Abstract
Background Several factors can affect the viscosity of a cement and therefore its handling characteristics. We performed an in vitro study to ascertain whether anecdotal observations of differences in handling between batches of the same brand of cement actually existed.
Methods 3 batches of Simplex P Tobramycin (SPT), Refobacin Bone Cement (RBC), SmartSet GHV (SSG) and Palacos R+G (PRG) were tested. 6 replicates of each batch were vacuum-mixed and their viscosity in relation to time was measured under laboratory conditions using a rheometer. We examined the handling characteristsics of 6 replicates of each batch after they were hand-mixed under theater conditions.
Results Inter- and intra-batch variability was seen in the viscosity of all brands of cement tested. Interbatch calculations were influenced by high intra-batch variability in viscosity. The viscosity of RBC cement was similar to that of SSG, but different to that of PRG (p = 0.01 at 5 N and p = 0.009 at 40 N).
Interpretation Our results suggest that in clinical practice, extrinsic factors such as preparation conditions and methods probably play a more important role than the intrinsic variability of cements. However, variability in handling and viscosity will exist in all brands of cement prepared under theater conditions and the surgeon must be aware of why they may act differently.
Antibiotic-loaded acrylic cements (ALAC) are produced by a range of companies and their different handling and mechanical characteristics have been well documented (Kuehn et al. Citation2005). In order to achieve a properly interdigitated cement mantle, it is essential for the surgeon to know the working time of the cement so as to correctly pressurize the cement and insert the components when at optimal viscosity (Breusch et al. Citation2002). Extrinsic factors such as pre-chilling (Jiranek Citation2005), mixing method (He et al. 2003, Mau et al. Citation2004), ambient temperature and humidity (Eyerer and Jin Citation1986, Davies et al. Citation1989, Askew et al. Citation1990, Dunne and Orr Citation2002) must be taken into consideration.
From clinical experience, but also from previous in vitro studies with ALAC, the authors have noticed considerable differences in handling between samples of the same brand of cement. Interestingly, our literature search showed there has been very little research addressing this subject. Thus, in this study we examined 4 commonly used antibiotic-loaded bone cements (Herberts et al. Citation2003) and assessed whether there was any inter- or intra-batch variability in their handling characteristics and their viscosity as they polymerize.
Materials
3 different batches of Simplex P Tobramycin (SPT) (LOT 849CL, 964JL, 913FL), Refobacin Bone Cement R (RBC) (LOT 547AO, 546AO, 546BO), SmartSet GHV (SSG) (LOT 2017644, 2017643, 2017957), and 3 batches of Palacos R+G (PRG) (6382, 6381, 6380) were obtained from manufacturers after informing them of the study protocol. SPT is a methylmethacrylate (MMA)-styrene copolymer cement, making it more voluminous and hydrophobic. It contains Tobramycin and is sterilized using gamma radiation, which is known to reduce the molecular weight of the polymer. The other 3 cements are similar in composition. They are MMA-MA copolymers with added Gentamicin and are sterilized using ethylene oxide. SSG contains twice as much Gentamicin than RBC and PRG.
Methods
Handling
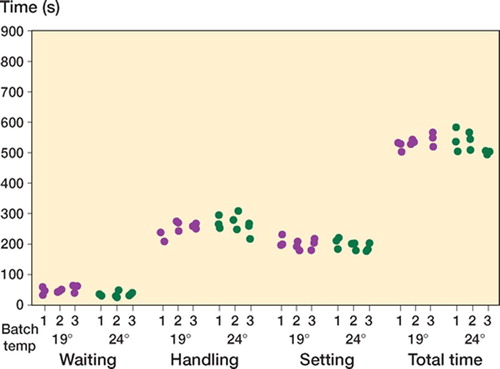
6 samples of each batch were tested for their handing characteristics at different temperatures.
The handling samples were made according to the manufacturer's instructions and hand mixed with a polymeric bowl and spatula. They were tested in a climate-controlled theater with a humidity of 50 ± 10% and a temperature of either 24 ± 2°C or 19 ± 2°C after being equilibrated for 24 h. For SPT, only 3 mixes per batch were available for handling and were tested at 19°C only. Conditions were stable throughout each individual session, in which 3 replicates of each batch of cement were tested. We chose this setup to resemble a situation as close to clinical routine as possible.
The tests were performed as defined by ISO and ASTM standards by one surgeon who was familiar with their use. The surgeon was blinded to the batch being tested and time elapsed to minimize both inter- and intra-observer bias.
