of study estimate of the difference in survival probability vs. year of publication with superimposed regression line. Y-axis values greater than zero favor uncemented fixation and the area of each circle is proportional to the sample size of the study. Slope = 1.4% per year (95% CI: 0.5–2.3)
Discussion
We have summarized the best evidence from comparative studies on the use of cemented vs. uncemented fixation in THR. 20 studies comparing cemented and uncemented fixation in THR met the criteria for inclusion in this systematic review. While meta-analysis did not demonstrate overall superiority of either method of fixation as measured by a difference in survival, subgroup analysis of the type A comparisons not restricted to young patients (less than or equal to 55 years of age) demonstrated a statistically significant survival advantage with cemented fixation. Among type B studies, a linear association between survival difference and year of publication was found, with uncemented fixation outlasting cemented comparators after 1995. Poor performance by cemented titanium stems and threaded and macro-ingrowth cups were found to lead subgroup estimates to favor uncemented stems and cemented cups in their respective subgroups. These findings offer important lessons for future investigations.
This analysis suggests that cemented fixation gives favorable results at the population level, though some caution in drawing inferences is advisable. These results may have limited generalizability to the United States or other countries where cemented fixation is performed much less frequently, where THR is performed at an earlier mean age (Lucht Citation2000, Puolakka et al. Citation2000, CDC Citation2002, Malchau et al. Citation2002), or where the population is not as socially or demographically uniform. Moreover, young patients suffer from higher failure rates (Berry et al. Citation2002, Malchau et al. Citation2002) and pose a dilemma in the choice of implant and fixation method. Lower revision rates with uncemented fixation at 8–10 years in patients who are 50 years old or younger (Capello Citation1990, Xenos et al. Citation1995, Kronick et al. Citation1997, Fink et al. Citation2004) has encouraged optimism. The 7% difference between the population level (age unrestricted) and younger subgroup estimates (: –0.038 vs. 0.031) means that prospective studies should be designed to compare the best available cemented implants against the best available uncemented implants without pooling all age groups, because results are likely to differ between groups.
Improvement in relative performance of uncemented fixation in recent years was found among type B studies. This is consistent with data from numerous uncontrolled studies (Zicat et al. Citation1995, Kim et al. Citation1999, Della Valle et al. Citation2004, Sinha et al. Citation2004). A study on the survival of more modern uncemented cups by Gaffey and colleagues (Citation2004) compared to the results from a historical cemented control group has provided some of the strongest evidence to this effect at 15 years of follow-up. That study is the most current of the 5 specifically addressing cup survival, and the only one to favor uncemented fixation, which may explain why its omission in the sensitivity analysis () led to a significant shift in the summary estimate of survival difference to favor cemented fixation. The study by Gaffey et al. (Citation2004) was designed to assess the importance of implant fixation with cemented vs. uncemented technique, and part of the difference in survival may be mediated through impact on wear rates. Uncemented fixation has been found to increase wear rates, which can lead to early failure (Tanzer et al. Citation1992, Xenos et al. Citation1995, McCombe et al. Citation2004). Improvements in polyethylene production, alternate bearing surfaces, and other design features may have contributed to the relatively improved survival of uncemented implants. Further studies will be necessary to confirm these assertions.
Cemented stems of titanium and threaded macroingrowth cups explain some inconsistency in the results of studies that were included in the metaanalysis. For series of cemented titanium stems, numerous authors have reported loosening rates of 10–49% at 3–5 years (Robinson et al. Citation1989, Tompkins et al. Citation1994, Rorabeck et al. Citation1996a). We found cemented fixation to be inferior when titanium stems were used and superior when a stainless steel or cobalt-chrome stem was used. Similarly, threaded macro-ingrowth cups have performed poorly with loosening rates of 25–55% at 10–15 years of follow-up (Kubo et al. Citation2001, Aldinger et al. Citation2004, Grant and Nordsletten Citation2004.). When these implants were tested against cemented cups, cemented cups outperformed them by 5%, whereas studies comparing porous-coated Harris-Galante I/II cups to cemented polyethylene cups moved the difference in survival in the direction of favoring uncemented fixation by 9%. The World Medical Association Declaration of Helsinki (World Medical Association Citation1997) requires that new treatments be tested against the best known current standard. We found that control groups have not always been selected with regard to the best available treatment or standard of care. Future comparative trials should avoid these past mistakes and use systematic reviews and comprehensive summaries of implant performance from the implant registries, with long-term followup in selecting comparator groups.
