Abstract
Background The question of whether fracture healing and mechanical properties of the callus are influenced by osteoporosis (OP) is still not settled. We therefore studied this issue in vitamin D-depleted ovariectomized (OVX) rats, an OP model previously shown to induce weakening of the femoral neck, and thus thought to be closer to the human condition than the classic OVX rat model.
Methods 72 female Wistar rats were randomized into two groups: ovariectomy and vitamin D-deficient diet (Ovx-D group) or sham operation and normal rat chow (Sham group). After 12 weeks, a closed tibial midshaft fracture was performed on the right side and fixed with an intramedullary nail. Bone loss and callus formation were monitored with DXA; serum levels of estradiol and vitamin D3 were measured and histomorphometric analyses were performed. Mechanical properties of callus, tibia, femoral shaft, and femoral neck were examined in 3-point cantilever bending 6 weeks after fracture.
Results The Ovx-D group showed reduced BMD in the spine and femoral neck, and reduced trabecular bone volume in the femoral head. There were no differences in BMD and mechanical properties of callus between the groups. Except for reduced stiffness of the right femoral neck in the Ovx-D group (p = 0.02), no differences in the mechanical strength of long bones were detected.
Interpretation Our results suggest that the systemic effects of estrogen and vitamin D deficiency are not crucial for fracture healing or mechanical properties of the callus.
The WHO has defined osteoporosis (OP) as a progressive systemic skeletal disease characterized by low bone mass and microarchitectural disturbance in bone tissue, with a consequent increase in bone fragility and susceptibility to fracture (Kanis Citation1994). The OP-related fracture incidence seems to rise, making OP a major health problem worldwide (Egermann et al. Citation2005). Increasing average life expectancy and the number of elderly in the western population have further aggravated the consequences for individuals and society. In addition, several lifestyle and health-related factors such as nutrition, physical activity, cigarette smoking and heavy alcohol consumption have been linked to the increased incidence of OP (The North American Menopause Society Citation2006). Among these, much attention has been paid to vitamin D. Inadequate 25-hydroxy-vitamin D (25(OH)D) levels are common in the elderly, and particularly so in patients with fragility fracture—especially among those with hip fracture (Moniz et al. Citation2005, Nurmi et al. Citation2005, Dixon et al. Citation2006).
Whether or not fracture healing and the mechanical properties of callus are influenced by OP is still unclear. Although impaired fracture healing in the aged is not generally assumed, the frequently observed failure of implant fixation in the presence of OP indicates reduced healing capacity (Barrios et al. Citation1993).
The number of studies on human fracture healing in OP is limited. On the other hand, several studies have investigated fracture healing in the ovariectomized (OVX) rat model (Egermann et al. Citation2005). The rat fracture model has been useful for elucidation of the process of fracture repair (McKibbin Citation1978, Einhorn Citation1998), and the OVX rat model has gained acceptance as being relevant for studying postmenopausal trabecular bone loss (Kalu Citation1991, Kimmel Citation1996). The validity of the OVX rat model for the human condition has been questioned, however, partly due to differences between how human and rodent OVX-related cortical bone loss proceeds (Egermann et al. Citation2005). In addition, the classic OVX model does not take into account the abovementioned lifestyle and health-related predisposing factors, such as diet. Thus, a model combining OVX and a vitamin D-deficient diet may be closer to what is observed in elderly women with hip fractures. In addition, reduced strength in the femoral neck—as seen in elderly patients—has been reported previously in this model (Kaastad et al. Citation2001).
We investigated the combined effects of OVX and vitamin D deficiency on the mechanical properties of a healing tibial midshaft fracture after 6 weeks. Estrogen is reported to be an important regulator of cartilage homeostasis, growth and maturation (Lee and Witchel Citation1997), and the finding of mRNA for estrogen receptors expressed in callus during fracture healing in rats would suggest that estrogen has a role in the healing process (Boden et al. Citation1989). On the other hand, vitamin D deficiency is known to induce secondary hyperparathyroidism, increased bone turnover, and bone mineral loss (Fraser Citation1995, Ooms et al. Citation1995, Mosekilde Citation2005). Furthermore, vitamin D and some of its metabolites may have roles in bone metabolism and fracture healing (Omeroglu et al. Citation1997). In addition, vitamin D deficiency has been shown to block formation of mature bone during fracture healing, although the deposition of osteoid remains unchanged (Lindgren et al. Citation1981).
Thus, our working hypothesis was that the combined withdrawal of estrogen and vitamin D will disturb and/or delay the fracture healing process, leading to callus with impaired mechanical properties.
Material and methods
Local guidelines were followed and the study protocol was approved by the Norwegian National Animal Research Authority.