The mixing phase was deemed to have started as soon as all constituents had been combined, and it lasted 30 sec. The period between mixing and the point the cement can be applied is the waiting phase. The end of the waiting phase was recorded when a Biogel powder-free latex surgical gloved finger (Regent Medical Co., Manchester, UK) could be inserted 5 mm into the cement for less than a second and not adhere to the mixture.
The working phase immediately followed waiting phase and ended when upon folding, the cement no longer adhered to itself. The end of the working time until the moment at which the cement had become completely hardened was recorded as the setting phase.
Viscosity
6 samples of each batch were tested to examine the viscosity of the cement as it cured. The viscosity tests were performed after samples were mixed according to the manufacturer's instructions under a vacuum of –200 millibars, using the Optivac System in a climate-controlled laboratory with a humidity of 50 ± 5% and a temperature of 23 ± 1°C. We chose laboratory conditions for this part of the study to minimize extrinsic influences. The SPT was stored at 23 ± 1°C before testing but the 3 high-viscosity cements were pre-chilled to 4 ± 2°C for 24 h to create comparable conditions. Pre-chill-ing is recommended when vacuum mixing Palacos (Lidgren et al. Citation1987), but not for SSG (according to its manufacturer). When the monomer was added after the powder (as is recommended by SPT and SSG), care must be taken not to suck some of the monomer into the suction tubing before it has had a chance to mix with the powder. We resolved this problem by briefly mixing the constituents before applying the vacuum.
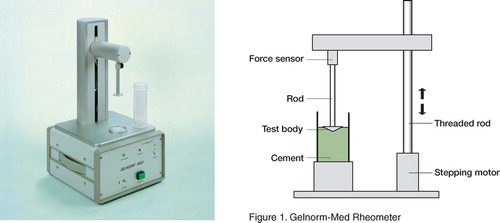
The samples were tested with the Gelnorm-Med device (Gel Instrumente AG, Thalwil, Switzerland; ) 150 sec after the addition of all the powder to the monomer. The cone-shaped measuring tool with a diameter of 28 mm and surface area of 6.1 cm2was dipped into the cement at a speed of 0.03 mm/sec. The force necessary to immerse the tool was recorded. The procedure was continued until the limiting force of 50 N. Two measurement points (5 and 40 N) were chosen for data analysis, as these had previously been found to correlate with the end of the working phase and the setting phase (Kuehn and Gopp Citation2001).
Statistics
The time at which 5 N is required to move plunger approximates the end of the working phase and 40 N the end of the setting phase (Kuehn and Gopp Citation2001). Thus, these two time points were extrapolated from the data and a one-way ANOVA was performed to determine whether there was a difference in the viscosities between different batches of a particular cement at these points. The level of intra-batch variability precluded the use of statistical tests in the analysis of the handling curves, and the results are presented as a graph.
Table 1. Handling phases
Results
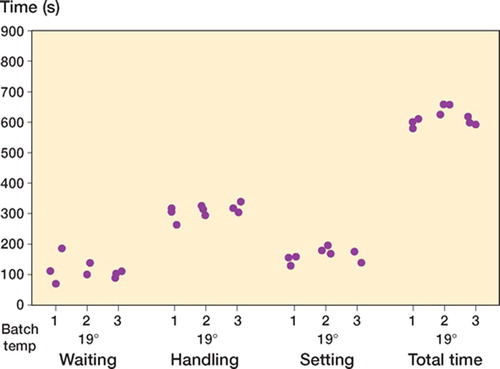
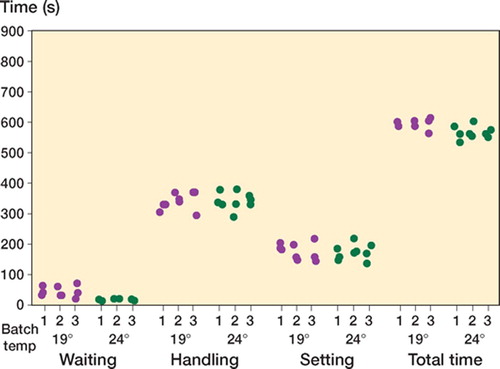
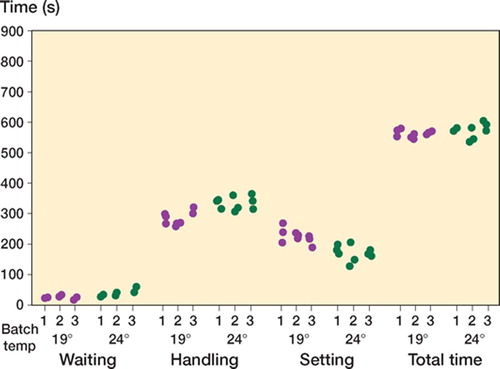
Handling ()
The combined handling and setting phases were similar in length at both ambient temperatures (). For all cements there was intrabatch handling variability, but no obvious interbatch handling variability was observed.
