Abstract
Background Despite the well-known effect of type-I collagen in promoting cartilage repair, the mechanism still remains unknown. In this study we investigated this mechanism using a rabbit model of cartilage defects.
Animals and methods 5-mm-diameter full-thickness defects were created on both patellar grooves of 53 Japanese white rabbits (approximately 13 weeks old). The left defect was filled with collagen gel and the right defect was left empty. The rabbits were killed and examined morphometrically until the twenty-fourth postoperative week, by (1) evaluation of matrix production, (2) enumeration of the total number of cells engaged in cartilage repair, (3) enumeration of the proliferating cells, (4) localization of mesenchymal stem cells, and (v) localization of apoptotic cells.
Results We found that type-I collagen enhances cell recruitment, and thereby increases the number of proliferating cells. A considerable proportion of the proliferating cells were identified as bone marrow-derived mesenchymal stem cells. However, type-I collagen does not prevent the chondrocyte precursors from undergoing apoptotic disengagement from the chondrogenic lineage.
Interpretation Type-I collagen promotes cartilage repair by enhancing recruitment of bone marrowderived mesenchymal stem cells. Additional use of agent(s) that sustain mesenchymal stem cells along the chondrogenic path of differentiation may constitute an appropriate environment for cartilage repair.
Articular cartilage has a limited capacity for repair. It has long been known that the way in which cartilage is repaired depends on its depth. No appreciable repair occurs in partial-thickness defects that are confined to the cartilage itself (Kim et al. Citation1991). Since the bone marrow is the reservoir of mesenchymal stem cells (MSCs), the chondrogenic progenitor cells are available only when the cartilage defect is in contact with the marrow space (Yoo et al. Citation1998). Thus, cartilage repair in fibrocartilage and sometimes in hyaline cartilage can only occur if the full-thickness defect penetrates into the bone marrow (Shapiro et al. Citation1993).
Because of the high prevalence of osteoarthritic disorders, the repair of articular cartilage has become one of the important targets of modern tissue engineering. Isolation and use of MSCs to regenerate bone and joint tissues is being vigorously investigated (Caplan Citation1991, Nishimura et al. Citation1999, Huang et al. Citation2005), as are the variety of signaling molecules implicated in the critical regulation of chondrogenic differentiation (Sato and Urist Citation1984, Hunziker et al. Citation2001). However, regeneration of large cartilage defects has not yet been fully achieved.
Local application of exogenous collagen has been known to promote repair of damaged cartilage, although the precise mechanism of promotion of cartilage repair has not been fully elucidated (Speer et al. Citation1979, Buma et al. Citation2003). In order to investigate the effects of exogenous collagen on chondrogenesis, we carried out a detailed examination of the cellular events during the repair of rabbit cartilage defects with and without the use of collagen.
Material and methods
Animals and collagen
53 Japanese White rabbits of about 13 weeks of age and weighting 2.5–3 kg (Kitayama labes, Nagano, Japan) were used. Collagen gel (Cellmatrix I-A, Nitta Gelatin, Osaka, Japan) was prepared from acid-soluble type-I collagen solution from porcine tendon. 8 volumes of collagen solution were mixed with 1 volume each of 10× concentrated PBS and reconstitution buffer (2.2 g NaHCO3 and 4.77 g HEPES in 100 mL of 0.05 N NaOH).
Articular cartilage defect model
The rabbits were anesthetized by intramuscular injection of a mixture of ketamine hydrochloride (60–70 mg/kg) and xylazine (6 mg/kg). 5-mm diameter full-thickness defects (5 mm in depth) were created on the patellar groove of the femur. The defect in the left knee was filled with cylindrical collagen gel (collagen group) and the defect in the right knee was left empty (control group). All rabbits were returned to their cages after the surgery and were allowed to move freely.