Animals
72 female Wistar rats (Taconic Europe, Ejby, Denmark), 10 weeks of age, with a mean weight of 235 (197–272) g were randomized into two groups. The animals in one group, termed Ovx-D, were ovariectomized and fed a vitamin D3-deficient diet (RM1; Special Diets Services, Witham, Essex, England (no vitamin D3, 0.55% Ca, and 0.50% P)) and the other group was sham-operated and fed normal rat chow (vitamin D3 at 1,500 IU/kg, 0.55% Ca, and 0.50% P). To avoid the excessive weight gain seen in OVX rats, pairwise feeding was started after the OVX/sham surgery. The food was weighed for both groups and the amount eaten by the Sham animals was used as a standard for all.
All animals had free access to tap water and were housed in an open system with 1 or 2 in wire-top plastic cages (2,000 cm2). The temperature was 21 ± 1°C, relative humidity was 55 ± 10%, and there were day/night cycles of 12 h with 20 exchanges of air every hour.
During surgery and prior to killing, the rats were anesthetized with a combination of Hypnorm (fluanisone 5 mg/ml and fentanyl citrate 0.1575 mg/mL) and Dormicum (midazolam 2.5 mg/mL). The dose was 0.2–0.35 mL per 100 g body weight subcutaneously. The animals were given analgesics for 1 day postoperatively (Temgesic; Schering-Plough, Kenilworth, NJ) at a dose of 0.05 mg per kg body weight subcutaneously.
In the Ovx-D group, bilateral ovariectomy was performed as previously described (Waynforth Citation1980), while the Sham group received only the skin incision followed by laparotomy.
After 12 weeks, a closed transverse fracture was made in the right tibial midshaft of all animals (Nordsletten et al. Citation1994b). During anesthesia, the heel of the right limb was placed on a platform with the distal part of the limb in-between 3 points in the jaws of a specially designed forceps (Ekeland et al. Citation1981). The jaws were rapidly closed to deliver a force that resulted in a standardized fracture of the right tibia. The fracture was then fixed with an intramedullary nail (Optiva 2 Smiths Medical 18 G 1.3 × 32 mm; Smiths Medical International Ltd., Kent, UK) as previously described (Nordsletten et al. Citation1994b). Animals for histomorphometric analysis were killed 3 weeks (7 Ovx-D and 7 Sham) or 6 weeks (6 Ovx-D and 5 Sham) after fracture. Under deep anesthesia, the tissues of the animals were fixed by vascular perfusion through the heart with phosphate-buffered (0.1 M) 2% paraformaldehyde. Tibia and femur were excised bilaterally and dissected free of soft tissues, and the intramedullary nail was carefully extracted from a proximal port of the tibia without damaging the fracture site.
6 weeks after surgery, 11 animals from each group were killed with an overdose of phenobarbital. Tibia and femur were then dissected free and the intramedullary nail was removed as described above. Prior to mechanical testing, the bones were kept moisturized in separate glass containers with 0.9% sodium chloride at 4°C.
Before death, blood was collected from all Ovx-D animals and 5 randomly chosen Sham animals by cardiac puncture, and then frozen for later determination of serum levels of vitamin D and estradiol. The 22 remaining animals were killed, and the bone tissue prepared and stored for later use in planned supplementary ultrastructural studies of fracture healing in osteoporotic rats.
Serum levels of 25-hydroxyvitamin D and estradiol
The serum levels of 25(OH)D were determined after acetonitrile extraction using a competitive radioimmunoassay (RIA) kit from DiaSorin (Stillwater, MN). The intra-assay variation was 6%. The serum levels of estradiol were determined after diethyl ether extraction using a competitive fluoroimmunoassay (DELFIA) kit from PerkinElmer, Turku, Finland. The intraassay variation was 3% for serum levels in the range of 0.43– 0.12 nmol/L and 5% for serum levels < 0.12 nmol/L. All measurements were performed by the Hormone Laboratory at Aker University Hospital, Oslo, Norway.
Dual-energy X-ray absorptiometry (DXA)
The bone mineral density (BMD) (g/cm2) was assessed in vivo by DXA using Lunar PIXImus (Lunar, Madison, WI) with hardware and software specially designed for bone densitometry in small animals. To evaluate bone loss prior to induction of fracture, BMD was measured in the right tibial midshaft of all animals. 2, 3 and 6 weeks after fracture, the following sites were scanned: the fracture area, tibial and femoral midshaft, femoral neck, and lower lumbal vertebra. The region of interest (ROI) was chosen as a longitudinal rectangle covering the outer cortices (tibial midshaft 11 × 25 pixels and femoral neck 11 × 11 pixels). For the callus area, a longitudinal rectangle was placed with the transverse fracture line right-angled in center, and the ROI was adjusted to cover the whole callus area. For the lower lumbar vertebra, a transverse rectangle was chosen (11 × 17 pixels). BMD of the intramedullary nail was measured after extraction, and subtracted from the in vivo values of the right tibial shaft and callus. The precision for the DXA measurements was estimated by duplicate measurements at the same time point, with intraassay variation of 3%.