4 randomized controlled trials assessing hybrid fixation (cementation of one component and uncemented fixation of the other) were excluded because they either only focused on polyethylene wear rates and component loosening or had inadequate follow-up to detect any failures resulting in revision (Godsiff et al. Citation1992, Karrholm et al. Citation1994, Onsten et al. Citation1998, McCombe and Williams, Citation2004). With respect to failure defined as revision of either or both components (type A), only the Danish and Swedish registries presented data on hybrid fixation as distinct from purely cemented or uncemented fixation and this was judged inadequate for independent subgroup meta-analysis. Thus, the hybrid fixation method was only assessed indirectly through analysis of studies comparing individual component failures.
While the majority of studies that were included were non-randomized and subject to significant bias and confounding, the potential for bias is not restricted to non-randomized studies. Of the 3 randomized controlled trials, only Laupacis et al. (Citation2002) documented proper randomization techniques and concealment of allocation, and discussed reasons for exclusion or non-participation. Loss to follow-up or non-response during data collection are also important sources of selection bias. Lack of attention to this problem was seen among both randomized and non-randomized studies in this review. Of the 3 randomized studies that mentioned the reasons for their exclusion and censoring, only Laupacis et al. provided the type of flow chart and accounting for withdrawals that the CONSORT statement (Altman Citation1996) requires in documentation of randomized controlled trials. Such clear and transparent reporting of all features related to validity of such trials ought to be enforced in orthopedic journals, as it is in many high-impact medical journals (Altman Citation1996, Moher et al. Citation2001a, Citationb).
Definition of a failure event in studies of implant survival is fraught with inconsistencies. While we attempted to use estimates based on revision undertaken for any reason—because this is less subjective than “aseptic loosening” or “mechanical failure”— the propensity for differential misclassification and resulting bias is present. This is because the decision to undertake a revision is influenced by the opinions of the surgeon and the patient. Moreover, this is not an adequately sensitive definition of all clinical failures. Revisions are occasionally performed on well-fixed implants without evidence of infection or mechanical failure, and many radiographically loose or symptomatic implants never come to be revised. Reporting of health-related quality of life and functional outcome in addition to standardized reporting of failure events in survival analyses will improve the accuracy and comparability of clinically relevant outcomes in future research. Randomized studies using radiostereometry (Mjöberg et al. Citation1986, Karr holm et al. 1994), a highly sensitive and specific computerized radiographic technique for quantifying implant migration and wear, may become useful surrogates in the future for detecting early failure and exposing fewer patients to new technologies that are potentially dangerous.
The studies reviewed here have shown that failure events in THR are rare, and that longterm follow-up is required to generate meaningful estimates of difference in survival probability. It is not uncommon for an implant being studied to be removed from the market or replaced by a new version before the scheduled endpoint of a trial, as was the case for the Mallory-Head prosthesis (Biomet, Warsaw, IN) used by Laupacis et al. (Citation2002). This can make clinical trials costly, logistically challenging, and in the end, potentially irrelevant. Some authors assert that national registries ought to be the research study design of choice to provide timely and relevant outcomes data to guide clinical practice, as it has in Scandinavia (Maloney and Harris Citation1990, Maloney Citation2002, Howard et al. Citation2004), and the results of this study underscore the need for this powerful tool for improvement of patient outcomes. Randomized clinical trials will, however, continue to be valuable when: (1) the question of relative superiority has been narrowed down to a few seemingly equivalent choices of fixation or implants, and a specific target population has been identified under which the experiment could be undertaken with equipoise; or when (2) the development of validated surrogate markers for early failure (such as radiostereometry) allows smaller sample sizes and shorter duration in the testing of a new strategy against an established control.
Several limitations in our work are important to note. In any systematic review or meta-analysis, there may be publication bias, incomplete ascertainment of studies, and errors in data extraction. The studies included in this review represent a diversity of designs, patient populations, surgical implants and approaches, and methods for assessing their efficacy. We believe that restricting our analysis to randomized studies alone would have ignored most of the comparative evidence on the subject. Also, certain potential predictors of out-come—such as race, rehabilitation program, and activity level—could not be explored, due to very limited information on these variables among the studies that were included. We did not find any statistical evidence of funnel plot asymmetry to suggest publication bias. We attempted to minimize errors in data extraction through cross-checking of all quantitative information by two of the authors. We used all sources of data that we could identify from a comprehensive literature search, without any restriction regarding language, to find studies for inclusion. Given the limitations in the published literature on this topic, the methods used in this systematic review and meta-analysis had limited bias and they explored sources of heterogeneity to the greatest degree possible.
