Abstract
Background Refobacin Palacos R (RPR) is no longer produced and Palacos R + G (PRG) and Refobacin Bone Cement R (RBC) have been introduced to supersede it. We performed an in vitro study to ascertain whether the handling and mechanical properties of the more recent cement preparations are different to those of their predecessor.
Methods The 3 cements were tested to ISO 5833 and German DIN 53435 standards. In addition, their gentamicin elution, shrinkage, viscosity, and handling characteristics were studied.
Results All 3 cements had comparable mechanical properties exceeding the ISO 5833 and DIN 53435 standards. They eluted similar amounts of gentamicin and had comparable shrinkage. In the batches tested, RBC showed statistically significantly longer handling curves. Both PRG and RBC had statistically significantly lower viscosities as they cured compared to their predecessor.
Interpretation Surgeons must be aware that both successor cements do not appear to have handling curves and viscoelastic properties identical to those of RPR.
Antibiotic-loaded Palacos bone cement with adjunctive systemic antibiotics in primary hip arthroplasty gives the lowest rate of revision due either to infection or aseptic loosening (Espehaug et al. Citation1997, Engesaeter et al. Citation2003). In 2005, production of gentamicin-loaded Refobacin Palacos R (RPR) (by Heraeus Kulzer GmbH; distributor and responsible manufacturer: Biomet Europe) ceased because of corporate reorganization. This has meant that both companies filled their own niches with follow-up products: Refobacin Bone Cement R (RBC) (produced by AAP Biomaterials GmbH & Co. KG, Dieburg, Germany; distributor and responsible manufacturer: Biomet Europe) and Palacos R + G (PRG) (produced by Heraeus Kulzer GmbH; distributed by Heraeus Medical GmbH). Some limited supplies of the “original” cement will still be available until stocks become exhausted.
Essentially, both companies claim that the “new” cements are equivalent to their predecessor. The early clinical experience of many surgeons across Europe suggests that the handling characteristics may not be identical, however. In clinical practice it is important to be familiar with the properties and handling of the bone cement used, as timing substantially influences operating technique and ultimately the outcome for the patient. We therefore conducted an in vitro study to investigate whether there are any detectable differences between the three cements in terms of their properties and handling characteristics.
Material
Refobacin-Palacos R (RPR) batch 6190, Palacos R + G (PRG) batch 6217 (6382 was used for the viscosity testing) and Refobacin Bone Cement R (RBC) batch 519B0 all list almost the same constituents.
All three monomers are provided in a 20-mL vial and contain 18.4 g MMA, 0.4 g N, N-dimethyl-p-toluidine (DmpT) and chlorophyll VIII. In PRG, refined peanut oil is used as a carrier for chlorophyll VIII, whereas RBC contains sunflower oil.
All three have 40.8 g of powder composed of: 0.84 g gentamicin sulphate (0.5 g active gentamicin), 33.6 g PMMA/MMA co-polymer, 6.1g zirconium dioxide, and 0.3 g benzoyl peroxide (BPO). RPR and PRG powder also contain 1 mg of chlorophyll VIII. RBC powder does not contain chlorophyll or its peanut oil carrier; thus, it is not green.
Methods
All preparations were made according to the manu-facturer's instructions in a climate-controlled room (temperature 23 ± 1°C and humidity 50 ± 5%).
Constituents were either stored at room temperature (23°C) or pre-chilled to 4 ± 2°C for 24 h before preparation. The samples were mixed for 30 s after the addition of all the powder to monomer, either by hand in porcelain bowls or under a vacuum of –200 millibar using the Easymix cement injector (Coripharm GmbH & Co KG, Dieburg, Germany) at 1 Hz.
Handling curves
The handling curves were examined for (1) cements prepared at room temperature that were hand-mixed, and (2) pre-chilled cements that were vacuum-mixed. The mixing phase was deemed to have started as soon as all the powder had been added to the liquid. For the hand-mixed samples it lasted 30 s, but for the vacuum mixes it took an additional 20 s to apply the vacuum and start combining the constituents.
The waiting phase immediately followed, and ended when a powder-free latex Biogel gloved finger (Regent Medical Co., Manchester, UK) would no longer adhere to the mixture. The samples that were prepared in the Easymix took additional time to be extruded from the syringe, so could not be evaluated before 105 s.
The working phase followed immediately and ended when the cement could be folded, joined together, and then separated without any self-adher-ence. The end of the working time until the setting time—which was moment at which the cement had become completely hardened—was taken as the setting phase (Kuehn Citation2000). 18 samples prepared at room temperature and 12 pre-chilled samples were tested.