Mechanical analysis
Mechanical testing of the fracture callus, the femoral midshaft, and the femoral neck was carried out on both the Ovx-D and the Sham group. According to recent data (Namkung-Matthai et al. Citation2001), the clinically relevant difference in means was considered to be 20% or more. Although 5 animals per group was calculated to be sufficient to evaluate a 20% difference in means by an independentsamples t-test (2-tailed) with a power of 80%, α = 0.05 and σ = 0.10 (Dupont and Plummer Citation1998), 11 animals were included in each group in order to make up for possible losses during the many different steps of the experiment.
The femoral midshaft was fractured 16 mm from the femoral condyles with 3-point cantilever bending as previously described (Engesaeter et al. Citation1978). The proximal femur was fixed in a clamp and the cam of the rotating wheel was used to engage the femoral condyles. A fulcrum positioned anteriorly 16 mm from the condyles was the third point of application of force. For testing of the femoral neck, the cam of the rotating wheel was placed against the femoral head with its central edge at the base of the femoral neck, and the wheel was rotated until the femoral neck fractured in combined bending and shear.
After demarcating the fracture line with a pen, the proximal tibia was fixed in the clamp, the cam of the rotating wheel was used to engage the distal end anteriorly, and the fulcrum was positioned posteriorly facing the demarcated fracture line in the callus area. After mechanical testing, the operators evaluated the bone fragments in each case to ensure that loading until failure actually occurred in the callus area. The midshaft of the tibia broke approximately 26 mm from the proximal tibia condyles, a measurement that was used later as a standard for positioning of the fulcrum during testing of the contralateral controls. The intact limb from both groups was used as an internal control. Nonunited fractures, defined as 2 separate bone fragments or a callus gap bridged only by soft tissue after extracting the intramedullary nail, were excluded from mechanical testing. All tests were performed at a loading rate of 12 radians/second (Nordsletten et al. Citation1994a). The load was measured with a load cell (250 N) connected to a computer via an amplifier. The load-deformation curves were recorded online in TestStar II (MTS Systems Corp., Eden Prairie, MN). Ultimate moment, ultimate energy absorption, stiffness and defiection were read directly or calculated from the computer recordings as described previously (Nordsletten et al. Citation1993), and group comparisons between the Ovx-D and Sham animals were carried out. Reproducibility of the test was 0.02% of maximum load (250 N).
Histomorphometry
Following dissection and removal of the intramedullary nail, the specimens were further fixed in 2% phosphate-buffered paraformaldehyde for 3 days, and then decalcified in 7% EDTA supplemented with 0.5% paraformaldehyde for approximately 40 days. After decalcification, transverse slices were taken from tibia at 2 different sites: 0.5-mm-thick samples for histomorphometric analysis were cut from the proximal midshaft, whereas 1.0-mm-thick slices were cut from the center of the callus. The callus specimens were prepared and stored for later ultrastructural analysis.
The femoral midshaft was harvested and thin transverse slices (of 0.5 mm) were cut. The femoral head and neck were split by a mid-longitudi-nal section. These specimens were further fixed in 2% glutaraldehyde, dehydrated in ethanol, and embedded in an epoxy resin according to a routine protocol. Semi-thin (1-μm) sections were stained with toluidine blue and examined in a light microscope.
2 sections from each of the tibial and femoral midshafts were studied in all animals. Anteroposterior and mediolateral outer diameter, cortical thickness, and cortical area were measured using a semiautomatic image analyzer program (Analy-SIS 3.0; Soft Imaging System, Münster, Germany). Images were obtained in a light microscope at a magnification of 1.25× using a digital camera. The anterioposterior and mediolateral outer diameters were measured with a right angle in-between, and the means of the diameters were calculated and used as the total outer diameter. The cortical thickness was measured at four randomly chosen points (with approximately 45° in-between) and the mean was calculated. The cortical area was calculated by measuring the area of the whole cross section minus the area of the bone marrow. Intraassay variation was less than 10%.
2 sections from the femoral head, bilaterally in all animals, were used for calculation of the trabecular bone volume density (VvTB). A light microscope with a digital camera was used to obtain images at a magnification of 2.5×, which were subsequently subjected to semiautomatic image analysis (AnalySIS 3.0; Soft Imaging System). A virtual square grid with test lines 300 μm apart was superimposed on images with one of the outer lines as close as possible to the growth plate and the other ones inside the cortical shell. The VvTB was calculated by point counting. Intraassay variation was 4%.