In conclusion, the published evidence suggests that cemented fixation still has superior survival among large subgroups of populations studied, and that survival of uncemented implants continues to improve. The effect on analyses of relative benefit from the use of suboptimal control groups (such as those with cemented stems of titanium and threaded cups) emphasizes the need for more uniform standards in the selection of control groups in future trials. Further research and improved methods are necessary to better define specific subgroups of patients in which the relative benefits of cemented and uncemented implant fixation can be more clearly demonstrated.
We thank Ms Gloria Won, Librarian at the H. M. Fishbon Memorial Library at the UCSF Medical Center, Mt. Zion, and Wayne Enanoria Ph.D., Division of Epidemiology at the University of California, Berkeley School of Public Health, for their assistance. Saam Morshed and Kevin J. Bozic are supported by an Orthopaedic Research and Education Foundation Clinical Research Training Fellowship.
No funder or sponsor participated in the design and conduct of the study: collection, management, analysis and interpretation of the data; or in preparation, review, and approval of the manuscript.
Contributions of authors
All authors participated in study design, execution, analysis and manuscript preparation.
References
- Agins H J, Salvati E A, Ranawat C S, Wilson P D, Jr., Pellicci P M. The nine- to fifteen-year follow-up of one-stage bilateral total hip arthroplasty. Orthop Clin North Am 1988; 19(3)517–30
- Aldinger P R, Thomsen M, Lukoschek M, Mau H, Ewerbeck V, Breusch S J. Long-term fate of uncemented, threaded acetabular components with smooth surface treatment: minimum 10-year follow-up of two different designs. Arch Orthop Trauma Surg 2004; 124(7)469–75
- Altman D G. Better reporting of randomised controlled trials: the CONSORT statement. BMJ 1996; 313(7057)570–1
- Barber T C, Healy W L. The hospital cost of total hip arthroplasty. A comparison between 1981 and 1990. J Bone Joint Surg (Am) 1993; 75(3)321–5
- Berry D J, Harmsen W S, Cabanela M E, Morrey B F. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg (Am) 2002; 84-A(2)171–7
- Callaghan J J, Templeton J E, Liu S S, Pedersen D R, Goetz D D, Sullivan P M. Results of Charnley total hip arthroplasty at a minimum of thirty years. A concise follow-up of a previous report. J Bone Joint Surg (Am) 2004; 86(4)690–5
- Capello W N. Uncemented total hip replacement. J Japanese Orthop Ass 1990; 64(2)S86
- CDC. National Hospital Discharge Survey, 1990–2002. United States Department of Health and Human Services, Centers For Disease Control, National Center for Health Statistics. 2002
- Charnley J. Surgery of the hip-joint: present and future developments. Br Med J 1960; 5176: 821–6
- Clark C R. Cost containment: total joint implants. J Bone Joint Surg (Am) 1994; 76(6)799–800
- Clohisy J C, Harris W H. Matched-pair analysis of cemented and uncemented acetabular reconstruction in primary total hip arthroplasty. J Arthroplasty 2001; 16(6)697–705
- Colditz G A, Burdick E, Mosteller F. Heterogeneity in metaanalysis of data from epidemiologic studies: a commentary. Am J Epidemiol 1995; 142(4)371–82
- D'Lima D D, Oishi C S, Petersilge W J, Colwell C W, Jr., Walker R H. 100 cemented versus 100 noncemented stems with comparison of 25 matched pairs. Clin Orthop 1998, 348: 140–8
- Della Valle C J, Berger R A, Shott S, Rosenberg A G, Jacobs J J, Quigley L. Primary total hip arthroplasty with a porous-coated acetabular component. A concise followup of a previous report. J Bone Joint Surg (Am) 2004; 86(6)1217–22
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 31(7109)629–34
- Emerson R H, Jr., Head W C, Emerson C B, Rosenfeldt W, Higgins L L. A comparison of cemented and uncemented titanium femoral components used for primary total hip arthroplasty: a radiographic and survivorship study. J Arthroplasty 2002; 17(5)584–91