Mechanical properties
5 cement samples stored at room temperature and 5 pre-chilled samples were prepared by vacuum mixing and tested according to the ISO 5833 4point bend and elastic modulus.
10 samples stored at both temperatures were prepared by vacuum mixing and tested to the ISO 5833 compression standards (International Standards Organisation Citation2002).
8 samples of cement stored at room temperature were prepared by vacuum mixing and their impact strength was examined according to German standard 53435 (Kuehn Citation2000).
8 samples of cement stored at room temperature were prepared by vacuum mixing to examine their 4-point bending strength according to German standard 53435.
8 specimens for tensile testing were prepared by vacuum mixing of cements stored at room temperature as described in ISO527-2 (5A). They were tested 24 h after preparation in a dry state in accordance with ISO 527-1 using a Zwick universal testing machine.
Shrinkage
The volume shrinkage of acrylic bone cement was calculated from the change in density, determined by the Archimedean principle, before and after the polymerization. A solid specimen immersed in water is exposed to the force of buoyancy. The value of this force is the same as that of the weight of the water displaced by the volume of the solid.
Three samples of cement stored at room temperature were hand-mixed. During the working phase, a small ball of the cement (2–3 g) was taken and tested using the same methods as used by previous investigators (Muller et al. Citation2002).
Antibiotic elution studies
5 discs 25 mm in diameter and 10 mm high were produced from standardized molds after vacuum mixing. Each disc was placed in 20 mL phosphate buffer (1.65 g KH2PO4 and 9.71 g Na2PO4 in 1 L H20, pH 7.4) and was transferred to an incubator at 37°C. No agitation was used. At 1, 6, 12, 24, 48, and 72 h the discs were removed from their solutions, the surfaces of the discs were patted dry, and they were transferred to 20 mL fresh phosphate buffer. The fresh samples were placed back into the incubator. 1 mL of eluate was then used to determine the antibiotic concentrations in solution using fluorescence polarization immunoassay (TDx system; Abbott, Abbott Park, IL).
The total cumulative quantity of gentamicin released by each was presented as a proportion of the sample's surface area in µg/cm2for ease of comparison with other studies.
Viscosity
The viscosity tests were performed with the Gel-norm-Med device (Gel Instrumente AG, Thalwil, Switzerland) (Dall et al. Citation2007). The pre-chilled bone cements were vacuum-mixed and tested 150 s after the addition of all the powder to the monomer. The cartridge was placed on the support of the measuring device. The cone-shaped measuring tool with a diameter of 28 mm and surface area of 6.1 cm2was attached to the surface of the cement by lowering the measuring unit (). The procedure was started and the test body was dipped vertically into the cement at a continuous speed of 0.03 mm/s. The force necessary to immerse the tool at the defined speed was recorded. The procedure was continued until the limiting force of 50 N had been reached. Two concurrent handling tests were performed for each cement and two measurement points (5 and 40 N) were chosen for data analysis, as these had previously been found to correlate approximately with the end of the working phase and the setting phase (Kühn and Gopp 2001). The median viscosity curves seen in are an average of the fifth and sixth curves seen in for each cement.
Results
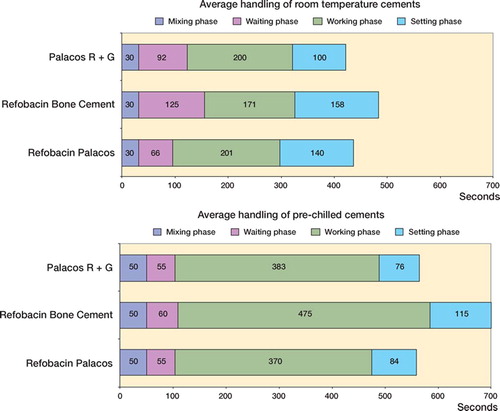
Handling characteristics (, and )
Room temperature, hand-mixed: Using the 2-tailed Student t-test, RBC had a longer waiting phase (p < 0.001), a shorter working phase (p = 0.03), and took a longer total time to set (p < 0.001) than room-temperature RPR. PRG also had a longer waiting phase (p = 0.004) than RPR, but a similar working phase and final setting time (p = 0.94 and p = 0.19, respectively).
Table 1. Handling characteristics of the three kinds of cement
Pre-chilled, vacuum-mixed:RBC had a longer working period (p = 0.02) and final setting time (p = 0.003) than RPR. PRG had a similar length of working phase and a similar setting time (p = 0.47 and p = 0.85)
ISO 5833 4-point bending strength ()
Table 2. Mechanical properties of the 3 cements
ISO 5833 4-point bending strength ()
The standard deviation of the pre-chilled RPR samples was larger than the other cements, so 4 additional samples were tested. There was no statistically significant difference in 4-point bending strength between the cements, either when prechilled or stored at room temperature.