Tissue preparation
The rabbits with large defects were killed with an overdose of sodium pentobarbital at postoperative weeks: 1 (n = 8), 2 (n = 8), 3 (n = 7), 4 (n = 8), 8 (n = 7), 12 (n = 8), and 24 (n = 7). One shot of 10 mM BrdU in PBS (20 mg/kg) was injected intramuscularly 1 h before the killing (Apte Citation1990, Miyaki et al. Citation1994). The entire knee was dissected and the distal part of the femur was then extirpated. One group of specimens was fixed in 20% paraformaldehyde at room temperature for 2 days, decalcified with 10% ethylenediamine tetraacetic acid (EDTA) for 6 weeks, and then embedded in paraffin. Each specimen was cut into 3-μm axial sections and deparaffinized, dehydrated, and used for subsequent staining with: hematoxylin and eosin, toluidine blue, bromodeoxyuridine (BrdU), and also TUNEL staining. The other group of specimens was immersed for 30 min in fixative containing 4% paraformaldehyde, 0.5% glutaraldehyde, and 0.2% picric acid in 0.1 M phosphate buffer (PB; pH 7.4) and then in a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 M PB at 4°C overnight. After being decalcified in 10% EDTA for 4 weeks, the specimens were embedded in 10% gelatin in 0.1 M PBS (pH 7.4). They were then washed in several changes of 0.1 M PB containing 15% sucrose, cut into 20-μm axial sections on a cryostat or 50-μm axial sections on a vibratome. The 20-μm cryostat sections were collected in 0.1 M PBS containing 0.3% Triton X- 100 (PBST) and used for study by confocal laser scanning microscopy, while the 50-μm vibratome sections were collected in 0.1 M PBS and used for electron microscopy.
Histological staining
For the immunostaining to detect the presence of BrdU, endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide. Antigen retrieval was done by heating with 0.01 M sodium citrate buffer (pH 6.0) and treatment with 0.05 mg protease type XXIV (Sigma) in 0.1 mL PBS. Unspecific staining was blocked by incubation with 10% normal pig serum (KHOJIN BIO, Saitama, Japan) in PBS. The sections were incubated at 4°C overnight with a monoclonal mouse anti-BrdU antibody (cat. no. 347580; Becton Dickinson, San José, CA) that had been diluted 1:500 with PBS and washed with PBS. Antibody binding was visualized by incubating with biotinylated rabbit anti-mouse IgG (Dako Cytomation, Glostrup, Denmark) and peroxidase-conjugated streptavidin in combination with diaminobenzidine (DAB) solution. The sections were then counterstained with hematoxylin.
For the TUNEL technique, endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide. The sections were treated with 20 μg/mL proteinase K (DAKO) and incubated with reaction reagent containing terminal transferase, biotin-16- dUTP, and TUNEL dilution buffer (Roche, Indianapolis, IN). Visualization was done with DAB solution. The sections were then counterstained with methyl green.
Confocal laser scanning microscopy
For triple-staining of CD44, CD45, and BrdU, the sections were incubated with a 1:1:1 [OK?] mixture of biotinylated mouse monoclonal anti-CD44 antibody (cat. no. CBL1411B; Chemicon), FITClabeled mouse monoclonal anti-CD45 antibody (cat. no. CBL1412F; Chemicon), and rat monoclonal anti-BrdU antibody (cat. no. ab6326; Abcam, Cambridge, UK), each diluted 1:1,000. After washing with PBS, they were then reacted with a 1:1 mixture of Texas red streptavidin (Vector Labs, Burlingame, CA) and cy5-labeled anti-rat IgG (Chemicon) diluted 1:1,000, and then observed under a confocal laser scanning microscope (LSM 510; Carl Zeiss Japan, Tokyo, Japan).
Transmission electron microscopy
The unstained vibratome sections were incubated for 1 h with 1% OsO4 in 0.1 M PBS at 4°C. The sections were then dehydrated with a graded series of ethanol and propylene oxide and embedded in Epon. Ultrathin sections were cut in an ultramicrotome (Ultra E; Reichert-Jung, Vienna, Austria) and stained for 20 min with 2% uranyl acetate, and for an additional 5 min with Reynold's solution. The ultrathin sections were then observed by electron microscopy (H-7500; Hitachi, Tokyo, Japan) and photographed.
Histological grading
The paraffin sections were stained with toluidine blue and each sample was graded according to Wakitani et al. (Citation1998) ().
Histological grading scale
Numbers of cells engaged in cartilage repair
Total cell number was determined using the sections stained with hematoxylin. 5 fields were randomly selected at a magnification of 400× and the cell number was expressed as mean number per optical field.
Number of proliferative cells engaged in cartilage repair
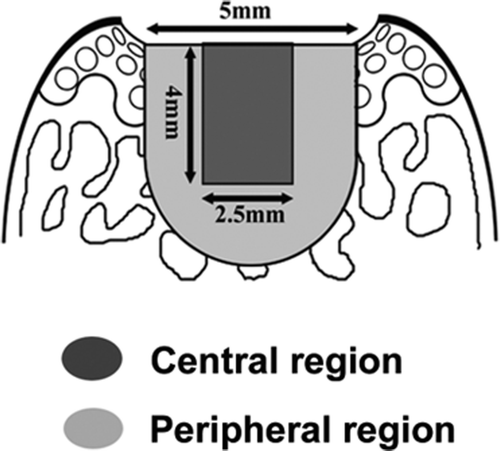
Number of proliferative cells was determined using the sections stained with BrdU. The defect was subdivided into the central region and the peripheral region. The central region was defined as the central 2 × 3-mm area and the peripheral region was defined as the remnant of the defect (). 5 fields were randomly selected at a magnification of 400× and the number of positive cells was counted.