Viscosity
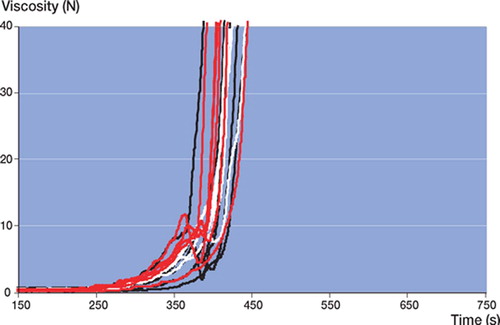
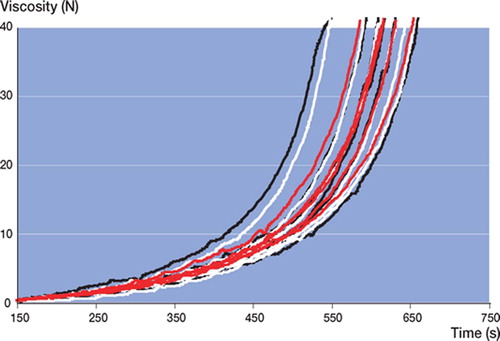
2 specimens of RBC and one specimen each of both SSG and SPT were discarded because of large air pockets and their extreme effect on the measurement. These were excluded from further analysis.
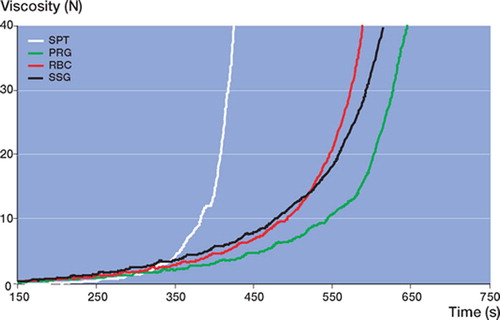
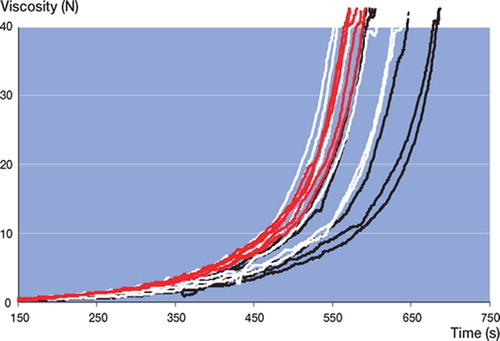
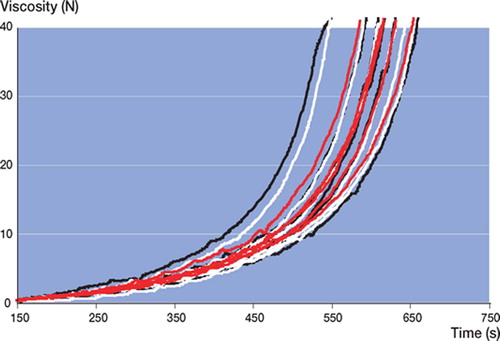
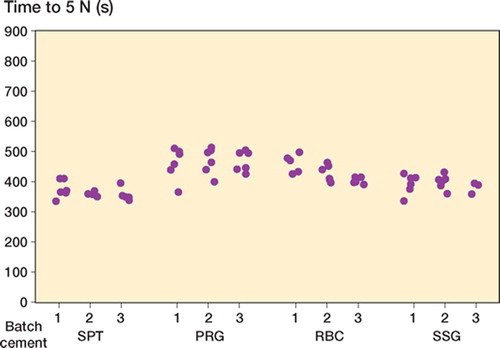
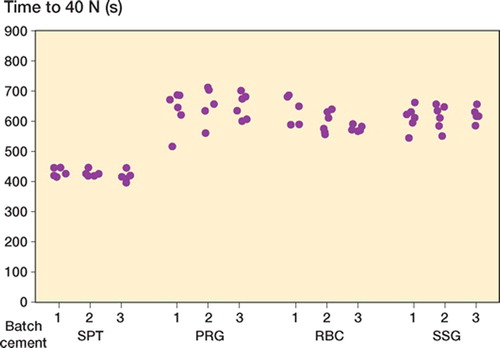
Simplex yielded the lowest initial viscosity and shortest curing time ( and ). All other cements showed comparable median viscosity curves in terms of shape (). Calculated median viscosity of RBC was similar to that of SSG, but was significantly different from that of PRG (p = 0.01 at 5 N and p = 0.009 at 40 N using Student's t-test, not corrected for multiple comparisons).