Statistics
All results are expressed as means and standard deviations (SDs). The mechanical data, which deviated slightly from a true normal distribution (illustrated by box plots; data not shown), were evaluated by an independent statistician (Department of Biostatistics, University of Oslo, Norway). An independent-samples t-test was considered to be suitable. Thus, the statistical differences between the groups for all parameters were calculated by the use of independent-samples t-test (two-tailed) in SPSS version 12.01 for Windows.
Concerning the histomorphometry, the mean of the measurements in each animal was calculated and used as a single observation to avoid statistical problems in managing dependent variables. A pvalue of ≤ 0.05 was considered significant.
Results
3 animals (1 Ovx-D and 2 Sham) were lost during the experimental period due to anesthesia or technical problems in establishing osteosynthesis. After randomization, but prior to ovariectomy and introduction of diet, mean body weight was 4.7% lower in the Ovx-D group compared to Sham (p = 0.02). However, despite pairwise feeding the Ovx-D group gained more weight than the Sham group. Mean body weight was 8.6% and 13% higher in the Ovx-D group after 3 and 6 weeks, respectively (p < 0.001).
Serum levels of 25-hydroxyvitamin D and estradiol
A 25(OH)D level of less than 25 nmol/L in serum was considered to be vitamin D deficiency (Lips Citation2001). The Ovx-D group had 25(OH)D levels of ≤ 18 nmol/L, and 94% had levels of 25(OH)D that were undetectable (i.e. ≤ 13 nmol/L).
Postmenopausal estrogen levels were defined as serum estradiol at < 0.10 nmol/L (Hormone Laboratory, Aker University Hospital, Oslo, Norway).
All Ovx-D animals presented serum estradiol in the range of the reference values for postmenopausal state (i.e. ≤ 0.08 nmol/L).
Dual-energy X-ray absorptiometry ( and )
Throughout the monitoring period, the Ovx-D group presented lower BMD in vertebra and femoral neck bilaterally than the Sham group (p < 0.001). At 6 weeks after fracture (18 weeks after OVX and introduction of diet), BMD was reduced by 26%, 15% and 24% in vertebra, and right and left femoral neck, respectively. Except for a slightly higher BMD of the right tibial shaft in the Ovx-D group at the time of fracture surgery (p = 0.03), no differences were detected in the tibial shafts. Importantly, no changes in BMD of callus were detected during the observation period.
Right-left comparison indicated bone loss in the fractured limb in both groups; on average, BMD was lower in the right femoral neck than in the left (Ovx-D: p < 0.001 after 2, 3, and 6 weeks; Sham: p = 0.05, 0.02, and 0.005 after 2, 3 and 6 weeks, respectively). In addition, at the end of the experiment, mean BMD of the right tibial shaft was reduced in the Ovx-D group (p = 0.008). Tibial BMD in the Sham group was unchanged at all time points.
Table 1. Bone mineral density (BMD) in vivo measured by DXA at different sites before and 2, 3, and 6 weeks after induction of a tibial midshaft fracture on the right side. BMD of the intramedullary nail was measured in vitro and subtracted from the in vivo BMD values of right tibia. The left limb was used as an internal control. Values are mean (SD)
Table 2. Right-left comparison of bone mineral density (BMD) in vivo 2, 3 and 6 weeks after induction of a tibial midshaft fracture on the right (R) side. BMD of the intramedullary nail was measured in vitro and subtracted from the in vivo BMD values of right tibia. The left limb (L) was used as an internal control. Values are mean (SD)
Mechanical testing
Except for one rat that was killed due to technical problems during fracture surgery, osteosynthesis was performed successfully in all animals. After 6 weeks, the healing rate was 55% and 64% in Ovx-D and Sham groups, respectively. Thus, 5 nonunions in Ovx-D group and 4 nonunions in Sham group were excluded from mechanical testing. Mechanical testing of the remaining calluses (6 Ovx-D and 7 Sham) showed no differences in mechanical properties between the two groups ().
Table 3. Comparison of biomechanical properties of callus and long bones in Ovx-D and Sham groups examined in a 3-point cantilever bending test. A standardized fracture was performed in the tibial midshaft on the right side of all animals, and fixed with an intramedullary nail. The nail was removed prior to mechanical testing. The left limb was used as an internal control. Values are mean (SD)
2 left femoral necks (1 from each group) were excluded due to damage of the femoral head during dissection. Mechanical testing of the right femoral neck showed a 16% decrease in bending stiffness in the Ovx-D group compared to the Sham group (p = 0.02), but there was no difference in other parameters between the groups.
No differences in mechanical properties of the femoral shaft were detected between the groups.
Because the average body weight of the 22 animals included in the mechanical testing was significantly higher in the Ovx-D group (p = 0.002),
normalizing of the mechanical strength by body weight was performed but did not alter the statistical outcome (data not shown).