Number of apoptotic cells disengaged from cartilage repair
Apoptotic cell number was counted using the sections stained with TUNEL. 5 fields were randomly selected at a magnification of 400× in each region, and the number of positive cells and the total cell number were counted. Apoptotic cell number was calculated as follows: apoptotic cell number (%) = (positive cell number / total cell number) × 100.
In vitro migration assay
It is known that bone marrow-derived mesenchymal stem cells (MSCs) give rise to progenitor cells of chondrocytes during cartilage repair (Shapiro et al. Citation1993). In order to compare the migration of MSCs and chondrocytes to the collagen matrix, we performed an in vitro migration assay ().
Bone marrow-derived mesenchymal stem cell preparations
Bone marrow was taken from the same kind of Japanese white rabbits as described above. Briefly, the proximal-medial surface of each tibia was exposed through a small incision. The soft tissue was retracted, and 2 mL of blood was aspirated from the bone marrow of the tibia (Wakitani et al. Citation1994) with use of an 18-gauge needle that was fastened to a 10-mL syringe containing 0.1 mL heparin (3,000 units per mL). The aspirate was washed twice with Tyrode balanced solution (cat. no. T1788; SIGMA) and centrifuged at 180 × g for 5 min. According to the Wakitani protocol for isolation of mesenchymal cells (Wakitani et al. Citation1994), the middle layer between the top clear layer and the lower red blood cell layer was aspirated and resuspended in DMEM containing 10% fetal bovine serum (FBS).
Chondrocyte preparations
Articular cartilage slices were taken from the same kind of Japanese white rabbit as described above. Distal femurs were dissected, cleared of adherent connective tissue, and cut into small pieces. Chondrocytes were then isolated by enzymatic digestion for 20 min in PBS containing 0.1% trypsin and 0.1% EDTA, and for 2 h in DMEM containing 0.5% collagenase. Cells were cultured as monolayers in DMEM containing 10% FBS.
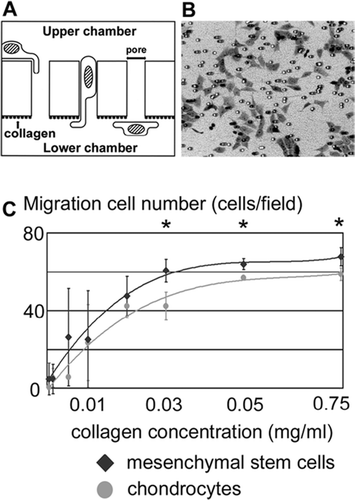
Migration assays were performed using Transwell permeable supports (Corning). The bottom of the upper well consists of polyester membrane with 8-μm pores (). The lower surfaces of the membranes were coated with different concentrations of type-I collagen (the same as implanted in the cartilage defect model) in PBS by incubation overnight at room temperature. The lower wells were filled with DMEM containing 1 mg/mL BSA. The upper wells were filled with 1.5 × 105 cells in 1.5 mL of DMEM containing 1 mg/mL BSA. The chamber was incubated for 5 h in a humidified incubator at 37°C. After the migration of cells, the membrane was removed, then fixed with 4% paraformaldehyde and stained with toluidine blue. The membranes were subsequently placed onto glass slides and the number of cells remaining on the upper surface and the number of cells migrated onto the lower surface of the membranes were counted by light microscopy at 400× magnification. For each experiment in triplicate, the number of cells in five microscope fields was determined and the mean was calculated.
Statistics
Results were expressed as mean values (± S.D). Student's t-test was used to compare the results between the two groups. A p-value of less than 0.05 was considered to be significant.
Results
Histological assessment of the effect of collagen matrix in cartilage repair
For both the collagen (+) and collagen (–) defects, the defect was first filled with fibrin network 1 week postoperatively. The superficial fibrous strands of the fibrin network formed an arc passing from one edge of the defect to the other (). The difference that was apparent during the first postoperative week was the abundance of spindleshaped cells, which were prominent in the peripheral region of the collagen (+) defect (, arrows).
