Abstract
Background It is unclear whether adult patients with high-grade non-metastatic osteosarcoma of the extremities, treated with neoadjuvant chemotherapy according to protocols designed for adults, have a different outcome than younger patients treated with conventional protocols.
Patients and methods From 1994 through 1999, we treated 34 patients with non-metastatic osteosarcoma of the extremities. These patients were aged mean 50 years (41–60), and received 4 cycles of multidrug chemotherapy (1 preoperatively and 3 postoperatively). Each cycle consisted of a combination of Cisplatin/Adriamycin, Ifosfamide/Cisplatinum and Ifosfamide/Adriamycin. 30 patients had limb salvage and 3 underwent amputation. During preoperative treatment, 1 died of toxicity. 16 patients had a good histological response to chemotherapy (≥ 90% tumor necrosis) and 17 had a poor response.
Results and interpretation With a median follow-up of 8 (5–11) years, 19/33 patients remained continuously disease-free and 14 relapsed (10 with metastases, 3 with local recurrence and metastases, and 1 with local recurrence alone). After further treatments, 2/14 relapsed patients are alive and disease-free, 11 died of tumor, and 1 is alive with uncontrolled disease. 5-year eventfree survival and overall survival were 56% and 70%, respectively. These results, which are similar to those of 296 patients under 40 years of age who were treated with conventional chemotherapy (5-year EFS 59% and 5-year OS 70%), indicate that neoadjuvant chemotherapy improves prognosis and also reduces amputations in patients aged over 40 with osteosarcoma of the extremities.
Adjuvant and neoadjuvant chemotherapy, introduced in the early 1970s, have significantly improved long-term survival in patients with osteosarcoma (OS) of the extremity. At the same time, the frequency of limb salvage surgery instead of amputation has also increased dramatically (Marina et al. Citation2004, Longhi et al. Citation2006). These results have, however, only been reported for young patients. Because of the low incidence of OS over the age of 40, the fear that these older patients may not tolerate aggressive modern chemotherapy—and the fact that in these patients, OS is usually associated with preexisting conditions such as Paget's disease or irradiated bones (Greditzer 3rd et al. 1983, Huvos et al. Citation1985)—patients over the age of 40 years who develop OS are often excluded from current trials of treatment. As a result, remarkably little is known about treatment and outcome for this age group.
In a previous paper (Bacci et al. Citation1998), we reported on 29 patients in the fourth and fifth decade of life who had non-metastatic OS of the extremity, and who were treated with neoadjuvant chemotherapy at our institution between 1986 and 1993. In that trial, chemotherapy was performed with Adriamycin (ADM) and Cisplatinum (CDP). Only poor responder patients also had Ifosfamide (IFO) added to the treatment postoperatively.
We now report the outcome in 34 patients aged between 41 and 60 who were treated (some time between 1994 and 1999) with a new protocol that in addition to ADM and CDP, also included IFO for all patients from the start of treatment. In this paper, the results obtained with this protocol are compared with those for 296 contemporary patients who were 40 years old or younger, and who were treated with more conventional protocols that also included high-dose methotrexate (MTX).
Material and methods
Patient selection
Between January 1994 and December 1999, 89 patients older than 41 years with newly diagnosed OS were seen at our institution. 21 of these patients were older than 60 years (mean 67 (61–77) years) and 68 were aged 60 or younger (mean 50 (41–60) years). A new neoadjuvant chemotherapy protocol was designed specifically for the latter group of patients, since at that age treatment with highdose MTX is usually not well tolerated. For this reason, of the 4 most active drugs in OS (ADM, CDP, IFO, and high-dose MTX), we included only the first 3 in this protocol. Criteria for entry into this new study, besides age between 41 and 60, were: (a) typical radiographic and histological features of primary, high-grade central OS; (b) tumor located in the extremity; (c) no prior treatments; (d) no evidence of metastases; and (e) no associated disease contraindicating chemotherapy with ADM, CDP, and IFO. Of the 68 patients in this age group who were observed during the period of the study (1994–1999), 37 were eligible. All eligible patients were invited to enter this trial after having been informed of the potential advantages and risks of the chemotherapy. Of the 37 eligible patients, 3 declined to enter the study and moved to other institutions for treatment. The remaining 34 patients were the object of this study ().