- Enanoria W T, Ng C, Saha S R, Colford Jr J M. Treatment outcomes after highly active antiretroviral therapy: a meta-analysis of randomised controlled trials. Lancet Infect Dis 2004; 4(7)414–25
- Fink B, Protzen M, Hansen-Algenstaedt N, Berger J, Ruther W. High migration rate of two types of threaded acetabular cups. Arch Orthop Trauma Surg 2004; 124(1)17–25
- Freeman M A, Plante-Bordeneuve P. Early migration and late aseptic failure of proximal femoral prostheses. J Bone Joint Surg (Br) 1994; 76(3)432–8
- Gaffey J L, Callaghan J J, Pedersen D R, Goetz D D, Sullivan P M, Johnston R C. Uncemented acetabular fixation at fifteen years. A comparison with the same surgeon's results following acetabular fixation with cement. J Bone Joint Surg (Am) 2004; 86(2)257–61
- Godsiff S P, Emery R J, Heywood-Waddington M B, Thomas T L. Cemented versus uncemented femoral components in the ring hip prosthesis. J Bone Joint Surg (Br) 1992; 74(6)822–4
- Goetz D D, Smith E J, Harris W H. The prevalence of femoral osteolysis associated with components inserted with or without cement in total hip replacements. J Bone Joint Surg (Am) 1994; 76(8)1121–9
- Grant P, Nordsletten L. Total hip arthroplasty with the Lord prosthesis. A long-term follow-up study. J Bone Joint Surg (Am) 2004; 86(12)2636–41
- Guerra E, Traina F, Antonietti B, Giardina F, Toni A. Cemented versus uncemented stem-to-bone fixation: a long-term survival comparison. Chir Organi Mov 2003; 88(3)253–5
- Havelin L I, Engesaeter L B, Espehaug B, Furnes O, Lie S A, Vollset S E. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand 2000; 71β(4)337–53
- Howard J L, Hui A J, Bourne R B, McCalden R W, Mac-Donald S J, Rorabeck C H. A quantitative analysis of bone support comparing uncemented tapered and distal fixation total hip replacements. J Arthroplasty 2004; 19(3)266–73
- Jordan R, Gold L, Cummins C, Hyde C. Systematic review and meta-analysis of evidence for increasing numbers of drugs in antiretroviral combination therapy. BMJ 2002; 324(7340)757–66
- Karrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemented, hydroxyapatite-coated, and porous-coated stems with roentgen stereophotogrammetric analysis. J Bone Joint Surg (Am) 1994; 76(11)1692–705
- Kim Y H, Kim J S, Cho S H. Primary total hip arthroplasty with the AML total hip prosthesis. Clin Orthop 1999, 360: 147–58
- Kim Y H, Oh S H, Kim J S, Koo K H. Contemporary total hip arthroplasty with and without cement in patients with osteonecrosis of the femoral head. J Bone Joint Surg (Am) 2003; 85(4)675–81
- Knessl J, Gschwend N, Scheier H, Munzinger U. Comparative study of cemented and uncemented hip prostheses in the same patient. Arch Orthop Trauma Surg 1989; 108(5)276–8
- Krismer M, Fischer M, Klestil T, Frischhut B. Uncoated polyethylene RM acetabular component versus Muller cemented acetabular component. A 4- to 8-year follow-up study. Arch Orthop Trauma Surg 1991; 110(4)195–9
- Kronick J L, Barba M L, Paprosky W G. Extensively coated femoral components in young patients. Clin Orthop 1997, 344: 263–74
- Kubo T, Inoue S, Maeda T, Arai Y, Hirakawa K, Wu Y. Uncemented Lord total hip arthroplasty: cup loosening common after minimum 10-year follow-up of 103 hips. Acta Orthop Scand 2001; 72(6)585–90
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg (Am) 2005; 87(7)1487–97
- Lau J, Ioannidis J P, Schmid C H. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127(9)820–6
- Laupacis A, Bourne R, Rorabeck C, Feeny D, Tugwell P, Wong C. Comparison of total hip arthroplasty performed with and without cement: a randomized trial. J Bone Joint Surg (Am) 2002; 84(10)1823–8
- Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand 2000; 71(5)433–9
- Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg (Am) (Suppl 2) 2002; 84: 2–20
- Maloney W J. An American implant registry: a clinical use trip wire. Orthopedics 2002; 25(9)923–4
- Maloney W J, Harris W H. Comparison of a hybrid with an uncemented total hip replacement. A retrospective matched-pair study. J Bone Joint Surg (Am) 1990; 72(9)1349–52
