Abstract
Introduction Treatment of osteoporosis is becoming more effective, but methods to identify patients who are most suitable for investigation and treatment are still being debated. Should any type of fracture have higher priority for investigation of osteoporosis than any other? Is the number of previous fractures useful information?
Material and methods We investigated 303 consecutive women patients between 55 and 75 years of age who had a newly diagnosed low-energy fracture. They answered a questionnaire on previous fractures which also dealt with risk factors. Bone mineral density (BMD) was measured at the hip, lumbar spine, and forearm.
Results The distribution of fracture location was: distal forearm 56%, proximal humerus 12%, vertebra 18%, and hip 13%, all with similar age. Half of the subjects had had at least one previous fracture before the index fracture, 19% had had two previous fractures, and 6% had had three or more previous fractures. Patients with vertebral or hip fracture had lower BMD and had had more previous fractures than patients with forearm or humerus fractures. There was an inverse correlation between number of fractures and BMD. Osteoporosis was present in one-third of patients with forearm fracture, in one-half of those with hip or humerus fracture, and in two-thirds of those with vertebral fracture.
Interpretation Vertebral fractures were the strongest marker of low BMD and forearm fractures the weakest. The number of previous fractures is helpful information for finding the most osteoporotic patient in terms of severity. Investigation of osteoporosis therefore seems warranted in every woman between the ages of 55 and 75 with a recent low-energy fracture, with highest priority being given to those with vertebral, hip, or multiple fractures.
Methods of pharmacological treatment of osteoporosis are becoming more effective. The optimal cost-benefit ratio is achieved when treatment is directed to the group with the highest fracture risk (Borgstrom et al. Citation2006). We therefore need valid instruments to identify this group in clinical routine. There is a continuous non-linear relationship between bone mineral density (BMD) and fracture risk. Measurement of bone mineral density is regarded as the single best predictor of fractures, but who should have a bone scan? Currently, general screening for osteoporosis by bone mineral measurement is not recommended, but simple risk markers should be used for selection of patients for bone scan (Kanis Citation1994). One strategy recommended by several recent expert committees is to use the occurrence of a recent low energy fracture in a postmenopausal woman as a major indication for investigation of osteoporosis (Fogelman Citation1999). Prior fragility fractures are strong determinants of subsequent fractures, which suggests that postmenopausal women presenting with a low-energy fracture may be an important target for finding early cases of osteoporosis (Karlsson et al. Citation1993, Honkanen et al. Citation1997, Huopio et al. Citation2000, Khan et al. Citation2001). This conclusion was confirmed in a meta-analysis of 11 prospective studies (Kanis et al. Citation2004), but several questions remain.
Is any type of fracture a stronger indication than any other? What significance has the number of previous fractures?
In an attempt to answer these questions, we compared 303 postmenopausal women who had sustained various types of recent low-energy fracture with 209 healthy controls. We also studied the predictive value (for osteoporosis) of numbers of prevalent fractures and of body mass index.
Subjects and methods
Study group
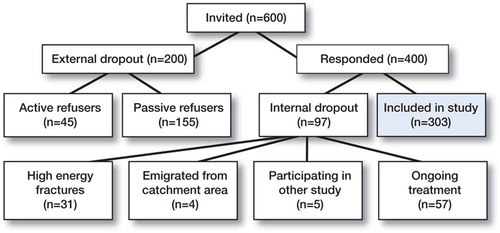
600 consecutive women patients 55–75 years of age who had recently sustained a low-energy fracture (in this paper called the index fracture) in the distal radius, proximal humerus, spine, or hip were invited to take part in the study. They were identified by the radiographic register of investigations at the Linköping University Hospital, or by the records at the emergency unit of Ryhov Hospital in the counties of Östergötland and Jönköping, Sweden. The inclusion period was January 1998 to February 2000. 303 women participated and were examined mean 3 months (6–170 days) after fracture diagnosis. For further details see , , and the paper by Hallberg et al. (Citation2004).
Table 1. Basic characteristics of the total fracture (F) and reference (R) groups
Reference (control) group
An age-matched reference population was recruited from the general population register by a random sampling procedure. Subjects with a history of any previous fracture were excluded from the reference group. 209 women (55–75 years old) were included in the reference group (Lofman et al. Citation1997) ().
