Abstract
Background The anterior cruciate ligament (ACL) constrains the anterior translation and axial rotation of the tibia. However, the effect of ACL injury on the mediolateral translation and varus-valgus rotation of the tibia is unknown. Because of the oblique orientation of the ACL, we hypothesized that ACL deficiency alters mediolateral translation and varus-valgus rotation.
Methods The kinematics of 9 cadavers from full extension to 90° of flexion under various loading conditions were measured before and after ACL resection using a robotic testing system.
Results ACL deficiency increased the medial translation of the tibia and valgus rotation, especially at 15° and 30° of flexion. For example, at 15°, ACL deficiency increased the medial translation from 1.2 (SD 0.9) mm to 1.8 (SD 1.1) mm in response to a quadriceps load. The valgus rotation also increased from 0.8° (SD 0.6) to 1.7° (SD 0.8).
Interpretation ACL deficiency altered both the mediolateral tibial translation and valgus-varus rotation under various loading conditions. The increased medial tibial translation could shift the contact in the medial compartment towards the medial tibial spine, a region where degeneration is observed in ACL-deficient patients. In addition to restoring anterior laxity, ACL reconstruction might need to restore the mediolateral translation of the tibia and varus-valgus rotation of the knee.
Many previously published studies have reported the effects of anterior cruciate ligament (ACL) deficiency on knee mechanics using in vitro and in vivo experiments. In vivo studies have indicated that ACL deficiency alters anteroposterior tibial translation and axial tibial rotation (Brandsson et al. Citation2001, Georgoulis et al. Citation2003, Zhang et al. Citation2003, Andriacchi and Dyrby Citation2005). Under anterior tibial loading, ACL deficiency increases tibial translation (Woo et al. Citation2002, Yoo et al. Citation2005) and under simulated muscle loads, ACL deficiency increases the anterior translation and internal rotation of the tibia (Yoo et al. Citation2005).
Long-term follow-up studies of patients with ACL injury have suggested a high incidence of joint degeneration ranging from 60% to 90%, between 10 and 15 years after injury (Beynnon et al. Citation2005). Degenerative changes are often observed in the medial compartment of the tibiofemoral joint (Fairclough et al. Citation1990, Buckland-Wright et al. Citation2000, Hill et al. Citation2005). Specifically, osteophytes have been noted on the lateral wall of the medial condyle and on the medial tibial spine (Fairclough et al. Citation1990). Although altered kinematics have been thought to contribute to joint degeneration (Roos et al. Citation1995, Buckwalter and Lane Citation1997), the specific ways in which altered kinematics might cause joint degeneration remain unknown.
Few data have been reported on the effects of ACL deficiency on the mediolateral translation and varus-valgus rotation of the knee. A recent in vivo study noted that the ACL was oriented so as to resist medial tibial translation throughout weight-bearing flexion from 0–90° (Li et al. Citation2005b). In vitro studies have also reported that the ACL force vector has a component that might resist medial tibial translation (Livesay et al. Citation1997, Li et al. Citation2006b). Thus, we hypothesize that ACL deficiency not only increases the anterior translation and internal rotation of the tibia, but also increases medial tibial translation. We propose that varus-valgus rotation is also altered as a result of the increased medial tibial translation and the geometry of the tibial plateau.
Material and methods
A previous study from our laboratory reported the effects of ACL deficiency on the anteroposterior translation and internal-external rotation of the tibia (Yoo et al. Citation2005). In this study, we re-ana-lyzed our previous in vitro experimental data to include mediolateral translation and varus-valgus rotation. We tested 9 fresh-frozen cadaveric human knee specimens (age range, 50–78 years) in our previous investigations (Yoo et al. Citation2005, Li et al. Citation2006b) using a robotic testing system (). Specimens with evidence of previous injury to the bones or soft tissues were excluded from the study. Specimens were examined using fluoroscopy (prior to testing) and dissection (after testing).
Figure 1. The knee specimens were tested on the robotic testing system. Anterior tibial loads were applied and muscle loads were simulated using a system of ropes and pulleys.