Histomorphometry
The Ovx-D group showed loss of trabeculae and trabecular connectivity in the femoral head (). This was confirmed by reduced VvTB (p < 0.001) (). Measurements of the cortical diameter, and thickness and area of the tibial and femoral midshaft showed only minor differences between the groups ().
Figure 1. Light microscopic pictures of sections of femoral head with a magnification of 2.5×. Panel A, from an Ovx-D rat, shows substantial loss of trabecular bone and trabecular connectivity compared to panel B, which was taken from a Sham rat.
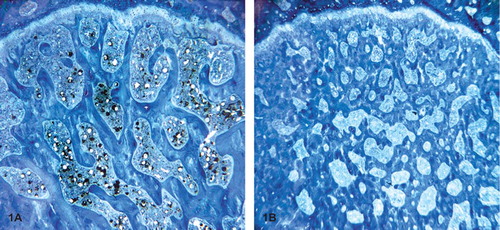
Figure 2. Trabecular bone volume density (VvTB) in the femoral head was significantly lower in the Ovx-group than in the Sham group, both 3 and 6 weeks after tibial shaft surgery. After 3 weeks, n = 14 in both right and left leg; after 6 weeks, n = 11/12 and 8/9 with respect to Ovx and Sham. * p < 0.001
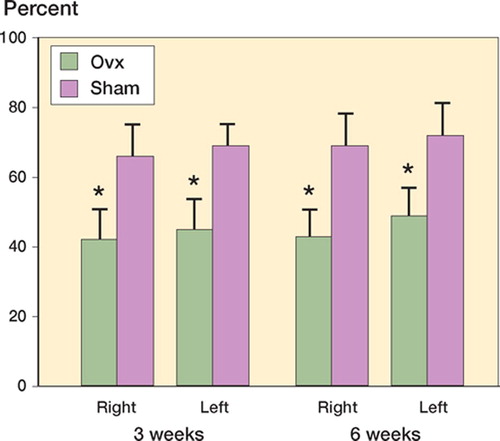
Table 4. Histomorphometry on tibial and femoral diaphyses 3 and 6 weeks after induction of tibial midshaft fracture. Values are mean (SD) × 10-1
Discussion
The fracture-healing process can be divided into 3 stages: the infiammatory, reparative, and remodeling phases (Buckwalter et al. Citation1996). In our model, the conditions 6 weeks after inducing the fractures corresponded to the reparative phase, when cartilaginous callus is the major component of healing. Our rat model with a combined vitamin D-and estrogen-deficient state was constructed in order to mimic the clinical picture of fracture healing commonly observed in elderly postmenopausal women with ovarian failure and vitamin D insufficiency.
Osteoporosis was successfully induced in trabecular bone; serum analyses confirmed artificial menopause and severe vitamin D deficiency, and significantly reduced BMD in proximal femur and spine, as well as reduced VvTB in the femoral head, confirmed loss of trabecular bone. Since no significant bone loss was detected in the midshaft of long bones in the Ovx-D group relative to the Sham group, this model is no different from what was previously observed in the conventional OVX model concerning cortical bone loss (Egermann et al. Citation2005). At the end of the experiment— after 6 weeks—there was an unexpectedly high rate of incomplete healing in both groups (but not significantly different). However, mechanical testing of the fractures that healed revealed no significant alterations in callus strength between the groups. In support of this, there was no difference in the mean BMD of callus between the groups.
Several methodological aspects may have influenced our results. Weakening of the femoral neck in the range of 20–60% compared to normal littermates has previously been reported in this OP model (Kaastad et al. Citation2001), but except for reduced bending stiffness of the femoral neck, no significant alterations in the mechanical strength of long bones were detected in the Ovx-D group compared to the Sham group. According to our results and the sample size, an undetectable but clinically relevant difference in mechanical strength of the femoral neck is unlikely. Thus, for some unknown reason we were not able to reproduce the results reported by Kaastad et al. (Citation2001).