ISO 5833 elastic modulus ()
There was no statistically significant difference in elastic modulus between the cements whether they had been pre-chilled or stored at room temperature.
ISO 5833 compression ()
One specimen each from the PRG and RBC samples had to be discarded because of macroscopic flaws. Both PRG and RBC were stronger than RPR when stored at room temperature and hand-mixed (p < 0.001), but they were weaker than RPR when they were pre-chilled and vacuum-mixed (p < 0.001).
German standard DIN 53435 bending strength ()
One specimen of PRG was discarded because of a macroscopic flaw. There were no statistically significant differences in the German standard bending strength between the cements stored and prepared at room temperature.
German standard DIN 53435 impact strength ()
2 of the RBC specimens were discarded because of macroscopic flaws. PRG was found to have a higher impact strength than RPR (p = 0.02).
Tensile strength ()
3 of the RBC samples, 3 of the PRG samples, and 1 of the RPR samples were unsuitable for testing. There were no statistically significant differences in tensile strength between the cements stored and prepared at room temperature.
Shrinkage ()
RBC shrank in volume by 2.67% after polymerization, which was similar to the result for RPR (p = 0.5). On the other hand, PRG only shrank by 2.25%—which was significantly less than the shrinkage of RPR (p = 0.03).
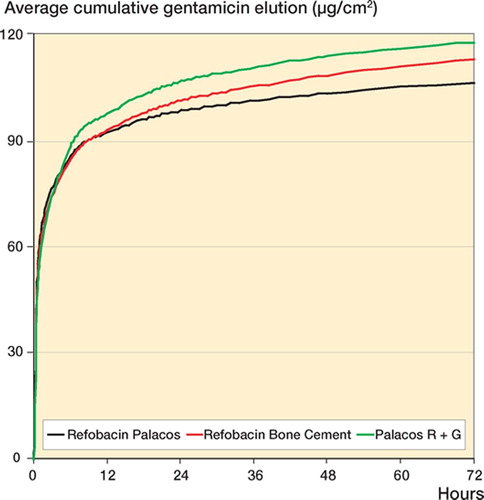
Gentamicin elution ( and )
PRG and RBC both lost more gentamicin by elution than their predecessor, but the differences were not statistically significant at 1 h (p = 0.4 and p = 0.5) or at 72 h (p = 0.2 and p = 0.2).
Table 3. Cement shrinkage, elution and viscosity
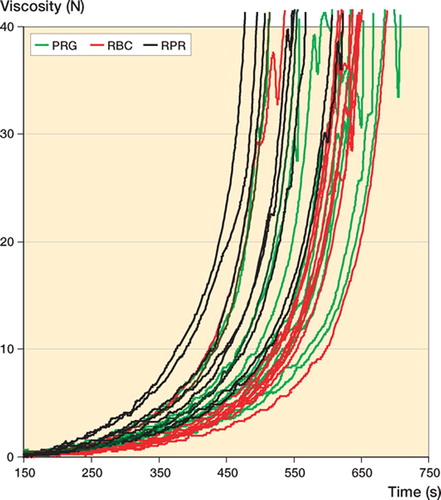
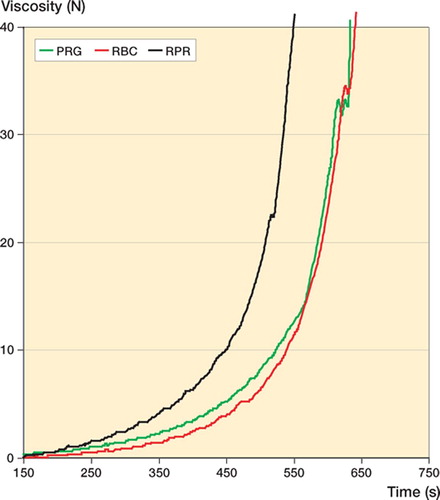
Viscosity ( and , and )
The time at which 5 N was required to move the plunger approximated the end of the working phase, and the time at which 40 N was required approximated the end of the setting phase. Thus, these two time points for PRG and RBC were extrapolated from the data and compared to that for RPR using Student's t-test.
To reach 5 N and 40 N, both RBC (p < 0.001 and p < 0.001) and PRG (p = 0.005 and p = 0.004) took longer than RPR. RBC yielded the lowest intrabatch variability (). The median viscosity curves of PRG and RBC in the batches tested were similar but both were obviously different to that of RPR, which had a higher viscosity ().