Table 1. Characteristics of the 34 patients in the study, and 5-year DFS
The results obtained in these 34 patients are compared with the results for 296 patients with non-metastatic osteosarcoma of the extremity who were 40 years old or younger and who were treated at our institute between April 1994 and August 1998. These patiente were treated with two sequential protocols (IOR/OS-4 and IOR/OS-5) that have been described in detail elsewhere (Bacci et al. Citation1998, 2002) (). These protocols included high-dose MTX, CDP, ADM, and IFO. The main differences between these two protocols and the one used in the present study were: (a) the addition of high-dose MTX to CDP, ADM and IFO pre- and postoperatively; (b) a higher cumulative dose of ADM (420 and 480 mg/m2 ADM vs. 300 mg/m2); (c) a higher number of courses of chemotherapy (19 and 18 vs. 12); and (d) a longer time of treatment, at least for poor responders (37 or 41 weeks vs. 35 weeks).
Table 2. Comparison of characteristics and dose intensity of treatment between patients aged 41-60 years and patients younger than 41
Diagnosis and pathological evaluation
Diagnosis of OS, established by clinical and radiological findings, was always confirmed using tumor tissue obtained from an open or trocar biopsy, as well as from the resected specimen. OS was classified as conventional, telangiectatic, or small-cell OS according to Mirra (Citation1989). Conventional osteosarcoma was subclassified as osteoblastic, fibroblastic, or chondroblastic. This distinction, always made on surgical specimens, was possible in all but 2 cases of “classic osteosarcoma” that were defined as “unclassifiable conventional osteosarcoma”. Tumor volume was evaluated retrospectively from CT-scan measurements of the 3 diameters of the lesion according to the method described by Gobel et al. (Citation1987).
Preoperative evaluation
Primary tumors were evaluated on standard radiographs, by Technetium 99-MDP bone scans, CT scans, and—in about one-half of the patients—also by MRI. Bone metastases were investigated with total body scans, whereas standard chest radiographs and CT scans of the chest were used to exclude lung metastases.
Chemotherapy
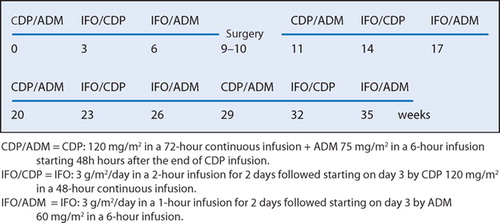
Chemotherapy consisted of 4 cycles of the following combinations of drugs given intravenously: Cisplatinum (CDP) and Adriamycin (ADM), Ifosfamide (IFO) and CDP, and IFO and ADM. The interval between each cycle was 3 weeks. 1 course was administered before surgery and 3 courses after surgery (). Dose intensity of chemotherapy was evaluated according to the method described by Hryniuk and Bush (Citation1984).
Surgery and pathological evaluation of response to chemotherapy
Type of surgery (amputation or limb salvage) and also type of reconstruction after resection of loadbearing bones (prosthesis, Küntscher rod or plate and cement, vascularized fibula combined with allograft, allograft and autograft) were chosen depending on tumor location and extension, involvement of neurovascular structure, desired lifestyle, and presence of complicating factors such as displaced pathologic fractures or infected biopsy sites. Limb salvage required that wide surgical margins could be achieved, and that the limb would be at least partially functional after reconstruction.
After surgery, surgeons and pathologists together reviewed gross specimens to determine surgical margins according to Enneking (Citation1987). Margins were classified as “adequate” if radical or wide, and “inadequate” if marginal, intralesional, or contaminated. Response to preoperative chemotherapy was evaluated following criteria previously reported (Picci et al. Citation1985) and it was graded as “good” (≥ 90% tumor necrosis) or “poor” (< 90% tumor necrosis). These two grades roughly correspond to grades III and IV and grades I and II (respectively) of the descriptive classification proposed by Rosen et al. (Citation1982).