Body weight in the Ovx-D group was higher than in the Sham group at the time of fracture, indicating that the calluses were loaded differently from the beginning and through the 6 subsequent weeks of fracture repair. Increased mechanical load has been reported to promote fracture healing and callus formation in mice (Gardner et al. Citation2006), and since body weight is proportional to the load applied to the callus in vivo, normalization of callus strength by body weight was an important correction, but did not alter the statistical outcome. Although OVX-related weight gain in rats is mainly caused by excess adipose tissue rather than increased/maintained bone mass (Gray and Wade Citation1981, Wade and Heller Citation1993, Sato et al. Citation1994), OVX has been reported to stimulate periosteal appositional growth at the long bone diaphysis (Turner et al. Citation1987, Miller et al. Citation1991) while at the same time increasing bone resorption at endosteal surfaces, leading to expansion of the medullar cavity (Turner et al. Citation1987, Jee et al. Citation1990, Peng et al. Citation1994, Pan et al. Citation1995, Aerssens et al. Citation1996). Since the bone resorbed at the endosteal surface close to the bone marrow is replaced at the periosteal adjacent surface, enlargement of the medullar cavity and cortical thinning may be the only sign of cortical bone loss in the long bone diaphyses (Danielsen et al. Citation1993, Jee and Yao Citation2001). However, the absence of geometrically measurable changes in long bone diaphyses in our study, supported by the fact that there were no differences in BMD, indicate that the excessive body weight in the Ovx-D group was probably not the cause of the maintained mechanical strength of long bones.
Both groups presented a low healing rate of the fractures at the end of the experiment. Thus, the nonunions are not likely to be caused by estrogen/ vitamin D depletion but rather by common factors, i.e. the model or the fracture/osteosynthesis procedure. Internal fixation of the fracture with an intramedullary nail may have been too rigid; too little motion between the fracture fragments can lead to insufficient callus formation (Carter et al. Citation1998, Thompson et al. Citation2002). Furthermore, recent data indicate that immobilization can reduce or delay fracture healing in mice (Uusitalo et al. Citation2005). This is supported in the present study, at least in part, by left-right comparison showing reduced BMD of the right fractured limb in both groups.
Despite several exclusions from the callus groups in mechanical testing, sample size was still sufficient to allow detection of a 20% difference in means according to existing literature (Dupont and Plummer Citation1998). However, widespread variance (i.e. large SDs) complicates the interpretation of the results. The callus presents the most pronounced variance, with SDs in range of 25–50% of means for both groups. The 3-point cantilever bending test has been reported to be a good test for examination of the properties of tibial fractures (Bak et al. Citation1990, Bak and Andreassen Citation1991, Bak and Jensen Citation1992). Thus, the explanation is more likely to be related to variance in the fractures and/ or the healing process than to complex biomechanics of the tibia. Slight variance in weight/size of the animals—and thus differences in length of the limbs—may have resulted in fractures at different sites along the tibial shaft. Furthermore, positioning of the fulcrum during mechanical testing is critical for the outcome; when optimally positioned, the fulcrum faces the center of the callus and loading until failure occurs in this area. Thus, inaccuracy in positioning of the fulcrum with respect to the callus area may have been a source of bias.
Even so, the widespread variance observed throughout the mechanical test results suggests a more generalized cause. Bending tests are strongly dependent on the operator’s testing skills and the orientation of specimens (Black et al. Citation1984), but all bending tests were performed by the same opera-tors—who were trained in the procedure to minimize this type of bias.
Although several studies support the previously held dogma that OVX impairs fracture healing at different stages of the repair process (Hill et al. Citation1995, Walsh et al. Citation1997, Namkung-Matthai et al. Citation2001), more recent data have indicated mainly unaffected mechanical properties of the callus (Kubo et al. Citation1999, Cao et al. Citation2002). Interestingly, we have shown that the additive effect of vitamin D depletion does not dramatically alter the results reported with OVX rats (Kubo et al. Citation1999, Cao et al. Citation2002). The BMD of callus, which is a widely used parameter of fracture healing, has previously been reported to be reduced in OVX rats (Walsh et al. Citation1997, Kubo et al. Citation1999). However, more recent evidence suggests that OVX-related increased bone turnover actually favors callus remodeling, and that callus size is increased in OVX rats after 6 weeks of fracture repair (Cao et al. Citation2002). In addition, after 16 weeks of healing OVX seemed to be advantageous in promoting the fracture repair by presenting non-visible fracture lines and fully remodeled callus into lamellar bone (Cao et al. Citation2002).
In summary, our results indicate that the mechanical properties of callus are mainly preserved even after the combined withdrawal of estrogen and vitamin D, suggesting that the whole callus appears to be able to compensate for the potentially negative effects of OVX and vitamin D deficiency on fracture healing. Thus, the maintenance of biomechanical integrity of callus in this OP model indicates a great capacity for biological adaptation and compensatory mechanisms in fracture repair.
On the other hand, this OP model may not be optimal for investigation of fracture healing due to low healing rate and considerable variance in the results of 3-point bending. Further studies are required to fully elucidate the clinical impact of estrogen and vitamin D on the healing of fractures, preferably at the molecular level.
Contributions of authors
LN, JEM, and FPR: initiated the study. GM and LBS: contributed equally to the study (shared first authorship). They wrote the experimental protocol under the supervision of LN and JEM. They also collected and analyzed the data under the supervision of LN and FPR and wrote the manuscript under the supervision of FPR (with input from LN and JEM during revision). GM, LBS, SD and JEM: performed the experiments.