Discussion
In clinical practice, variation in cement handling and viscosity are well known phenomena but despite this, relatively little has been published on this topic. Ideally, to allow for optimal operative technique, the behavior of cement should beconsistent and predictable. Both intrinsic factors (cement composition) and extrinsic factors will affect the curing process. Examples of extrinsic factors include the temperature at which the two components have been stored, ambient temperature and humidity, the mixing method and technique, or whether the dough is vigorously manipulated during curing. These factors were controlled for in our study and can therefore be disregarded.
Intrinsic factors that tend not to vary between batches but are responsible for differences seen between different cement brands are the type of polymer used and its degree of hydrophilia, the proportion of polymer powder to monomer liquid, andthe method of sterilization. One important factor that determines the handling characteristics of the cement is the rate at which the polymer is dissolved by the monomer. The rate of polymerization only becomes more important during the latter stages of curing. Higher proportions of small hydrophilic polymer molecules and higher polymer-to-monomer ratios increase the dissolution rate and have been shown to achieve a higher initial cement viscosity (Farrar and Rose Citation2001, Milner Citation2004).
Intrinsic factors that can vary slightly between batches of the same cement and alter the handling characteristics are the sizes (and therefore relative surface areas) of polymer beads, and also the concentrations of BPO initiator and DMPT accelerator(Milner Citation2004). Because the chemical composition of the polymer and co-polymer and their relative proportions can vary from batch to batch, the end product reaching the customer will depend on the in-house quality control process of the cement producingcompany.
Every manufacturer is obliged to present a graphical representation of the effect of temperature on the length of the handling phases during cement curing, but these can be misleading to the surgeon. The current requirements do not include exact specifications for the viscosity of the material. The handling curve is both subjective to measure and can be significantly influenced by the brand of latex glove used during determination of the waiting time (He et al. Citation2003). Hence, there is no objective way of describing the viscoelastic properties of cement in a useful way. However, the handling curves obtained in this study have still provided important information—in showing thatin the batches tested, pre-chilled RBC took the longest time to cure. Interestingly, the viscosity data showed a shorter curing time for RPR.
All 3 cements tested showed similar mechanical properties that exceeded international requirements. Although RBC did fall short of the traditional 3 kJ/m2limit that was previously specified for RPR by Heraeus Kulzer, it is important to note that all 3 cements tested yielded much lower impact strengths than previously reported (approx. 4.7–4.8 kJ/m2) (Kuehn et al. Citation2005). The ISO 5833 and DIN 53435 standards of mechanical strength are only conditionally useful, as they cannot predict the long-term clinical success of the cement. ASTM fatigue testing, RSA subsidence data, and arthroplasty registers will give a clearer picture as to what cements are associated with the best prosthetic survivorship. Although we found a statistically significant difference in the compression testing, the results are unlikely to be clinically important as cements tend to fail initially during tensile stresses. Fatigue data cannot be provided in this study, as these are much more time-consum-ing, but further tests are being carried out.
All 3 cements appear to have very similar gentamicin release rates. The elution occurred in a typical biphasic manner with most of the antibiotic being released in the first hour, and then continuing to elute at low levels for months to years. The amount of gentamicin eluted from PMMA is proportional to its initial concentration in the powder (He et al. Citation2002), surface area, surface roughness, and its porosity (van de Belt et al. Citation2000).
We tested our samples for shrinkage after they had been hand-mixed and our results are comparable to those of previous studies (Muller et al. Citation2002). The statistical differences found in our study must be considered irrelevant, as much higher shrinkage values are commonly found. If they had been vacuum-mixed, thus reducing the porosity of the cement, we would have expected up to 7% shrinkage (Kwong and Power Citation2006).
The fact that we used 2 separate batches of PRG for determination of handling characteristics and measurement of viscosity is an important weakness of our study protocol. However, the differences between the two “successor “ cements seen in handling, but not in viscosity, has raised an interesting question of inter-batch variability, which has been addressed in a subsequent study (Dall et al. Citation2007).
We used the Gelnorm-Med rheometer to qualitatively compare the cements in relation to time and not to provide quantative descriptions of the complex viscoelastic properties of the two cements, where an oscillating parallel cone plate at a low shear rate would be considered the gold standard (Farrar and Rose Citation2001, Kühn and Gopp 2001). Our method was simple, with the added benefit that the sample could be tested in the mixing device, thus reducing artefact. However, from the irregular shape of some of the viscosity curves, its reproducibility must be questioned. Unfortunately no validation of this rheometer is available. This is certainly another important criticism.