Bone mineral density, anthropometry, and questionnaire
BMD of the hip, lumbar spine, and forearm was measured by dual-energy X-ray absorptiometry (DXA) (Hologic QDR 4500A; Hologic Inc., Bedford, MA).
As a reference for BMD in the hip, we used the Third National Health and Nutrition Examination Survey (NHANES III) (Looker et al. Citation1998) and for BMD in the spine, we used reference data published by Favus (Citation1993). The decision to use the machinespecific databases was made to permit comparision between studies. BMD in Swedish women was compared to different machine-specific reference values in two previous studies (Lofman et al. Citation1997, Citation2000). In general, the BMD values in Swedish women were found to be somewhat lower in both the hip and spine—with the exception of premenopausal spine BMD, which were no different.
A patient was classified as being osteoporotic if the T-score for the lumbar spine or the whole hip (total hip) was below –2.5, and osteopenic if the lowest of these values was between –1 and –2.5. Definitions of osteoporosis and osteopenia at the respective sites were made from site-specific cutoff values for T-score according to the machine-spe-cific reference databases. Body height and weight were measured in indoor clothing without shoes, and body mass index (BMI) was calculated. Spine radiographs were performed at diagnosis in vertebral fracture patients, but not in the other patients. Hence, the true prevalence of vertebral compressions is unknown. A questionnaire that focused on index fracture, previous fractures, diseases, drugs, and other risk factors for osteoporosis and fractures was answered by the patient before visiting the osteoporosis units. Subjects who smoked on a daily basis were classified as current smokers.
Statistics
Student's t-test or analysis of variance (ANOVA) when there were more than 2 groups was used for comparison of mean values between groups. Correlations were calculated with Pearson coefficients. The null hypotheses were rejected at the 5% level (p < 0.05). In comparisons between multiple groups, the Bonferroni correction was applied before significance was considered. The Medical Products Agency of Sweden has recommended pharmacological treatment to be considered if the T-score is below –2.0 in fracture patients and below –2.5 in non-fracture patients. We therefore studied the outcome of these two different cut-off limits. Odds ratios (ORs) and the respective confidence intervals for risk of osteoporosis were calculated from a 2 × 2 cross table for the different fracture types in accordance with a case-control design.
Ethics
This study was approved by the Ethic Research Committee of the Faculty of Health Sciences, University of Linköping, and it was performed in accordance with the Declaration of Helsinki.
Results
Of the 303 fracture patients in the study group, 171 (56%) had a forearm fracture, 55 (18%) had a vertebral fracture, 40 (13%) had a hip fracture, and 37 (12%) had a fracture of the humerus. The mean age of patients with fracture of the forearm or humerus was 67 years and the mean age of patients with vertebral or hip fractures were 69 years. Mean age was the same for the dropout group (68) and the study group (68). There was, however, a higher percentage of hip fractures in the dropout group than in the study group: 19% as opposed to 13%. The rate of vertebral fractures was 22% in the dropout group and 18% in the study group. The corresponding figures for forearm fractures were 46% and 56%, and the figures for humerus fractures were similar.
The fracture patients were slightly taller than reference patients (difference +1.5 cm, p < 0.01), but no difference was seen for weight, BMI or hip axis length (HAL) (). The fracture group had a significantly lower mean BMD in all sites measured, compared to the reference group. Almost half (148/303) of the fracture patients had sustained at least one previous fracture and 57 (18%) had sustained at least two previous fractures. Interestingly, 11 of 40 patients with a hip fracture before the age of 75 years had sustained two or more previous fractures, yet had not been offered tests or treatment for osteoporosis. Since only a few patients had been examined using radiography of the spine, we know little about the true prevalence of vertebral fractures or deformities. 79 of the fracture patients (26%) had suffered from medical conditions or sequelae, which may have influenced their bone mass. A past history of thy reotoxicosis (n = 14) was the most common condition associated with significantly lower values of BMD (T-score < 2.0) compared to the other fracture cases or the reference group.
When we used diagnostic BMD cut-off principles according to the WHO criteria (T-score < –2.5 and T-score –1), there was a significantly different proportion of osteoporotic or osteopenic subjects in the fracture group and in the non-fracture reference group (). According to these criteria 46% of the fracture patients had osteoporosis, while only 16% were osteoporotic in the non-fracture reference group. The lowest BMD was seen in the vertebral fracture group, where 64% had osteoporosis and 35% had osteopenia.