This project was supported by grants from EU project no. 502941, OSTEOGENE, funds from the Norwegian Association against Osteoporosis and Helse Øst.
- Aerssens J, van Audekercke R, Talalaj M, Geusens P, Bramm E, Dequeker J. Effect of 1alpha-vitamin D3 and estrogen therapy on cortical bone mechanical properties in the ovariectomized rat model. Endocrinology 1996; 137: 1358–64
- Bak B, Andreassen T T. The effect of growth hormone on fracture healing in old rats. Bone 1991; 12: 151–4
- Bak B, Jensen K S. Standardization of tibial fractures in the rat. Bone 1992; 13: 289–95
- Bak B, Jorgensen P H, Andreassen T T. Dose response of growth hormone on fracture healing in the rat. Acta Orthop Scand 1990; 61: 54–7
- Barrios C, Brostrom L A, Stark A, Walheim G. Healing complications after internal fixation of trochanteric hip fractures: the prognostic value of osteoporosis. J Orthop Trauma 1993; 7: 438–42
- Black J, Perdigon P, Brown N, Pollack S R. Stiffness and strength of fracture callus Relative rates of mechanical maturation as evaluated by a uniaxial tensile test. Clin Orthop 1984, 182: 278–88
- Boden S D, Joyce M E, Oliver B, Heydemann A, Bolander M E. Estrogen receptor mRNA expression in callus during fracture healing in the rat. Calcif Tissue Int 1989; 45: 324–5
- Buckwalter J A, Einhorn T A, Bolander M E, Cruess R L. Healing of the Musculoskeletal Tissues. Fractures in adults4th, C A Rockwood, D P Green, R W Bucholz, J D Heckman. Lippincott-Raven, Philadelphia 1996
- Cao Y, Mori S, Mashiba T, Westmore M S, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 2002; 17: 2237–46
- Carter D R, Beaupre G S, Giori N J, Helms J A. Mechanobiology of skeletal regeneration. Clin Orthop (Suppl) 1998, 355: 41–55
- Danielsen C C, Mosekilde L, Svenstrup B. Cortical bone mass, composition, and mechanical properties in female rats in relation to age, long-term ovariectomy, and estrogen substitution. Calcif Tissue Int 1993; 52: 26–33
- Dixon T, Mitchell P, Beringer T, Gallacher S, Moniz C, Patel S, Pearson G, Ryan P. An overview of the prevalence of 25-hydroxy-vitamin D inadequacy amongst elderly patients with or without fragility fracture in the United Kingdom. Curr Med Res Opin 2006; 22: 405–15
- Dupont W D, Plummer W D. Power and sample size calculations for studies involving linear regression. Control Clin Trials 1998; 19: 589–601
- Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int (Suppl 2) 2005; 16: 129–38
- Einhorn T A. The cell and molecular biology of fracture healing. Clin Orthop (Suppl) 1998, 355: 7–21
- Ekeland A, Engesaeter L B, Langeland N. Mechanical properties of fractured and intact rat femora evaluated by bending, torsional and tensile tests. Acta Orthop Scand 1981; 52: 605–13
- Engesaeter L B, Ekeland A, Langeland N. Methods for testing the mechanical properties of the rat femur. Acta Orthop Scand 1978; 49: 512–8
- Fraser D R. Vitamin D Lancet. 1995; 345: 104–7
- Gardner M J, van der Meulen M C, Demetrakopoulos D, Wright T M, Myers E R, Bostrom M P. In vivo cyclic axial compression affects bone healing in the mouse tibia. J Orthop Res 2006; 24: 1679–86
- Gray J M, Wade G N. Food intake, body weight, and adiposity in female rats: actions and interactions of progestins and antiestrogens. Am J Physiol 1981; 240: 474–81
- Hill E L, Kraus K, Lapierre K P, Ryan S, Smith T, Martin R B, Finkelman R D. Ovariectomy impairs fracture healing after 21 days. Trans Orthop Res Soc 1995; 20: 230
- Jee W S, Mori S, Li X J, Chan S. Prostaglandin E2 enhances cortical bone mass and activates intracortical bone remodeling in intact and ovariectomized female rats. Bone 1990; 11: 253–66
- Jee W S, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 2001; 1: 193–207
- Kaastad T S, Reikeras O, Halvorsen V, Falch J A, Obrant K J, Nordsletten L. Vitamin D deficiency and ovariectomy reduced the strength of the femoral neck in rats. Calcif Tissue Int 2001; 69: 102–8
- Kalu D N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner 1991; 15: 175–91
- Kanis J A. Assessment of fracture risk and its application to ascreening for postmenopausal osteoporosis: synopsis of a WHO report WHO Study Group. Osteoporos Int 1994; 4: 368–81