In all batches of all cement brands tested, large intra-batch variability (wide confidence intervals) in viscosity (not to be confused with chemical composition) was found (, and ). Batch 3 of RBC showed the lowest intra-batchvariability (, , and ) and PRG batch 1 and 2 showed the widest confidence intervals (, , and ). Inter-batch variability was calculated, but did not show statistical significance (due to the large intra-batch variability in viscosity),except for RBC. There was a significant difference in the means of the 3 RBC batches at 5 N (p = 0.009) and 40 N (p = 0.03) ().
Table 2. Cement viscosity
Discussion
Cement handling and viscosity are highly dependent on both intrinsic factors, i.e. related to the composition of the cement itself, and extrinsic factors.
Intrinsic variations
Viscosity and therefore the handling characteristics are initially determined by the speed at which the powder dissolves and swells in the monomer (Farrar and Rose Citation2001). Variables that affect this diffusion process are: how hydrophilic the co-polymermolecules are, the relative surface area of the polymer beads, the powder/liquid ratio, the concentrations of activators and initiators, and the way in which the cement is sterilized.
Simplex P is an MMA-Styrene co-polymer and the other 3 cements are MMA-MA co-polymers. Styrene cements are more hydrophobic than MMA-MA, and so take longer to dissolve in the monomer—resulting in a lower initial viscosity, as also found here. An increased proportion of the small hydrophilic MA molecules in the MMA-MA copolymer results in faster dissolution and therefore higher initial viscosity.
The handling of a cement can be altered if necessary by changing the proportion of large and smallsized co-polymer molecules in the powder component (Pascual et al. Citation1996, Lewis and Carroll Citation2002). Small beads have a large relative surface area and are dissolved in the monomer more rapidly, thus increasing the cure rate. Waiting time is substantially independent of higher DmpT (accelerator in liquid monomer) or BPO (initiator in powder) concentrations, but both will shorten final setting times by speeding up polymerization (Milner Citation2004). SPT powder is sterilized using γ-radiation, which is known to reduce the molecular weight by around 50%. The other 3 cements are sterilized using ethylene oxide, which does not alter their molecular weight.
The manufacturing process that bone cement undergoes before a surgeon uses it in theater— and in particular, the ways in which its handling characteristics can be manipulated—is important to understand. Approximately 3 tons of polymer, from one of a low number of PMMA manufacturers worldwide, is sufficient to produce around 10–20 batches. Each batch is unique, and can be regarded as only having minimal variations after it is divided into 8,000 mixes of 40 g that are packaged and sterilized ready for use. There are small variations in the polymer beads produced, and so all batches of cement are tested in-house by quality control to try to ensure product standardization before mass production. It is common practice that prior to release, all cements are fine-tuned to try to reduce inter-batch variability.
Extrinsic factors
Examples of extrinsic factors that alter the handling of cement include the temperature at which the two components are stored, ambient temperature and humidity during preparation, the mixing method chosen, the mixing technique used, how vigorously the dough is manipulated during curing, and how handling itself is measured.
Pre-chilling of high-viscosity cements has become common practice as it reduces peak temperatures during polymerization (Iesaka et al. Citation2004), allows easier application via a syringe, prolongs the working phase, and minimizes variations in handling due to different ambient temperatures during preparation (Jiranek Citation2005). The sensitivity of the final setting time to temperature has been found to be more dependent on swelling and dissolution than on the polymerization process (Milner Citation2004). High relative humidity shortens the working phase; thus, surgeons must expect the cement to cure more quickly in warm, humid conditions. Surgeons can choose to prepare the cement by hand mixing or under a vacuum using one of the many commercially available devices (Mau et al. Citation2004).
He et al. (Citation2003b) found that reducing O2 concentration (as occurs by applying a vacuum during mixing) does not affect the waiting time but does reduce the setting time by 10%. Monomer that is sucked up the vacuum tubing, powder left in the recesses of some mixing systems (Dunne and Orr Citation2002), or spillage of either constituent before mixing will all alter the handing of the cement produced. The more kinetic energy transferred from the person mixing or handling the cement will shorten the setting time. A constant mixing time and rate must be used if reproducible results are to be expected.