Post-relapse treatment
Type of treatment for metastases and/or local recurrence in relapsed patients was not standardized, but performed on an individual basis considering the site of local recurrence, site and number of metastases, length of free interval, and whether local recurrence and metastases were isolated or combined. Second-line chemotherapy, usually performed with drugs not used in the first-line treatment (MTX) or with higher doses of drugs previously used (IFO), was given only to patients in whom it was not possible to achieve complete surgical removal of metastases, in patients with a disease-free interval between the first treatment and relapse shorter than 2 years, or when there were more than 2 recurrences. 5 of the 14 relapsed patients were not treated at Rizzoli after the relapse but at other institutions, and for these 5 patients our data were drawn from indirect information.
Statistics
Because of a lack of uniformity in the therapeutic regimen performed after relapse and considering that all but 2 patients of the 14 who relapsed died or are alive with uncontrolled disease (see later), the prognostic significance of the variables investigated was evaluated regarding only event-free survival (EFS). Post-relapse outcome and overall survival was also reported, but these data should be considered with caution. In fact, as reported before, when recurrent disease occurred, post-relapse treatment was not homogeneous—and of the 5 patients not treated at our institution, we have only indirect data and important details are often lacking.
EFS was established from the start of treatment to the date of recurrence (local and/or systemic). EFS curves were calculated according to the Kaplan-Meier method and compared by means of the log rank test. Significance was set at p < 0.05.
Results
Treatment compliance and dose intensity of the chemotherapy
All but 2 patients completed their treatment. Among the 391 courses delivered, 76 (19%) were administered with a delay longer than 5 days (median 9 (6– 22) days). Reasons for these delays were: delayed bone marrow recovery in 67 courses (88%), other abnormal laboratory findings in 3 cases, surgical complications in 2, and organizational problems in 4. Although the protocol did not cover dose reductions, in 20 courses doses were reduced (median 18% (10–25)). Due to delays in courses and/or dose reductions, of the 33 assessable patients only 2 received the exact planned dose intensity, while in the other 31 the mean dose intensity was between 90% and 95% in 6 cases, between 80% and 89% in 10, and less than 80% in 15 cases. The mean dose intensity was similar to that in the group of patients younger than 41 years ().
Surgery, surgical margins, and histological response to chemotherapy
Surgery consisted of amputation in 3 patients, and 30 patients were treated with limb salvage. 1 patient was not treated because of death following chemotherapy toxicity during preoperative treatment.
Surgical margins were inadequate (1 marginal and 1 intralesional) in 2 patients and adequate (2 radical and 29 wide) in 31 patients. Both inadequate surgical margins were observed in patients treated with limb salvage.
Chemotherapy-related tumor necrosis was good in 16 patients and poor in 17.
Event-free survival (EFS)
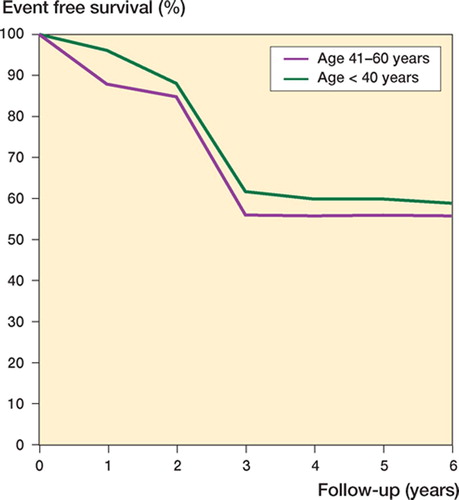
At a median follow-up of 8 (5–11) years, 19 patients remained continuously event-free, 14 relapsed, and 1 died from chemotherapy toxicity during the preoperative treatment. Because no patients had relapse after 5 years, the 5-year EFS was 56% as reported above. Although the number of patients was too small for us to provide prognostic factors, EFS was unrelated to sex and age of patients, presence or absence of pathologic fracture, site, tumor volume and histologic subtype, serum value of alkaline phosphatase at presentation, type of surgery and surgical margins, histological response to preoperative chemotherapy, and total dose intensity of treatment.