Figure 1. Effect of collagen matrix on cartilage repair. Transverse sections of collagen (-) defect (A, C, E, G, I, K, and M) and collagen (+) defect (B, D, F, H, J, L, and N). (A) and (B) show the defects at the first postoperative week. Arrows indicate the regions where there is an abundance of spindle-shaped cells. (C) and (D) show the defects at the second week. Arrowheads indicate the initial site of proteoglycan production. (E) and (F), (G) and (H), (I) and (J), (K) and (L), and (M) and (N) show those at the fourth, eighth, twelfth, and twenty-fourth week, respectively. Note the collagen(-) defect does not produce sufficient cartilage matrix, whereas the collagen(+) defect succeeds in resurfacing of the articular cartilage (toluidine blue).
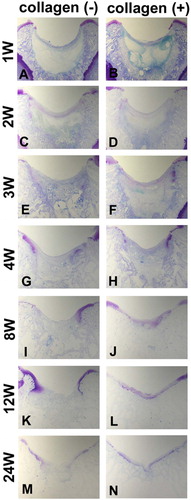
The earliest proteoglycan production was found in both the collagen (+) and collagen (–) defects 2 weeks postoperatively. The initial site of proteoglycan production was found to be at the edges of the cartilage defects ( and , arrowheads). The proteoglycan-containing cartilage matrix extended from both edges of the defect. In the collagen (–) defects, however, the amount of cartilage matrix was insufficient to cover the whole defect during the time points that followed (). In turn, the collagen (+) defects allowed continuous growth of the proteoglycancontaining cartilage, which finally covered the defect ().
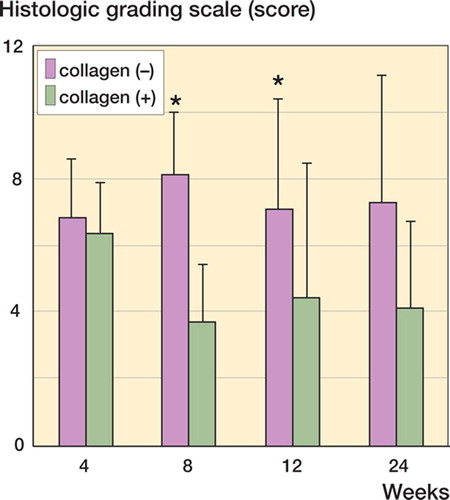
According to the Wakitani's histological grading score (), the collagen (+) defects were repaired by cartilage tissue of superior quality relative to the collagen (–) defects (). However, the grading score of the collagen (+) defect did not improve after the eighth postoperative week (), suggesting that use of type-I collagen matrix alone does not guarantee complete repair of the cartilage.
Figure 2. Histological evaluation using Wakitani's histological grading score (Wakitani et al. Citation1998). The collagen (+) defect is repaired with cartilage tissue of superior quality as compared to the collagen (–) defect. However, the grading score of the collagen (+) defect does not improve after the eighth postoperative week. Data are shown as mean ± SD. Asterisks indicate statistical significance (Student's t-test, p < 0.05).
Total number of cells under different conditions of repair
Local application of collagen matrix promotes repair of cartilage ( and ), although the exact mechanism has not been fully elucidated (Speer et al. Citation1979, Buma et al. Citation2003). Since an abundance of spindle-shaped cells in the peripheral region of the defect was a characteristic finding during repair of the collagen (+) defects, the cells distributed in the cartilage defects were carefully counted.
The total numbers of cells in the collagen (–) and collagen (+) defects showed distinct time courses (). The numbers of cells in the collagen (–) defects showed a moderate and steady increase until the fourth postoperative week. Then there was a decrease in cell number during the fourth to twenty-fourth postoperative weeks.
Figure 3. Total number of cells under different repair conditions. The total cell number distributed in the collagen (–) and collagen (+) defects display distinct time courses. There is an early increase in cell number in the collagen(+) defect at the second week (asterisk), followed by a decrease to a level comparable to that of the collagen (–) defect. Data are shown as mean ± SD. Asterisk indicates a significant difference (Student's t-test, p < 0.05).