Table 2. Diagnostic classification of fracture cases and non-fracture controls using two cut-off levels of the T-score
Use of a higher cut-off level (T-score < –2.0) increased the apparent prevalence of osteoporosis, particularly in the groups with forearm fracture and hip fracture (). Thus, the Z-score for total hip differed by 1.07 standard deviations (0.38 vs. –0.69) between groups (p < 0.001) (). Z-score for the lumbar spine was –0.22 and 0.70 respectively, but the mean Z-score for forearm was not significantly different between groups.
Table 3. Hip- and vertebral fracture groups compared to the age-matched references
In the vertebral fracture group, Z-score for lumbar spine, total hip, and forearm differed from the reference group by 1.09, 1.00, and 0.71 standard deviations, respectively (p < 0.01, ).
In the forearm fracture group, the corresponding differences between the Z-scores for fracture and reference groups were smaller (0.44, 0.38, and 0.19), but yet significant in the spine and hip ().
Table 4. Forearm- and humerus fracture groups compared to the age-matched references
The outcome of the comparative analysis between fracture and control groups () yielded high odds ratios for prevalence of osteoporosis. Although the confidence intervals were wide due to the small size of the study groups, OR was separated from –1.0 in all subgroups. The odds of having either osteopenia or osteoporosis were < 20 for patients with hip fracture and 75 for patients with fractures in the spine (mean values), whereas the OR for the forearm fracture group was slightly above 10 (). As expected, the OR depended on the cut-off limits used. For T-score –2.5 and –2.0 the OR for the hip fracture group was 8.2 and 9.5 respectively at the lower end of the 95% confidence interval. For the spine and the forearm, the corresponding odds ratios were 16–17 and 7–9 (CIlow).
Table 5. Odd ratio of osteopenia and osteoporosis in different fracture groups (95% confidence limits for OR)
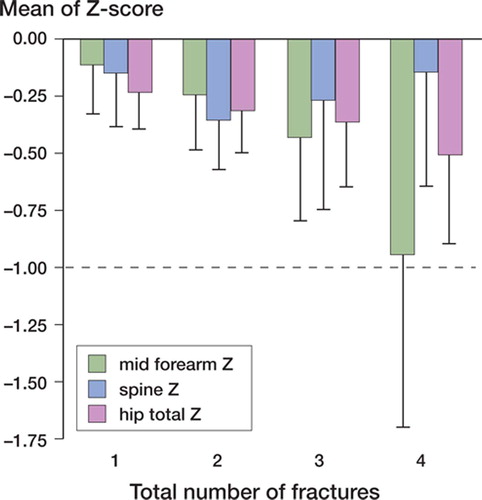
There was a strong association between number of previous fractures and bone mineral density. Patients with 3 or more previous fractures had the lowest Z-score for the hip and forearm (). Mean BMD of the lumbar spine (Z-score) decreased for the first two fractures but was higher in the group who had sustained 3 or more fractures (n = 57).
Figure 2. Number of previous fractures in relation to Zscore of BMD at various sites. Error bars depict CIlow.
Hip axis length (HAL) was greater in the hip fracture patients and forearm fracture patients than in age-matched controls ( and ). The hip fracture patients were slightly, but also significantly, taller than their reference counterparts: 163 cm as opposed to 161 cm (p < 0.01). A similar difference was also seen between the forearm fracture patients and the reference group (p = 0.05).
There was no significant difference in BMI between the fracture and reference groups— whether considering the total group or specific fracture subgroups. Within each fracture subgroup, however, there was a significant positive correlation between BMI and BMD.
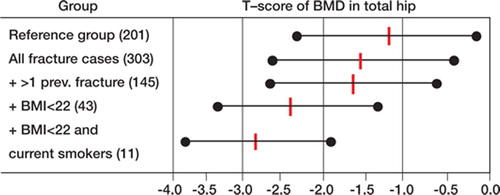
A comparison of BMD was done in subgroups with the following additional risk factors: multiple fractures, low BMI (defined as a BMI of < 22), and current smoking (). The lowest BMD was seen in the subgroup with low BMI, and particularly if the individuals were smokers. Stratification in further subgroups was not meaningful due to small cell numbers.