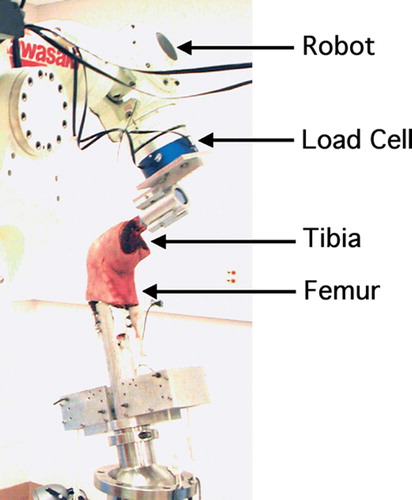
Details of the robotic testing system have been described in detail in previous publications (Li et al. Citation2002, Yoo et al. Citation2005). Briefly, each specimen was first thawed overnight and the ends of the femur and tibia were potted in bone cement. The soft tissues surrounding the knee joint were left intact. Before installing the specimen on the robotic testing system, it was preconditioned by flexing it between full extension and full flexion 10 times manually. The tibia was then fixed in a clamp attached to the load cell and the femur was fixed to a rigid pedestal.
Coordinate systems were created on the knee joint using a digitizing stylus (Microscribe 3DX; Immersion Technologies, San Jose, CA). Flexionextension was defined as rotation about the transepicondylar line, internal-external rotation was defined as rotation about the longitudinal axis of the tibia (internal and external rotation axis), and varus-valgus rotation was defined as rotation about an axis formed by the cross product of the transepicondylar line and the longitudinal axis of the tibia. The origin of the coordinate system was the center of the transepicondylar line. Mediolateral translation was defined as translation of the tibia along the transepicondylar line.
A 6-degrees-of-freedom (DOF) load cell recorded the forces and moments applied to the knee as the robotic manipulator measured the motion of the knee in response to the loads applied. First, a series of passive positions was measured from full extension to 90° of flexion using the force-moment control mode of the robotic testing system. Starting with the knee fixed at full extension, the knee was moved in the remaining 5 DOF until the forces and moments applied to the origin of the knee coordinate system were minimal (less than 5 N and 0.5 Nm, respectively). The knee was then fiexed by 1° and another passive position was found until a passive path was measured from full extension to 90° of flexion. The passive path was repeated for 5 cycles by the robot to further precondition the specimen. The passive path served as a reference position for measuring the motion of the knee in response to the applied loads.
The knee then was loaded at full extension, 15°, 30°, 60°, and 90° of flexion along the passive path. An anterior tibial load of 130 N was applied through the origin of the knee coordinate system to simulate clinical examinations of the knee, such as Lachman and anterior drawer tests. With the flexion angle fixed, the tibia moved in the remaining 5 DOF until it reached an equilibrium position in response to the 130 N anterior load. The robotic manipulator recorded the equilibrium position of the knee at each flexion angle.
Next, two different muscle loading conditions were applied to the knee: an isolated quadriceps force of 400 N and a combined quadriceps and hamstrings (semitendinosus, semimembranosus, and biceps femoris) load of 400 N and 200 N, respectively. The proportions of these applied loads were based on an inverse dynamic study (Li et al. Citation1999). The quadriceps and hamstring forces were applied using a system of ropes and pulleys. The loads were aligned along the femoral shaft. The kinematics of the knee in response to the muscle loads were measured by the robot at each flexion angle.
After the intact knee kinematics had been measured in response to the 3 loading conditions, the ACL was resected to simulate an ACL-deficient knee. The ACL was resected using a medial parapatellar arthrotomy, with the knee fixed in the robotic testing system. The arthrotomy was then closed, and the loads (anterior tibial load and both muscle loads) were applied to the knee using the same protocol for the intact knee.