- Kimmel D. Animal models for in vivo experimentation in osteoporosis research Osteoporosis 2 vols. R Marcus, D Feldman, J Kelsey. Academic Press, San Diego 1996
- Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, Kitajima I, Semba I, Hirasawa Y. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol 1999; 68: 197–202
- Lee P A, Witchel S F. The influence of estrogen on growth. Curr Opin Pediatr 1997; 9: 431–6
- Lindgren J U, Narechania R G, McBeath A A, Lange T A, DeLuca H F. Effects of 1,24 dihydroxyvitamin D3 and calcitonin on fracture healing in adult rats Clin Orthop. 1981, 160: 304–8
- Lips P, Vitamin D. deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001; 22: 477–501
- McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br 1978; 60: 150–62
- Miller S C, Bowman B M, Miller M A, Bagi C M. Calcium absorption and osseous organ-, tissue-, and envelope-spe-cific changes following ovariectomy in rats. Bone 1991; 12: 439–46
- Moniz C, Dew T, Dixon T. Prevalence of vitamin D inadequacy in osteoporotic hip fracture patients in London. Curr Med Res Opin 2005; 21: 1891–4
- Mosekilde L. Vitamin D and the elderly. Clin Endocrin 2005; 62: 265–81
- Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason R S, Murell G A, Diwan A D, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 2001; 28: 80–6
- Nordsletten L, Kaastad T S, Narum S, Madsen J E, Haug E, Reikeras O. Training increases the in vivo strength of the lower leg: an experimental study in the rat. J Bone Miner Res 1993; 8: 1089–95
- Nordsletten L, Kaastad T S, Madsen J E, Reikeras O, Ovstebo R, Stromme J H, Falch J. The development of femoral osteopenia in ovariectomized rats is not reduced by high intensity treadmill training: a mechanical and densitometric study. Calcif Tissue Int 1994a; 55: 436–42
- Nordsletten L, Madsen J E, Almaas R, Rootwelt T, Halse J, Konttinen Y T, Hukkanen M, Santavirta S. The neuronal regulation of fracture healing - effects of sciatic nerve resection in rat tibia. Acta Orthop Scand 1994b; 65: 299–304
- Nurmi I, Kaukonen J P, Luthje P, Naboulsi H, Tanninen S, Kataja M, Kallio M L, Leppilampi M. Half of the patients with an acute hip fracture suffer from hypovitaminosis D: a prospective study in southeastern Finland. Osteoporos Int 2005; 16: 2018–24
- Omeroglu S, Erdogan D, Omeroglu H. Effects of single high-dose vitamin D3 on fracture healing. An ultrastructural study in healthy guinea pigs. Arch Orthop Trauma Surg 1997; 116: 37–40
- Ooms M E, Lips P, Roos J C, van der Vijgh W J, Popp-Sni-jders C, Bezemer P D, Bouter L M. Vitamin D status and sex hormone binding globulin: determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res 1995; 10: 1177–84
- Pan Z, Jee W S, Ma Y F, McOsker J E, Li X J. Intermittent treatments of prostaglandin E2 plus risedronate and prostaglandin E2 alone are equally anabolic on tibial shaft of ovariectomized rats. Bone. 1995; 17(4 Suppl)291–6
- Peng Z, Tuukkanen J, Vaananen H K. Exercise can provide protection against bone loss and prevent the decrease in mechanical strength of femoral neck in ovariectomized rats. J Bone Miner Res 1994; 9: 1559–64
- Sato M, McClintock C, Kim J, Turner C H, Bryant H U, Magee D, Slemenda C W. Dual energy x-ray absorptiometry of raloxifene effects on the lumbar vertebrae and femora of ovariectomized rats. J Bone Miner Res 1994; 9: 715–24
- The North American Menopause Society. Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 2006; 13: 340–67
- Thompson Z, Miclau T, Hu D, Helms J A. A model for intramembranous ossification during fracture healing. J Orthop Res 2002; 20: 1091–8
- Turner R T, Vandersteenhoven J J, Bell N H. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res 1987; 2: 115–22
- Uusitalo H, Rantakokko J, Vuorio E, Aro H T. Bone defect repair in immobilization induced osteopenia: a pQCT, biomechanical, and molecular biologic study in the mouse femur. Bone 2005; 36: 142–9
- Wade G N, Heller H W. Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am J Physiol 1993; 264: 1219–23
- Walsh W R, Sherman P, Howlett C R, Sonnabend D H, Ehrlich M G. Fracture healing in a rat osteopenia model. Clin Orthop 1997; 342: 218–27
- Waynforth H B. Experimental and surgical technique in the rat. Academic Press, London 1980