Discussion
Our findings indicate that osteoporosis was a neglected health problem at the time of recruitment of our patient group (1998–1999). Half of the total fracture group had osteoporosis (T < –2.5) while only 16% of the women in the age-matched non fracture group (i.e. without a low-energy fracture) had osteoporosis at this age.
Despite the prevalence of fracture(s), few had been referred for BMD measurement and only 57 (14%) of 400 subjects with fracture who had responded to the invitation had in fact been treated for osteoporosis. This finding is in accordance with other reports (Freedman et al. Citation2000, Andrade et al. Citation2003, Feldstein et al. Citation2003).
The age range of the present study material was limited to 55–75 years to reduce confounding from co-morbidity, which is often a problem in patients who are even older. The average ages in the four fracture type subgroups were almost identical, allowing valid comparisons. Our series was fairly small, but it was examined in depth in a standardized manner.
One limitation of the study was that the dropout rate was 33%. A random sample (n = 36) of the passive dropout group at baseline examination was traced two years after the fracture, and four of the patients were found to have died. The passive dropout group also had more severe fractures, greater co-morbidity, but were in similar age (Hallberg et al. Citation2004). Another limitation was that radiographic examination of the spine was not performed in patients other than those with vertebral fracture as index fracture.
We used machine-specific reference values to allow comparison between studies. BMD for Swedish women was compared to different machinespecific reference values published in two previous studies (Lofman et al. Citation1997, Citation2000). In general, the BMD values for Swedish women were found to be somewhat lower in both hip and spine, with premenopausal spine BMD being an exception (in that the Swedish values and reference values corresponded well) (Lofman et al. Citation1997).
Is the number of previous fractures a good marker for risk of osteoporosis and fracture?
An important finding in our material was that there was a strong inverse correlation between the number of previous fractures and BMD in the forearm and the hip. Women with a recent hip fracture also had sustained a greater number of previous fractures than women with a recent forearm fracture. On the other hand, there was a less clear correlation between vertebral BMD and the number of previous fractures, which may be ascribed to vertebral compressions and degenerative diseases of the spine (Rand et al. Citation1997, Kanis and Gluer Citation2000, Muraki et al. Citation2004). Although Torgerson et al. (Citation1996) reported a 6-times increased risk of fracture in perimenopausal women with two or more previous fractures, and Ross et al. (Citation1993) reported that the presence of one or two fractures increased the risk of new vertebral fractures by a factor of 7, the predictive value of multiple fractures has seldom been studied or considered in other reports. We consider that since information on the number of previous fractures is usually easily accessible, it may be of value in the clinical routine when selecting patients for investigation of bone mineral density.
Previous fracture can be used as a predictor of future fractures
Despite the relatively low age range (55–75 years), many of our study patients with different types of fracture had already sustained previous fractures. The rate for patients with fracture of the forearm or humerus was 46%, and for hip and vertebral fracture patients it was 48% and 60%, respectively. Several studies have shown that one low-energy fracture is associated with an increased risk of future fractures. Pooled estimates suggest a doubled overall risk of future fracture for a patient who has already sustained a fracture compared to nonfracture subjects (Klotzbuecher et al. Citation2000, Kanis et al. Citation2004).
Huopio et al. (Citation2000) found that women with a previous fracture had an increased relative risk of 70% (RR 1.7; 95% CI 1.3–2.2) of a future fracture, compared to age-matched women without a fracture. However, different fractures seem to have different predictive value. After a forearm fracture, the risk of future hip fractures was found to be increased by only 40% according to Cuddihy et al. (Citation1999), while a vertebral fracture in a postmenopausal woman has been reported to predict a 4-times higher risk of new vertebral fractures and a 2-fold higher risk of hip fracture than in women with no previous fracture (Klotzbuecher et al. Citation2000).
Fracture predicts osteoporosis and osteoporosis predicts fracture
As could be expected, patients with an incident fracture had lower BMD on average than controls without fracture, but usefulness in predicting low BMD appears to vary between different fracture types. In our study fracture of the spine, hip and humerus were stronger predictors of osteoporosis than fracture of the forearm, although the latter was also associated with reduced BMD compared to controls.
Studies on the relationship between BMD at one site and the risk of fracture at the same or another site provide information on site-specific fracture estimates (De Laet et al. Citation1998, Masud et al. Citation2001).