As well as all of the aforementioned factors that can alter the viscosity of a cement, measurement of the handling curve is notoriously inaccurate for a number of reasons. For example, the brand of the powder-free latex glove used has been noted to have a significant effect on the waiting time (He et al. Citation2003a). Also, the observer may press his or her finger a variable distance into the dough when measuring the doughing time, a subjective amount of force is applied to the dough when it is folded together to measure the end of the working phase, and the observer may handle the dough more vigorously and thus speed up its curing rate. We therefore used one blinded observer wearing the same brand of glove as routinely used to perform arthroplasty in theater for all of the handling tests. This person tested each sample every 10 seconds in order to minimize potential observer error.
The theater that the handling tests were performed in over several sessions was air-conditioned, but was subject to a greater range of temperature and humidity than laboratory conditions would have provided. Thus, when analyzing the data, we felt that comparisons could only be made between the 3 batches of the particular cement being tested at one temperature during one of these sessions. Our results suggest that there is as much intra-batch as inter-batch variability in the handling curves. In contrast to what might be expected, in general the batches prepared at the higher temperature did not cure more rapidly. This can only be explained by the variability in the storage and theater conditions, although the same rooms were used for all samples. As mentioned previously, we chose conditions that were as close to the clinical routine as possible. This can be both considered a weakness and a strength of this study, as the findings have highlighted the importance of extrinsic factors. This would not have been so clear if laboratory conditions had been implemented for the entire study protocol.
We used the Gelnorm-Med rheometer (Gel Instrumente AG, Thalwil, Switzerland) to qualitatively compare the cements in relation to time—and not to provide quantative descriptions of the complex viscoelastic properties of the two cements, where an oscillating parallel cone plate at a low shear rate would be considered the gold standard (Farrar and Rose Citation2001). Our method was simple and had the added benefit that the sample could be tested in the mixing device, thus reducing artefact, but its reproducibility must be questioned considering the irregular shape of some of the viscosity curves, which was also observed in our preceding study (Dall et al. Citation2007). Unfortunately, no other publication on validation of this rheometer is available (Kuehn and Gopp Citation2001).
All of the cement manufacturers had seen the study protocol before supplying the cement samples to be tested. With the exception of SPT, the batches supplied were concurrent—and so were probably as similar in composition as the manufacturers could make them. A wider inter-batch variation might have been seen if the batches had been produced months apart. This would have meant that the manufacturers would have been supplied with slightly different PMMA bead sizes, so they would have had to modify the proportions of the other constituents to try to maintain a consistent handling curve whilst maintaining minimal ISO 5833 standards. This must be considered the main flaw of this study, as it probably reflects the best case scenario for the manufacturers. Despite this, our results showed significant variability in viscosity between the three batches of RBC. However, this was most likely the effect of the reduced intrabatch variability in batch 3. We might have found similar results in the other cement brands if there had been less intra-batch variability. We do not therefore consider this finding—although statistically significant—to be relevant to clinical practice.
The term intra-batch variation reflects all extrinsic, intrinsic, and measurement-related influences. As there is only negligible intrinsic variation of the product composition within each batch, the intrabatch variation seen in our study is most likely due to alterations in the extrinsic factors discussed above, including any possible measurement errors. Intra-batch variability must not be interpreted as poor cement composition and production quality. The inter-batch batch variation is affected by the aforementioned extrinsic factors, but is also influenced by the subtle intrinsic differences between (but not within) different batches of the same cement.
Although not the main objective of our study, we found shorter curing times for RBC compared to PRG. This finding contrasts with results from our previous study using older batches (Dall et al. Citation2007), where both RBC and PRG yielded similar viscosity curves but had longer curing times when compared to the predecessor Refobacin Palacos R (RPR). It would appear that the more recent batches produced resemble the viscoelastic properties of RPR more closely.
Our findings are still somewhat surprising, as we found far less inter-batch variability than expected. This suggests that extrinsic factors probably play a more important role in clinical practice, as evidenced by the relatively high intra-batch variability. However, inter- and intra-batch variability will exist in all brands of cements prepared under theater conditions and the surgeon must be aware of why they may act differently.
Further research into the viscoelastic properties of bone cement is required to allow manufacturers to correlate these rheological findings with the optimal moment for the surgeon to apply the cement, pressurize it, and insert the prosthesis—in order for the best cement mantle to be produced.