Kinematics were reported by using the intact passive path as the reference position. In our previous studies, we reported anteroposterior tibial translation and internal-external tibial rotation (Yoo et al. Citation2005, Li et al. Citation2006b). We re-analyzed previous data to include the mediolateral tibial translation and varus-valgus tibial rotation before and after ACL resection. A repeated-measures analysis of variance and Student-Newman-Keuls test were used to analyze the effect of ACL deficiency on the translation and rotation under the loads applied. Differences were considered statistically significant at p < 0.05.
Results
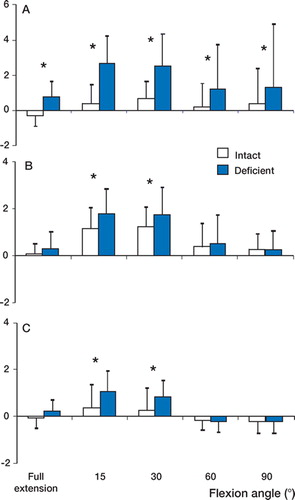
Anterior cruciate ligament deficiency caused a medial translation of the tibia, particularly at low flexion angles (). Under the anterior tibial load, ACL deficiency increased medial tibial translation at all selected flexion angles (). For example, at 15° of flexion, the medial tibial translation in the ACL-deficient knee was significantly higher (p < 0.05) than in the intact knee (2.7 (SD 1.6) mm versus 0.4 (SD 1.1) mm). Under the quadriceps load, ACL deficiency increased (p < 0.05) medial tibial translation at 15° and 30° of flexion (). At 15°, the tibia translated medially by 1.2 (SD 0.9) mm in the intact knee and by 1.8 (SD 1.1) mm in the ACL-deficient knee (p < 0.05). Under the combined quadriceps and hamstring load, ACL deficiency again increased (p < 0.05) medial tibial translation at 15° and 30° of flexion (). At 15° of flexion, the tibia translated medially by 0.3 (SD 1.0) mm in the intact knee and by 1.1 (SD 0.9) mm in the ACL-deficient knee (p < 0.05).
Figure 2. (A) After ACL deficiency, medial tibial translation (denoted by positive values in the graphs) increased at all flexion angles under the anterior tibial load. (B) Under the isolated quadriceps load, medial translation increased at 15° and 30°. (C) Under the combined quadriceps and hamstring load, medial translation increased at 15° and 30°. (* p < 0.05).
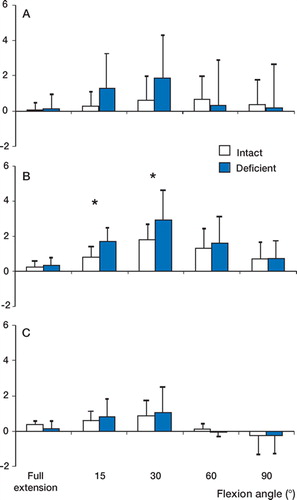
Only a slight effect of ACL deficiency on varusvalgus rotation was observed (). Under the anterior tibial load, we observed a valgus rotation of the tibia in the ACL-deficient knee relative to the intact knee from full extension to 90° of flexion (). No statistically significant differences were observed at any flexion angle, however. Under the isolated quadriceps load, ACL deficiency significantly increased (p < 0.05) the valgus rotation of the tibia at 15° and 30° of flexion (). At 15° of flexion, the valgus rotation in the intact knee was 0.8° (SD 0.6), whereas the ACL-deficient knee was rotated by 1.7° (SD 0.8). Under the combined quadriceps and hamstring load, no statistically significant difference in varus-valgus rotation was observed ().
Figure 3. (A) After ACL deficiency, no differences in varusvalgus rotation were observed under the anterior tibial load. (B) Under the isolated quadriceps load, valgus rotation (denoted by positive values in the graphs) increased at 15°. (C) No differences in varus-valgus rotation were observed under the combined quadriceps and hamstrings load. (* p < 0.05).
Discussion
Our principal finding was that ACL deficiency increased medial tibial translation between 15° and 30° under simulated muscle loads and between 0° and 90° under anterior tibial loads. ACL deficiency also increased valgus rotation at 15° and 30° under the isolated quadriceps load. Under the combined quadriceps and hamstring load, however, the valgus rotation of ACL-deficient knees was similar to that of the intact knees. These data support our hypothesis that ACL deficiency alters mediolateral translation and varus-valgus rotation of the knee.