A meta-analysis of prospective studies showed that at the age of 65 years, risk ratio for subsequent fractures increased by 3 in both men and women for each decrease of 1 SD in BMD, exponentially (Johnell et al. Citation2005). These authors and others also reported that fracture history and BMD were to a great extent independent predictors of future fractures. The usefulness of low BMD in predicting fracture declined with age (Tromp et al. Citation2000, The European Prospective Osteoporosis Study (Epos) Group (Anonymous Citation2002), Johnell et al. Citation2005).
Ross et al. (Citation1991) investigated the independent contributions of bone mass and existing vertebral fractures as predictors of the risk of new vertebral fractures, and reported that a single fracture at the baseline examination increased the risk of new vertebral fractures by 5-fold. In their study, the presence of two or more vertebral fractures increased the risk by 12-fold. A combination of low bone mass (below the thirty-third percentile) and the presence of two or more current fractures increased the risk by 75-fold relative to women with the highest bone mass (above the sixty-seventh percentile) and no current fractures. A history of low-energy fractures in addition to BMD measurement therefore adds significant information for the prediction of future fracture risk and for grading of the priority regarding treatment.
Fracture prediction: relative and combined contributions from multiple factors
Body weight and body mass index and a “smallframe body constitution” are also valuable predictors of osteoporosis that may be used in the selection of patients for osteoporosis investigation and treatment. In our study, normal or high BMD was mainly seen in overweight or obese women. The group with BMI < 29 had a significantly higher Z-score for BMD than the group with BMI < 22 in the lumbar spine, in accordance with previous studies (Michaelsson et al. Citation1996). The lack of a difference in BMI between the fracture and control groups could be a result of shrinking body height with age. Tobacco smoking and heredity are other factors that have been reported to have predictive value for osteoporosis and fracture.
Although our series was small, adding multiple risk factors suggests increased power for osteoporosis prediction (). Research aimed at establishing composite algorithms that include several risk determinants for the prediction of future fractures is under way (Cummings et al. Citation1995, De Laet et al. Citation1998, Kanis et al. Citation2005, Sornay-Rendu et al. Citation2005) but as far as we know, the contribution of multiple fractures as opposed to single fractures has not been considered to date.
In summary, osteoporosis is still a neglected health problem. Patients with hip and vertebral fractures—at least those in the 55–75 age range— should be investigated for osteoporosis with higher priority than patients with fractures at other skeletal sites. Patients with multiple previous fractures also constitute an important risk group that should be given more attention than has been the case hitherto. This is a risk factor that is easy to assess in the clinical interview, and a strong marker of osteoporosis.
We thank Marit Andersson, Bibbi Gurung, and Helene Hall of Linköping University Hospital for all their help with administration, bone mineral density measurement, and care of the patients in the study. This study was supported by grants from the County Council of Östergötland, Sweden.
Contributions of authors
OL: study design, statistical and clinical evaluation, and reporting. IH: study design, patient recruitment, clinical evaluation, reporting, and project management. KB: statistical analysis and reporting. OW: clinical analysis and reporting. LK: patient recruitment, clinical evaluation, and project management. AMR: patient recruitment and clinical evaluation. LL: study design and reporting. GT: study design, clinical evaluation, reporting, and project supervisor.