Pattern of relapse
In the 14 patients who relapsed, the first recurrences were metastases in 10 cases and local recurrence (LR) in 4. In one case only, the LR remained isolated while metastases developed in the other 3 despite immediate surgical treatment of the LR. First site of metastases—considering also the 3 patients who developed metastases after local relapse—were lung in 11 patients, spine in 1 patient, and both lung and bone (pelvis) in 1. In the 11 patients who relapsed in the lung, the mean number of metastatic nodules seen by CT scan was 4 (1–7); secondary lesions were monolateral in 6 cases and bilateral in 5. Mean time to relapse was 17 (2–30) months.
Local recurrence
As stated before, local relapse occurred in 4 patients at an average of 9 (6–12) months from the beginning of treatment. In all but 1 case local recurrence occurred with systemic relapse, which followed the LR in 2 cases and preceded it in 1. In these 4 patients, the primary tumor was treated with limb salvage. Margins were wide in 3 and intralesional in 1, and histological response was good in 1 patient (the one with intralesional margins) and poor in the other 3.
Treatment of relapse and post-relapse outcome
The 4 local recurrences were all treated with amputation. One of these patients is alive and free of disease after 6 years, while the other 3 subsequently developed metastases and died 4–16 months after the second relapse.
Lung metastases were treated with wedge resection in 8 cases and lobectomy in one. In 4 of these patients, metastectomy was followed by secondline chemotherapy. The remaining patient had only palliative treatment; this also applied to the patients who relapsed with bone metastases and the patient who relapsed with lung and bone metastases.
Of the 9 patients who first relapsed with lung metastases, 1 is alive and free of disease 9 years after the last treatment while the other 8 had one or more further relapses. Of these 8 patients, 1 is alive with uncontrolled disease 22 months after the last relapse whereas the other 7 died of the tumor 6–64 months after the first relapse. The 5-year overall survival was 65%.
Toxicity from chemotherapy
1 patient died of sepsis during a severe neutropenia that followed the last preoperative cycle. Among the 391 courses of chemotherapy performed, a grade 4 thrombocytopenia without associated episodes of bleeding was reported in 10% of the courses. Incidence of WHO grade 4 neutropenia was seen in 19% of courses. Admissions to hospital for treatment of febrile neutropenia or platelet transfusions were recorded after 4% of all courses. Episodes of WHO grade 1–2 renal toxicity were recorded in 0.6% of courses. The main type of extra-hemato-logical toxicity was acute nephrotoxicity (WHO grade 2, creatinine clearance 25 mL/min) observed in a 58-year-old woman after the third cycle of CDP. No other cases of severe nephrotoxicity or second tumor were observed. Neurological disturbance after IFO was reported in 4 patients, while Cisplatin-related neuropathy or ototoxicity was reported in 2 cases.
Comparison with a more aggressive protocol in younger patients
In a comparison of the above results with the results achieved in 296 patients with non-metastatic osteosarcoma of the extremity, aged between 4 and 40 years old and treated with a more aggressive protocol of chemotherapy at our institution, we found no differences between the two groups in terms of rate of limb salvage, histological response to preoperative treatment, 5-year disease-free survival and overall survival, or local recurrence. The mean time to relapse and the mean time to death were longer in the younger group of patients (; ).
Table 3. Comparison between results obtained in 34 a patients of the present study (Jan 1994–Dec 1999) and those obtained in 296 younger patients treated during the same period (Apr 1994–Aug 1998) with Rizzoli's conventional protocols (IOR/OS-4 and IOR/OS-5)
Discussion
Efficacy of adjuvant and neoadjuvant chemotherapy in non-metastatic osteosarcoma of the extremity has been well established for children, adolescents, and young adults (Rosen et al. Citation1982, Winkler et al. Citation1984, 1988, Meyers et al. Citation1992, Provisor et al. Citation1997, Fuchs et al. Citation1998, Bacci et al. Citation1998, 2002), whereas it seems to be ineffective for patients over the age of 60 years (Huvos Citation1986). This is probably due to reduction of dose/intensity of treatments. For patients aged between 41 and 60 years, who have generally been excluded from single-institutional or multicenter trials of neoadjuvant chemotherapy for osteosarcoma, there are very few data in the literature. To our knowledge, there have only been 3 papers on this issue and these were quite different with regard to patient selection and (above all) treatment.