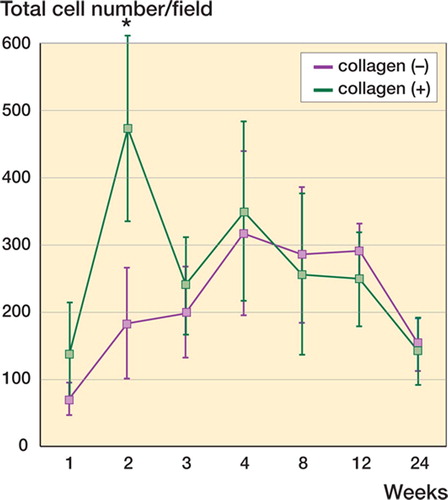
The collagen (+) defects, in turn, showed an early increase in cell numbers (, asterisk) at the second week, followed by a decrease to levels comparable to those of the collagen (–) defects (). Since this early increase in cell numbers was a characteristic finding during repair of the collagen (+) defects, we attempted to characterize the cells involved in this increase.
Spatiotemporal distribution of the proliferating cells
To characterize the cells responsible for the early increase in cell density (, asterisk), distribution of the proliferating cells was studied using the BrdU technique (). For detailed cellular localization, the cartilage defect was differentiated further into the central and peripheral regions ().
Figure 4. BrdU-positive cells during different phases of cartilage repair. Proliferating cells are identified by BrdU technique: (E), (F), (G), and (H); 200× magnification. Hematoxylin and eosin stained sections of corresponding regions are shown to demonstrate cellularity of the each condition: (A), (B), (C), and (D); 100× magnification.
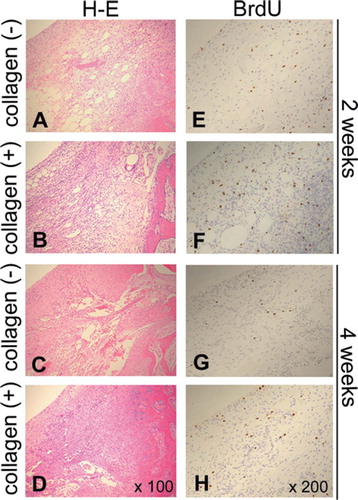
Figure 5. The central and peripheral regions for localization of proliferating cells. To characterize the cells responsible for the early increase in cell number (asterisk, ), the distribution of proliferating cells was studied using BrdU staining. For detailed localization of cells, the cartilage defect was divided further into the central and peripheral regions.
The peripheral region of the collagen (–) defects had a moderate increase in proliferating cells by the second week (, bars second from left). The central region of the collagen (–) defect was characterized by having very few proliferating cells throughout the entire time period studied (, left-hand columns). The failure in resurfacing of the cartilage defect in the collagen (–) defect () may have resulted from the paucity of proliferating cells in the central region of the defect.
Figure 6. Spatiotemporal distribution of the proliferating cells. BrdU-positive cells were counted in the central and peripheral regions. Densities of proliferating cells are presented as cells/field for collagen(-) defects and collagen(+) defects. Data are shown as mean. Bars indicating SD are omitted for simplicity. Asterisk indicates significant differences between peripheral regions in collagen(+) and collagen(-) defects (Student's t-test, p < 0.05).
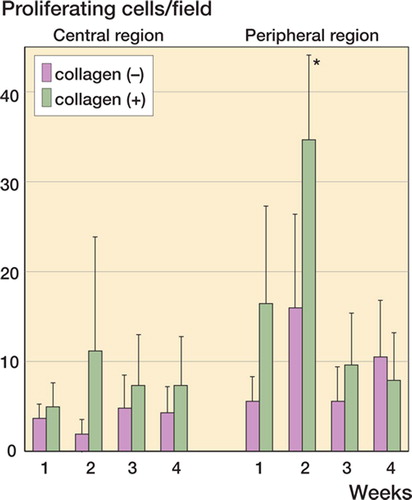
The peripheral region of the collagen (+) defect, in turn, was characterized by a greater increase in proliferating cells at the second week (, asterisk). The central region of the collagen (+) defect also had a larger number of proliferating cells than the collagen (–) defect (, columns second from right). These observations led us to the hypothesis that the success of cartilage resurfacing in the collagen (+) defect was attributable to the large population of proliferating cells recruited by the collagen matrix.
In vitro recruitment assay on bone marrowderived mesenchymal stem cells
Use of the collagen matrix in the cartilage defect apparently caused the increase in proliferating cells at the second postoperative week (, asterisk). In good agreement with in vivo studies, the cultured MSCs were recruited to the substrate- bound collagen in a clearly dose-dependent manner in vitro (). The migratory response of mature chondrocytes used as a control was significantly weaker than that of the MSCs ().