- Andrade S E, Majumdar S R, Chan K A, Buist D S, Go A S, Goodman M, Smith D H, Platt R, Gurwitz J H. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 2003; 163: 2052–7
- Anonymous. The relationship between bone density and incident vertebral fracture in men and women. J Bone Miner Res 2002; 17: 2214–21
- Borgstrom F, Johnell O, Kanis J A, Jonsson B, Rehnberg C. At what hip fracture risk is it cost-effective to treat? International intervention thresholds for the treatment of osteoporosis. Osteoporos Int 2006; 17: 1459–71
- Cuddihy M T, Gabriel S E, Crowson C S, O'Fallon W M, Melton L J, 3rd. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int 1999; 9: 469–75
- Cummings S R, Nevitt M C, Browner W S, Stone K, Fox K M, Ensrud K E, Cauley J, Black D, Vogt T M. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995; 332: 767–73
- De Laet C E, Van Hout B A, Burger H, Weel A E, Hofman A, Pols H A. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res 1998; 13: 1587–93
- Favus M J. Primer on the metabolic bone diseases and disorders of bone mineral metabolism: Bone density reference data. Raven Press, New York 1993
- Feldstein A, Elmer P J, Orwoll E, Herson M, Hillier T. Bone mineral density measurement and treatment for osteoporosis in older individuals with fractures: a gap in evidence-based practice guideline implementation. Arch Intern Med 2003; 163: 2165–72
- Fogelman I. Screening for osteoporosis. No point until we have resolved issues about long term treatment. Bmj 1999; 319: 1148–9
- Freedman K B, Kaplan F S, Bilker W B, Strom B L, Lowe R A. Treatment of osteoporosis: are physicians missing an opportunity. J Bone Joint Surg (Am) 2000; 82: 1063–70
- Hallberg I, Rosenqvist A M, Kartous L, Lofman O, Wahlstrom O, Toss G. Health-related quality of life after osteoporotic fractures. Osteoporos Int 2004; 15: 834–41
- Honkanen R, Tuppurainen M, Kroger H, Alhava E, Puntila E. Associations of early premenopausal fractures with subsequent fractures vary by sites and mechanisms of fractures. Calcif Tissue Int 1997; 60: 327–31
- Huopio J, Kroger H, Honkanen R, Saarikoski S, Alhava E. Risk factors for perimenopausal fractures: a prospective study. Osteoporos Int 2000; 11: 219–27
- Johnell O, Kanis J A, Oden A, Johansson H, De Laet C, Delmas P, Eisman J A, Fujiwara S, Kroger H, Mellstrom D, Meunier P J, Melton L J, 3rd, O'Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res 2005; 20: 1185–94
- Kanis J A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368–81
- Kanis J A, Gluer C C. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int 2000; 11: 192–202
- Kanis J A, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey E V, Mellstrom D, Melton L J, Pols H, Reeve J, Silman A, Tenenhouse A. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004; 35: 375–82
- Kanis J A, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int 2005; 16: 581–9
- Karlsson M K, Hasserius R, Obrant K J. The ankle fracture as an index of future fracture risk. A 25–40 year follow-up of 1063 cases. Acta Orthop Scand 1993; 64: 482–4
- Khan S A, de Geus C, Holroyd B, Russell A S. Osteoporosis follow-up after wrist fractures following minor trauma. Arch Intern Med 2001; 161: 1309–12
- Klotzbuecher C M, Ross P D, Landsman P B, Abbott T A, 3rd. Berger M Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15: 721–39
- Lofman O, Larsson L, Ross I, Toss G, Berglund K. Bone mineral density in normal Swedish women. Bone 1997; 20: 167–74
- Lofman O, Larsson L, Toss G. Bone mineral density in diagnosis of osteoporosis: reference population, definition of peak bone mass, and measured site determine prevalence. J Clin Densitom 2000; 3: 177–86
- Looker A C, Wahner H W, Dunn W L, Calvo M S, Harris T B, Heyse S P, Johnston C C, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 1998; 8: 468–89
- Masud T, Jordan D, Hosking D J. Distal forearm fracture history in an older community-dwelling population: the Nottingham Community Osteoporosis (NOCOS) study. Age Ageing 2001; 30: 255–8
- Michaelsson K, Bergstrom R, Mallmin H, Holmberg L, Wolk A, Ljunghall S. Screening for osteopenia and osteoporosis: selection by body composition. Osteoporos Int 1996; 6: 120–6
- Muraki S, Yamamoto S, Ishibashi H, Horiuchi T, Hosoi T, Orimo H, Nakamura K. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos Int 2004; 15: 724–8
- Rand T, Schneider B, Grampp S, Wunderbaldinger P, Migsits H, Imhof H. Influence of osteophytic size on bone mineral density measured by dual X-ray absorptiometry. Acta Radiol 1997; 38: 210–3
- Ross P D, Davis J W, Epstein R S, Wasnich R D. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114: 919–23
- Ross P D, Genant H K, Davis J W, Miller P D, Wasnich R D. Predicting vertebral fracture incidence from prevalent fractures and bone density among non-black, osteoporotic women. Osteoporos Int 1993; 3: 120–6
- Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas P D. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 2005; 20: 1813–9
- Torgerson D J, Campbell M K, Thomas R E, Reid D M. Prediction of perimenopausal fractures by bone mineral density and other risk factors. J Bone Miner Res 1996; 11: 293–7
- Tromp A M, Ooms M E, Popp-Snijders C, Roos J C, Lips P. Predictors of fractures in elderly women. Osteoporos Int 2000; 11: 134–40