The increased medial tibial translation after ACL deficiency can be explained by the geometry of the ACL during knee motion. Our recent study on in vivo ACL kinematics indicated that the ACL is oriented obliquely, so as to resist medial tibial translation as well as anterior tibial translation (Li et al. Citation2005b). Previous in vitro studies have also noted that the ACL has a force component that might restrain the medial translation of the tibia (Livesay et al. Citation1997, Li et al. Citation2006b). All of these data suggest that ACL deficiency may increase knee laxity in the mediolateral direction in addition to the anteroposterior direction.
A medial shift of the tibia relative to the femur might explain the increased valgus rotation of the tibia after ACL deficiency. If the medial femoral condyle moves toward the upward slope of the tibial spine and the lateral femoral condyle moves away from the tibial spine, a relative valgus rotation might result. This mechanism is consistent with the increased valgus rotation observed in ACL-deficient knees under quadriceps load. However, a combined hamstrings and quadriceps load reduced this difference compared to the isolated quadriceps. These data suggest that strengthening the hamstring muscles might reduce the valgus rotation caused by ACL deficiency.
The increase in medial tibial translation could have an important effect on tibiofemoral joint contact mechanics. A small difference in translation might alter the contact stress distributions due to the complex geometry of the tibiofemoral cartilage (Li et al. Citation2005a). The increased medial translation may shift the regions of contact laterally, resulting in contact between the femoral condyle and the medial tibial spine. The possibility of this mechanism is supported by recent in vivo studies of ACL-deficient patients, in which an increase in medial tibial translation and a lateral shift in the cartilage contact (toward the medial tibial spine in the medial compartment) were observed (DeFrate et al. Citation2006, Li et al. Citation2006a).
Altered cartilage contact patterns after ACL deficiency might predispose the knee to degenerative changes. Previous reports have described cartilage lesions in the medial compartment of patients with ACL injury (Fairclough et al. Citation1990, Murrell et al. Citation2001, Tandogan et al. Citation2004, Hill et al. Citation2005). In ACL-deficient patients, Fairclough et al. observed osteophytes on the lateral surface of the medial femoral condyle inside the intercondylar notch and on the medial tibial spine (Fairclough et al. Citation1990). These patterns of degeneration might be caused by impingement of the tibial spine on the medial femoral condyle, due in part to increased medial tibial translation (Fairclough et al. Citation1990, DeFrate et al. Citation2006, Li et al. Citation2006a).
One limitation of the current study was that a cadaveric model was used. It is difficult to simulate the complex loading conditions that occur in vivo using a cadaver. We therefore applied an anterior tibial load, an isolated quadriceps load, and a combined quadriceps and hamstrings load, in order to simulate different types of loading. The simulated muscle loads were also lower in magnitude than in vivo muscle loads, which have been estimated to reach several times body weight (Li et al. Citation1999, Yoo et al. Citation2005). Despite these limitations, we consistently observed increases in medial tibial translation after ACL deficiency in response to each of the various loading conditions.
In conclusion, we found that in addition to altering anteroposterior tibial translation and internal tibial rotation, ACL deficiency increases medial tibial translation. Valgus rotation was also increased under isolated quadriceps loading at 15° and 30° of flexion, but not under combined quadriceps and hamstring loading. These kinematic changes may shift the tibiofemoral articular cartilage contact locations toward the tibial spine in the medial compartment, resulting in altered articular cartilage contact stresses. This mechanism might contribute to the medial compartment degeneration often observed in patients with ACL deficiencies. Thus, surgical reconstruction of the ACL should restore 6-degrees-of-freedom knee kinematics.
The authors gratefully acknowledge the financial support of the National Institutes of Health (R21 AR051078), the National Football League Charities Foundation, and the Department of Orthopaedic Surgery at the Massachusetts General Hospital.