Figure 7. Recruitment assay on the bone marrow-derived mesenchymal stem cells and chondrocytes. (A) Scheme of in vitro migration assay. Different concentrations of collagen were used to coat the lower surfaces of porous membranes. Cells were seeded on the upper surface and those that migrated to the lower surface were counted. (B) Microscopic appearance of the migrated cells. (C) Numbers of migrated mesenchymal cells and chondrocytes at different concentrations of substrate-bound collagen. Data are expressed as mean ± SD. Asterisk indicates a statistically significant difference between mesenchymal cells and chondrocytes (Student's t-test, p< 0.05).
Characterization of the cell types recruited by collagen
The use of collagen matrix in the cartilage defects apparently caused the increase in proliferating cells () by enhancing the migratory response of MSCs (). In order to characterize the type(s) of cells recruited to cartilage repair, cell surface markers were studied. Despite the numerous studies that have been carried out to characterize mesenchymal stem cells, no single cell surface marker for identification of marrow-derived MSCs has been found yet. Instead, combinations of cell surface markers have been used to identify these cells (Djouad et al. Citation2005, Wagner et al. Citation2005). CD44-positive and CD45-negative cells are cur- rently considered to be marrow-derived MSCs (Djouad et al. Citation2005, Wagner et al. Citation2005). MSCs give rise to the chondrocyte progenitors. Thus, CD44-positive, CD45-negative and BrdU-positive cells are considered to be the MSCs that are undergoing self-renewal.
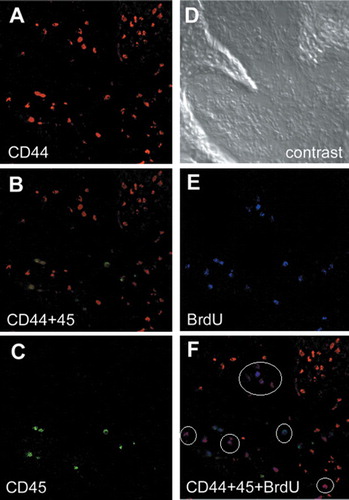
Triple staining for CD44, CD45, and BrdU of the peripheral region at the second week (corresponding to the asterisk in the ) indicated that approximately half of the BrdU-positive cells were CD44-positive and CD45-negative (, white circles), indicating that the half of the recruited cells were MSCs that still retained mitotic activities. Use of the collagen in the cartilage defect apparently caused the increase in proliferating cells () by enhancing cell migration (), and a considerable proportion of the recruited proliferating cells were MSCs that were undergoing self-renewal mitosis.
Figure 8. Characterization of the cell types recruited by collagen. Combinations of cell-surface markers are used to identify the mesenchymal stem cells (MSCs), and the CD44-positive and CD45-negative cells are considered to be the marrow-derived MSCs. Immunofluorescent detection of CD44 (A), CD45 (C), and BrdU (E). B. double staining of CD44 (red) and CD45 (green). F. triple staining of CD44 (red), CD45 (green), and BrdU (blue). Approximately half of the BrdU-positive cells are CD44-positive and CD45-negative (white circles), indicating that a considerable proportion of the recruited cells are MSCs that still retain their mitotic activities. D. phase-contrast view.
Distribution of apoptotic cells during chondrogenesis
The histological grading score of the collagen (+) defect did not improve after the eighth postoperative week, suggesting that the use of collagen does not guarantee complete repair of cartilage (). In order to address the incomplete repair of collagen (+) defects, the outcome of the recruited MSCs, or MSC-derived cells, must be assessed. Chondrocyte-committed MSCs can probably give rise to mature chondrocytes at multiple stages along the path of differentiation. It has been well established that apoptosis reflects disengagement from the axis of differentiation (Morioka et al. Citation1998). To gain better insight into the outcome of the recruited MSCs, apoptotic activities were evaluated using TUNEL staining.
For both the collagen (–) and collagen (+) defects, there was a sharp increase in TUNEL-positive cells at the second postoperative week, followed by a decrease during the second to eighth postoperative weeks (). Electron microscopy revealed the ultrastructural characteristics of the apoptotic cells, confirming that the TUNEL-positive cells do indeed reflect apoptosis ( and ). Since the amount of TUNEL-positive cells was not altered by the use of collagen, we assumed that the use of collagen does not affect the differentiation activity.
Figure 9. Apoptotic cell distribution during chondrogenesis. Densities of TUNEL-positive cells during cartilage repair. For both the collagen (–) and collagen (+) defects, there are sharp increases in numbers of TUNEL-positive cells at the second postoperative week, followed by a decrease during the second to eighth postoperative week. Since the amount of TUNEL-positive cells is not altered by the use of collagen, it is likely that the collagen usage does not affect differentiation activity. Data are expressed as mean ± SD.