- McCombe P, Williams S A. A comparison of polyethylene wear rates between cemented and uncemented cups. A prospective, randomised trial. J Bone Joint Surg (Br) 2004; 86(3)344–9
- Mendenhall S. 2003 Hip and knee Implant Review. Orthopedic Network 2004; 14(3)1–16
- Mjoberg B, Selvik G, Hansson L I, Rosenqvist R, Onnerfalt R. Mechanical loosening of total hip prostheses. A radiographic and roentgen stereophotogrammetric study. J Bone Joint Surg (Br) 1986; 68(5)770–4
- Moher D, Cook D J, Eastwood S, Olkin I, Rennie D, Stroup D F. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 1999; 354(9193)1896–900, Quality of Reporting of Meta-analyses
- Moher D, Schulz K F, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001a; 285(15)1987–91
- Moher D, Schulz K F, Altman D G. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001b; 357(9263)1191–4
- Onsten I, Carlsson A S, Besjakov J. Wear in uncemented porous and cemented polyethylene sockets: a randomised, radiostereometric study. J Bone Joint Surg (Br) 1998; 80(2)345–50
- Puolakka R, Pitkanen M T, Rosenberg P H. Comparison of three catheter sets for continuous spinal anesthesia in patients undergoing total hip or knee arthroplasty. Reg Anesth Pain Med 2000; 25(6)584–90
- Reigstad A, Rokkum M, Bye K, Brandt M. Femoral remodeling after arthroplasty of the hip. Prospective randomized 5-year comparison of 120 cemented/uncemented cases of arthrosis. Acta Orthop Scand 1993; 64(4)411–6
- Robinson R P, Lovell T P, Green T M, Bailey G A. Early femoral component loosening in DF-80 total hip arthroplasty. J Arthroplasty 1989; 4(1)55–64
- Rorabeck C H, Bourne R B, Mulliken B D, Nayak N, Laupacis A, Tugwell P. The Nicolas Andry award: comparative results of cemented and uncemented total hip arthroplasty. Clin Orthop 1996a, 325: 330–44
- Rorabeck C H, Bourne R B, Mulliken B D, Nayak N, Laupacis A, Tugwell P. The Nicolas Andry award: comparative results of cemented and uncemented total hip arthroplasty. Clin Orthop 1996b, 325: 330–44
- Sharp S, Sterne J. sbe16.1: New syntax and output for the meta-analysis command. Stat Tech Bull 1998; 42: 6–9
- Sinha R K, Dungy D S, Yeon H B. Primary total hip arthroplasty with a proximally porous-coated femoral stem. J Bone Joint Surg (Am) 2004; 86(6)1254–61
- Stroup D F, Berlin J A, Morton S C, Olkin I, Williamson G D, Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283(15)2008–12
- Tanzer M, Maloney W J, Jasty M, Harris W H. The progression of femoral cortical osteolysis in association with total hip arthroplasty without cement. J Bone Joint Surg (Am) 1992; 74(3)404–10
- Tompkins G S, Lachiewicz P F, DeMasi R. A prospective study of a titanium femoral component for cemented total hip arthroplasty. J Arthroplasty 1994; 9(6)623–30
- Volkmann R, Schneider M A, Bretschneider C, Weise K. More failures of uncemented acetabular screw-rings than of cemented polyethylene cups in total hip arthroplasties. Int Orthop 1999; 23(3)138–9
- Weidenhielm L R, Mikhail W E, Nelissen R G, Bauer T W. Cemented collarless (Exeter-CPT) versus uncemented collarless (PCA) femoral components. A 2- to 14-year follow-up evaluation. J Arthroplasty 1995; 10(5)592–7
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277(11)925–6
- Wykman A, Olsson E, Axdorph G, Goldie I. Total hip arthroplasty. A comparison between cemented and pressfit noncemented fixation. J Arthroplasty 1991; 6(1)19–29
- Xenos J S, Hopkinson W J, Callaghan J J, Heekin R D, Savory C G. Osteolysis around an uncemented cobalt chrome total hip arthroplasty. Clin Orthop 1995, 317: 29–36
- Zicat B, Engh C A, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg (Am) 1995; 77(3)432–9
- Zimmerman S, Hawkes W G, Hudson J I, Magaziner J, Hebel J R, Towheed T. Outcomes of surgical management of total HIP replacement in patients aged 65 years and older: Cemented versus uncemented femoral components and lateral or anterolateral versus posterior anatomical approach. J Orthop Res 2002; 20(2)182–91