Thanks to Catriona Graham of the WTCRF Epidemiology and Statistics Core for statistical advice.
The study was conducted using Heraeus Kulzer's laboratory equipment and facilities, Wertheim, Germany. All tests were performed by the authors. All companies provided materials at no charge and they did not participate in the design of the study, in the evaluation of the results, or in the writing of the manuscript. No funding or remuneration was received by the authors for this study.
Contributions of authors
GD: study design, laboratory work, manuscript preparation. PS: laboratory work, logistics. SM: laboratory work. SB: study design, manuscript preparation.
- Askew M J, Kufel M F, Fleissner P R, Jr., Gradisar I A, Jr., Salstrom S J, Tan J S. Effect of vacuum mixing on the mechanical properties of antibiotic-impregnated polymethylmethacrylate bone cement. J Biomed Mater Res 1990; 24(5)573–80
- ASTM International. F451-99ae1 Standard specification for acrylic bone cement. ASTM 2006; 13.01(13.01)1–2
- Breusch S, Heisel C, Muller J, Borchers T, Mau H. Influence of cement viscosity on cement interdigitation and venous fat content under in vivo conditions: a bilateral study of 13 sheep. Acta Orthop Scand 2002; 73(4)409–15
- Dall G F, Simpson P M.S, Breusch S J. In vitro comparison of Refobacin-Palacos R with Refobacin Bone Cement and Palacos R+G. Acta Orthop 2007; 78: 404–11
- Davies J P, O'Connor D O, Burke D W, Harris W H. Influence of antibiotic impregnation on the fatigue life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res 1989; 23(4)379–97
- Dunne N J, Orr J F. Curing characteristics of acrylic bone cement. J Mater Sci Mater Med 2002; 13(1)17–22
- Eyerer P, Jin R. Influence of mixing technique on some properties of PMMA bone cement. J Biomed Mater Res 1986; 20(8)1057–94
- Farrar D F, Rose J. Rheological properties of PMMA bone cements during curing. Biomaterials 2001; 22(22)3005–13
- He S, Scott C, DeLuise M, Edwards B, Higham P. Effect of choice of surgical gloves on dough time measurements of acrylic bone cement. Biomaterials 2003a; 24(2)235–7
- He S, Scott C, Higham P. Mixing of acrylic bone cement: effect of oxygen on setting properties. Biomaterials 2003b; 24(27)5045–8
- Herberts P, Malchau H, Garellick G. Swedish National Hip Arthroplasty Register – Annual Report. 2003
- Iesaka K, Jaffe W L, Kummer F J. Effects of the initial temperature of acrylic bone cement liquid monomer on the properties of the stem-cement interface and cement polymerization. J Biomed Mater Res B Appl Biomater 2004; 68(2)186–90
- International Standards Organisation. ISO 5833: Implants for surgery-acrylic resin cements3rd. International Standards Organisation, BernSwitzerland 2002
- Jiranek W. Thermal manipulation of bone cement. Orthopedics (8 Suppl) 2005; 28: 863–6
- Kuehn K D, Gopp U. A new method for the determination of the flow characteristics of bone cements. ESB Conference London. 2001; 49, Proceedings
- Kuehn K D, Ege W, Gopp U. Acrylic bone cements: composition and properties. Orthop Clin North Am 2005; 36(1)17–28
- Lewis G, Carroll M. Rheological properties of acrylic bone cement during curing and the role of the size of the powder particles. J Biomed Mater Res 2002; 63(2)191–9
- Lidgren L, Bodelind B, Moller J. Bone cement improved by vacuum mixing and chilling. Acta Orthop Scand 1987; 58(1)27–32
- Mau H, Schelling K, Heisel C, Wang J S, Breusch S J. Comparison of various vacuum mixing systems and bone cements as regards reliability, porosity and bending strength. Acta Orthop Scand 2004; 75(2)160–72
- Milner R. The development of theoretical relationships between some handling parameters (setting time and setting temperature), composition (relative amounts of initiator and activator) and ambient temperature for acrylic bone cement. J Biomed Mater Res B Appl Biomater 2004; 68(2)180–5
- Pascual B, Vazquez B, Gurruchaga M, Goni I, Ginebra M P, Gil F J, Planell J A, Levenfeld B, SanRoman J. New aspects of the effect of size and size distribution on the setting parameters and mechanical properties of acrylic bone cements. Biomaterials 1996; 17(5)509–16