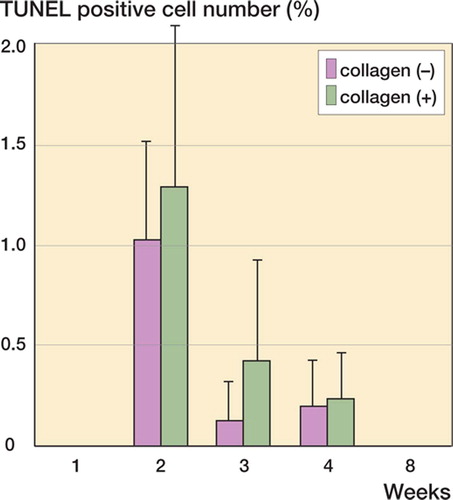
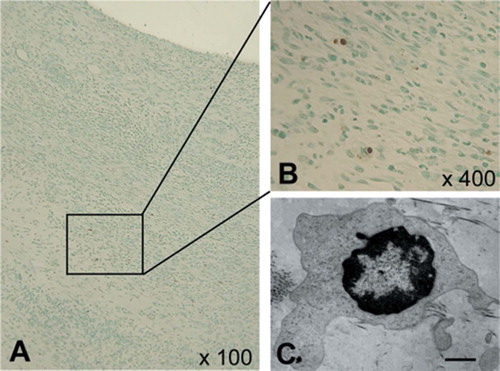
Figure 10. Confirmation of apoptotic cells. A and B: TUNEL staining sections showing the localization of apoptotic cells; 100× and 400× magnification, respectively. The rectangle indicates the area of panel B. C: Electron microscopy showing findings compatible with apoptosis (bar represents 1.0 μm).
The triple staining for CD44, CD45, and BrdU indicated that approximately half of the MSCderived cells (CD44-positive and CD45-negative) were BrdU-positive (), suggesting that these MSC-derived cells probably undergo selfrenewal proliferation. However, it noteworthy that apoptotic disengagement of the precursor cells also takes place in parallel.
Taken together, the results of the present study demonstrate that the use of collagen in cartilage defects causes an increase in cell proliferation () by enhancing cell migration (). A considerable proportion of the recruited proliferating cells are MSCs undergoing self-renewal mitosis (). However, the use of collagen may not affect the chondrocytic differentiation, and the MSC-derived progenitor cells undergo apoptotic disengagement from the chondrocytic lineage ( and ).
Discussion
The classical statement by Hunter in Citation1743, “From Hippocrates to the present age it is universally allowed that ulcerated cartilage is a troublesome thing and that, once destroyed, is not repaired”, still eloquently describes present-day difficulties when attempting to repair damaged cartilage. Application of exogenous collagen to the cartilage defect is known to facilitate cartilage repair (Wakitani et al. 1989, Breinan et al. Citation2000). Our study has demonstrated that collagen (+) defects are repaired with cartilage tissue of superior quality compared to collagen (–) defects (). However, the grading scores of the collagen (+) defects did not improve after the eighth postoperative week (), indicating that application of type-I collagen matrix alone does not guarantee complete repair of the cartilage. Dorotka et al. (Citation2005) used a collagen complex consisting of a dense layer of type-IV collagen and a porous layer of type-II collagen for chondral defects. The effectiveness of collagen implantation was not confirmed in the latter study, which compared the result of treatment with microfracture alone to the one with microfracture and collagen implantation. Thus, the effectiveness of different kinds of collagen may vary.
In order to achieve desirable repair of cartilage, a variety of signaling molecules implicated in the critical regulation of chondrogenesis have been used (Seller et al. Citation2000). However, there is no clearcut consensus as to which signaling molecules can result in superior cartilage regeneration (Hickey et al. Citation2003). In the present study, by investigating the detailed cellular events occurring during cartilage repair, we have tried to provide an insight into better use of molecular and biological agents. In addition, despite the favorable effects of collagen (Speer et al. Citation1979, Wakitani et al. 1989, Breinan et al. Citation2000, Buma et al. Citation2003), the detailed mechanism of action of exogenous collagen in facilitating cartilage repair has not been fully addressed in the literature.
MSCs can differentiate into different cell types, including fibroblasts, osteocytes, adipocytes, and chondrocytes (Pittenger et al. Citation1999). Because mature chondrocytes are immotile cells that are buried in the matrix that they produce themselves, differentiation along the chondrogenic lineage probably follows a spatiotemporally stringent course (Shum and Nuckolls Citation2002). During the early stages of chondrogenic differentiation, MSCs give rise to motile precursor cells for chondrocytes, which must be recruited at least to the vicinity of the future cartilage. After arriving at the site of future cartilage, they differentiate into immotile chondrocytes that produce cartilage matrix. We also found that the use of collagen in the cartilage defect apparently caused an increase in proliferating cells () by enhancing cell migration (), and a considerable proportion of the recruited cells are MSCs that are undergoing selfrenewal mitosis ().