We believe that the differences seen between the cements in this study are largely a result of the variation in polymer/co-polymer particle sizes and ratios, which depends both on the production process and the experience of the scientist releasing the final product. This hypothesis is supported by the findings of Tidehem and Hassander Citation(2005). All cements undergo “fine tuning” before being released to the customer, by adjusting the ratio between the polymer and co-polymer. Minor alterations in the composition of subsequent batches will have been made to optimize their handling characteristics. Small differences in viscosity curves were also found between RPR and RBC using an oscillating rheometer in a study commissioned by Biomet from Lund University, Sweden (Tidehem and Hassander Citation2005). These authors also found that RBC seemed to have a similar chemical composition and particle size to RPR, but different particle shape and a wider range of molecular weights.
The main and clinically important findings of our study are that RBC and PRG appear to have different handling/viscosity curves compared to RPR—at least in the batches we tested, which were chosen and provided by each manufacturer. Thus, these results have should be interpreted with caution, as this study did and could not take into account any inter-batch variability (Dall et al. Citation2007).
Surgeons must be aware that a change to another cement may be associated with different handling characteristics; thus, intraoperative timing may have to be adjusted. A new learning curve must also be anticipated. It is impossible to predict whether these findings will have implications for the long-term outcome. This seems unlikely, considering the almost identical chemical composition of all three cements. RSA studies will provide further insight in the creep behavior, but ultimately data on long-term outcome will be needed. Ideally, in the national arthroplasty registries the type of “new” cement used should be noted.
We are grateful to Catriona Graham, of the WTCRF Epidemiology and Statistics Core for statistical advice.
The study was conducted using Heraeus Kulzer's laboratory equipment and facilities, Wertheim, Germany. All tests were performed by the authors. All companies provided materials at no charge. The companies did not participate in the design of the study, in the evaluation of the results, or in the writing of the manuscript. No funding or remuneration was received by the authors for this study.
Contributions of authors
GD: study design, laboratory work, manuscript preparation. PS: laboratory work, logistics. SB: study design, manuscript preparation.
References
- Dall G F, Simpson P M.S, Mackenzie S P, Breusch S J. Inter- and intra-batch variability in the handling characteristics and viscosity of commonly used antibiotic-loaded bone cements. Acta Orthop 2007; 78: 412–20
- Engesaeter B, Lie A, Espehaug B, Furnes O, Vollset E, Havelin I. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003; 74(6)644–51
- Espehaug B, Engesaeter L B, Vollset S E, Havelin L I, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J.Bone Joint Surg (Br) 1997; 79(4)590–5
- Farrar D F, Rose J. Rheological properties of PMMA bone cements during curing. Biomaterials 2001; 22(22)3005–13
- He Y, Trotignon P, Loty B, Tcharkhtchi A, Verdu J. Effect of antibiotics on the properties of poly(methylmethacrylate)-based bone cement. J Biomed Mater Res 2002; 63(6)800–6
- He S, Scott C, DeLuise M, Edwards B, Higham P. Effect of choice of surgical gloves on dough time measurements of acrylic bone cement. Biomaterials 2003; 24(2)235–7
- International Standards Organisation. ISO 5833: Implants for Surgery-Acrylic Resin Cements. 3rd. International Standards Organisation, BernSwitzerland 2002
- Kuehn K D. Bone cements. Up to date comparison of physical and chemical properties of commercial materials. 1st. Springer-Verlag, HeidelbergGermany 2000
- Kuehn K D, Gopp U. A new method for the determination of the flow characteristics of bone cements ESB Conference. London 2001; 49, Proceedings
- Kuehn K D, Ege W, Gopp U. Acrylic bone cements: composition and properties. Orthop Clin North Am 2005; 36(1)177–28
- Kwong F N, Power R A. A comparison of the shrinkage of commercial bone cements when mixed under vacuum. J Bone Joint Surg (Br) 2006; 88(1)120–2
- Milner R. The development of theoretical relationships between some handling parameters (setting time and setting temperature), composition (relative amounts of initiator and activator) and ambient temperature for acrylic bone cement. J Biomed Mater Res B Appl Biomater 2004; 68(2)180–5
- Muller S D, Green S M, McCaskie A W. The dynamic volume changes of polymerising polymethyl methacrylate bone cement. Acta Orthop Scand 2002; 73(6)684–7
- Tidehem J, Hassander H. Comparison of Refobacin-Palacos R and Refobacin Bone Cement R. Dec, 2005, <http://www.bonecement.com/index.php?id=15108&no_cache=1>
- van de Belt H, Neut D, Uges D R, Schenk W, vanHorn J R, van der Mei H C. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000; 21(19)1981–7