In an evaluation of 47 patients with osteosarcoma who were older than 40 (median 53 (41–80) years) and who were treated at the Mayo Clinic between 1977 and 1998, Carsi and Rock (Citation2002) reported a 5-year disease-free survival and overall survival rate of 32% and 41%, respectively. It must be stressed, however, that this group included 18 cases with axial lesions, all of whom initially had synchronous metastases, and 4 with lowgrade osteosarcoma. Moreover, 20 patients did not receive any form of systemic treatment and only 12 received neoadjuvant chemotherapy. Diseasefree survival and overall survival for the subset of patients with high-grade non-metastatic osteosarcoma were 33% and 52%, respectively.
Naka et al. (Citation1995) reported a dismal survival rate of 18% at 5 years in 20 patients older than 40 with osteoblastic osteosarcoma. The authors did not report whether these patients received chemotherapy.
In a retrospective review covering 220 patients with high-grade non-metastatic osteosarcoma of the extremity who were aged over 40 and who had been treated in 12 different institutions, Grimer et al. (Citation2003) reported an overall 5-year survival rate of 46%. The figure was 53% when considering only patients aged between 40 and 60. 69 of these patients (31%) did not receive chemotherapy.
The policy of our institute has been to treat highgrade osteosarcoma of the extremity in patients ≤ 40 years old with aggressive neoadjuvant chemotherapy (also including high doses of MTX), in patients older than 60 with surgery only, and in patients aged 41–60 with surgery and aggressive chemotherapy (excluding high-dose MTX, however).
In a previous paper (Bacci et al. Citation1998), we reported the results of treating 29 patients aged 40–60 who had non-metastatic osteosarcoma of the extremity with a chemotherapy regimen including only ADM and CDP. The patients had been treated between 1986 and 1993. We found that the outcome in these 29 patients was significantly better than that achieved in 24 patients of the same age who were treated with surgery alone between 1975 and 1985.
The results of the present study confirm the above data. This is by far the largest analysis of outcome in a homogenous group of patients aged 41–60 with non-metastatic osteosarcoma of the extremity to be treated with the same neoadjuvant protocol in a single institution. The results appear to demonstrate that when treated with neoadjuvant chemotherapy, the outcome in patients aged 41–60 is no different from that of younger patients. In fact, the rate of limb salvage, EFS, and overall survival in the 44 patients aged 41–60 in the present study were essentially the same as the results achieved in 260 younger patients treated during almost the same period. The results obtained in the present study, in which all patients received a 3-drug regimen (CDP, ADM, and IFO) from the start of treatment, were also similar to those of the previous study in which IFO was used only postoperatively in poor responders (Bacci et al. Citation2000).
In conclusion, our results suggest that in patients over 40 years of age, high-grade osteosarcoma of the extremity should be treated with neoadjuvant chemotherapy and that chemotherapy-induced toxicity in the age group evaluated (40–60 years) is acceptable when high-dose MTX is avoided.
Contributions of authors
GB, PP: wrote the manuscript. SF, AL, MS: chemotherapy and data management. MM, NF: surgical treatment. SG: radiological evaluation. AB: translation and revision. CF: registered nurse (chemotherapy).