Contributions of authors
GL: designed the study, analyzed data and prepared the manuscript. RP: conducted experiments, processed and analyzed data and assisted in manuscript writing. LED: participated in experiments, analyzed data and assisted in manuscript writing. JDY and SEP: prepared specimens, performed surgeries and contributed to drawing clinical relevancies. TJG: designed study, provided surgical guidance and clinical relevancies and contributed in prepration of the manuscript.
- Andriacchi T P, Dyrby C O. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech 2005; 38(2)293–8
- Beynnon B D, Johnson R J, Abate J A, Fleming B C, Nichols C E. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med 2005; 33(10)1579–602
- Brandsson S, Karlsson J, Eriksson B I, Karrholm J. Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthop Scand 2001; 72(4)372–8
- Buckland-Wright J C, Lynch J A, Dave B. Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis 2000; 59(8)641–6
- Buckwalter J A, Lane N E. Athletics and osteoarthritis. Am J Sports Med 1997; 25(6)873–81
- DeFrate L E, Papannagari R, Gill T J, Moses J M, Pathare N P, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med 2006; 34(8)1240–6
- Fairclough J A, Graham G P, Dent C M. Radiological sign of chronic anterior cruciate ligament deficiency. Injury 1990; 21(6)401–2
- Georgoulis A D, Papadonikolakis A, Papageorgiou C D, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient knee during walking. Am J Sports Med 2003; 31(1)75–9
- Hill C L, Seo G S, Gale D, Totterman S, Gale M E, Felson D T. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum 2005; 52(3)794–9
- Li G, Kaufman K R, Chao E Y, Rubash H E. Prediction of antagonistic muscle forces using inverse dynamic optimization during flexion/extension of the knee. J Biomech Eng 1999; 121(3)316–22
- Li G, Gill T J, DeFrate L E, Zayontz S, Glatt V, Zarins B. Biomechanical consequences of PCL deficiency in the knee under simulated muscle loads-an in vitro experimental study. J Orthop Res 2002; 20(4)887–92
- Li G, Park S E, DeFrate L E, Schutzer M E, Ji L, Gill T J, Rubash H E. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-car-tilage contact. Clin Biomech 2005a; 20(7)736–44
- Li G, Defrate L E, Rubash H E, Gill T J. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res 2005b; 23(2)340–4
- Li G, Moses J M, Papannagari R, Pathare N P, Defrate L E, Gill T J. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg (Am) 2006a; 88(8)1826–34
- Li G, Papannagari R, DeFrate L E, Yoo J D, Park S E, Gill T J. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop 2006b; 77(2)267–74
- Livesay G A, Rudy T W, Woo S L, Runco T J, Sakane M, Li G, Fu F H. Evaluation of the effect of joint constraints on the in situ force distribution in the anterior cruciate ligament. J Orthop Res 1997; 15(2)278–84
- Murrell G A, Maddali S, Horovitz L, Oakley S P, Warren R F. The effects of time course after anterior cruciate ligament injury in correlation with meniscal and cartilage loss. Am J Sports Med 2001; 29(1)9–14
- Roos H, Adalberth T, Dahlberg L, Lohmander L S. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage 1995; 3(4)261–7
- Tandogan R N, Taser O, Kayaalp A, Taskiran E, Pinar H, Alparslan B, Alturfan A. Analysis of meniscal and chondral lesions accompanying anterior cruciate ligament tears: relationship with age, time from injury, and level of sport. Knee Surg Sports Traumatol Arthrosc 2004; 12(4)262–70
- Woo S L, Kanamori A, Zeminski J, Yagi M, Papageorgiou C, Fu F H. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon A cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg (Am) 2002; 84(6)907–14
- Yoo J D, Papannagari R, Park S E, DeFrate L E, Gill T J, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med 2005; 33(2)240–6
- Zhang L Q, Shiavi R G, Limbird T J, Minorik J M. Six degrees-of-freedom kinematics of ACL deficient knees during locomotion-compensatory mechanism. Gait Posture 2003; 17(1)34–42