References
- Bacci G, Ferrari S, Donati D, Longhi A, Bertoni F, Di Fiore M, Comandone A, Cesari M, Campanacci M. Neoadjuvant chemotherapy for osteosarcoma of the extremity in patients in the fourth and fifth decade of life. Oncol Rep 1998; 5: 51259–63
- Bacci G, Ferrari S, Mercuri M, Longhi A, Capanna R, Tienghi A, Brach del Prever A, Comandone A, Cesari M, Bernini G, Picci P. Neoadjuvant chemotherapy for extremity osteosarcoma—preliminary results of the Rizzoli's 4th study. Acta Oncol 1998; 37(1)41–8
- Bacci G, Ferrari S, Bretoni F, Ruggieri P, Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M, Campanacci M. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Riz-zoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol 2000; 18(24)4016–27
- Bacci G, Ferrari S, Longhi A, Picci P, Mercuri M, Alvegard T A, Saeter G, Donati D, Manfrini M, Lari S, Briccoli A, Forni C. Italian Sarcoma Group/Scandinavian Sarcoma Group. High dose ifosfamide in combination with high dose methotrexate, adriamycin and cisplatin in the neoadjuvant treatment of extremity osteosarcoma: preliminary results of an Italian Sarcoma Group/Scandina-vian Sarcoma Group pilot study. J Chemother 2002; 14: 2198–206
- Carsi B, Rock M G. Primary osteosarcoma in adults older than 40 years. Clin Orthop 2002; 397: 53–61
- Enneking W F. A system for the evaluation of the surgical management of musculoskeletal tumors. Limb salvage in musculoskeletal oncology, W F Enneking. Churchill Livingstone, New York 1987; 113: 187–91
- Fuchs N, Bielack S S, Epler D, Bieling P, Delling G, Korholz D, Graf N, Heise U, Jurgens H, Kotz R, Salzer-Kuntschik M, Weinel P, Werner M, Winkler K. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol 1998; 9(8)893–9
- Gobel V, Jurgens H, Etspuler G, Kemperdick H, Jungblut R M, Stienen U, Gobel U. Prognostic significance of tumor volume in localized Ewing's sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol 1987; 113: 2187–91
- Greditzer H G, 3rd, McLeod R A, Unni K K, Beabout J W. Bone sarcomas in Paget disease. Radiology 1983; 146(2)327–33
- Grimer R J, Cannon S R, Taminiau A M, Bielack S, Kempf-Bielack B, Windhager R, Dominkus M, Saeter G, Bauer H, Meller I, Szendroi M, Folleras G, San-Julian M, van der Eijken J. Osteosarcoma over the age of forty. Eur J Cancer 2003; 39(2)157–63
- Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol 1984; 2(11)1281–8
- Huvos A G. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer 1986; 57(7)1442–9
- Huvos A G, Woodard H Q, Cahan W G, Higinbotham N L, Stewart F W, Butler A, Bretsky S S. Postradiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patients. Cancer 1985; 55: 61244–55
- Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 2006; 32(6)423–36
- Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004; 9(4)422–41
- Meyers P A, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol 1992; 10(1)5–15
- Mirra R. Bone tumors. Lea & Fabiger, London-Philadelphia 1989; 2: 255–6
- Naka T, Fukuda T, Shinohara N, Iwamoto Y, Sugioka Y, Tsuneyoshi M. Osteosarcoma versus malignant fibrous histiocytoma of bone in patients older than 40 years. A clinicopathologic and immunohistochemical analysis with special reference to malignant fibrous histiocytomalike osteosarcoma. Cancer 1995; 76(6)972–84
- Picci P, Bacci G, Campanacci M, Gasparini M, Pilotti S, Cerasoli S, Bertoni F, Guerra A, Capanna R, Albisinni U, Galletti S, Gherlinzoni F, Calderoni P, Sudanese A, Baldini N, Bernini M, Jaffe N. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy Regional mapping of viable and nonviable tumor. Cancer 1985; 56(7)1515–21
- Provisor A J, Ettinger L J, Nachman J B, Krailo M D, Makley J T, Yunis E J, Huvos A G, Betcher D L, Baum E S, Kisker C T, Miser J S. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol 1997; 15(1)76–84
- Rosen G, Caparros B, Huvos A G, Kosloff C, Nirenberg A, Cacavio A, Marcove R C, Lane J M, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer 1982; 49: 61221–30
- Winkler K, Beron G, Kotz R, Salzer-Kuntschik M, Beck J, Beck W, Brandeis W, Ebell W, Erttmann R, Gobel U, Havers W, Henze G, Hinderfeld L, Hocker P, Jobke A, Jurgens H, Kabish H, Preusser P, Prindull G, Ramach W, Ritter J, Sekera J, Treuner J, Wust G, Landbeck G. Neoadjuvant chemotherapy for osteogenic sarcoma: results of a Cooperative German/Austrian study. J Clin Oncol 1984; 2(6)617–24
- Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfurst C, Berger J, Ritter J, Jurgens H, Gerein V, Graf N, Russe W, Gruemayer E R, Ertelt W, Kotz R, Preusser P, Prindull G, Brandeis W, Landbeck G. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 1988; 6(2)329–37