Apoptosis is a form of regulated cell death under stringent genetic control. Apart from the regulation of physiological cell turnover (Morioka et al. Citation1998), it has been well established that apoptosis reflects disengagement from the path of differentiation (Wong et al. Citation1999). Our study demonstrates that irrespective of collagen usage, the MSC-derived progenitor cells undergo apoptotic disengagement from the chondrocytic lineage ( and ). Thus, the use of type-I collagen may not affect the chondrocytic differentiation.
In order to enhance the process of repair of cartilage injury, a wide variety of signaling molecules have been used to promote proliferation and/or differentiation of MSCs. Using an experimental setup similar to ours, Hiraki et al. (Citation2001) proposed that the poor repair of large full-thickness defects is due to insufficient concentrations of fibroblast growth factor-2 (FGF-2). Recently, Chuma et al. (Citation2004) reported that only 1 day of exposure to FGF-2 (residual ratio of 50% within 30 min) led to cartilage resurfacing instead of the long-advocated 2 weeks of administration of FGF-2. These authors concluded that there was promotion of cartilage repair because FGF-2 enhanced recruitment of MSCs. The present study has, however, demonstrated that proliferating cells were abundantly recruited by the collagen without any use of exogenous FGF-2 (, asterisk), suggesting that the use of collagen may substitute for FGF-2 in cell recruitment.
We found that the use of collagen enhances cell migration () and increases proliferation of cells (), a considerable proportion of which are MSCs undergoing self-renewal mitosis (). However, use of collagen does not affect chondrocytic differentiation, and the MSC-derived progenitor cells undergo apoptotic disengagement from the chondrocytic lineage. Taken together, phase-specific usage of the growth factors may be advocated for better regeneration of damaged cartilage. Our findings suggest that in addition to the collagen that enhances initial recruitment of MSCs, agent(s) that sustain the MSC-derived progenitor cells along the chondrogenic linage are also required.
We gratefully acknowledge Yoko Uratani for her technical assistance and we thank Takefumi Yamamoto (Central Research Laboratory, Shiga University of Medical Science) for technical support regarding histological examination. This work was supported by grants from the Japanese Health Foundation for Prevention of Chronic Diseases and the Improvement of Quality of Life of Patients.
Contributions of authors
YM: supervised the whole study. SI: supervised the whole study and proofread the manuscript. MF: gave technical advice regarding histological examination. EI, KA, and TM: performed the vitro study.
- Apte S S. Ki-67 monoclonal antibody (MAb) reacts with a proliferation associated nucler antigen in the rabbit Oryctolagus cuniculus. Histochemistory 1990; 94: 201–4
- Breinan H A, Martin S D, Hsu H-P, Spector M. Healing of canine articular cartilage defects treated with microfracture, a Type-II ollagen matrix, or cultured autologous chondrocytes. J Orthop Res 2000; 18: 781–9
- Buma P, Pieper J S, van Tienen T, van Susante J L C, van der Kraan P M, Veerkamp J H, van den Berg W B, Veth R P H, van Kuppevelt T H. Cross-linked type-I and type-II collagenous matrices for the repair of full-thickness articular cartilage defects-A study in rabbits. Biomaterials 2003; 24: 3255–63
- Caplan A I. Mesenchymal stem cells. J Orthop Res 1991; 9: 641–50
- Chuma H, Mizuta H, Kudo S, Takagi K, Hiraki Y. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cartilage 2004; 12: 834–42
- Djouad F, Bony C, Häupl T, Uzé G, Lahlou N, Louis-Plence P, Apparailly F, Canovas F, Rème T, Sany J, Jorgensen C, Noël D. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res Ther 2005; 7: R1304–15
- Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage 2005; 13(8)655–64
- Hickey D G, Frenkel S R, Di Cesare P E. Clinical applications of growth factors for articular cartilage repair. Am J Orthop 2003; 32: 70–6
- Hiraki Y, Shukunami C, Iyama K, Mizuta H. Differentiation of chondrogenic precursor cells during the regeneration of articular cartilage. Osteoarthritis Cartilage (Suppl A) 2001; 9: S102–8
- Huang J I, Kazmi N, Durbhakula M M, Hering T M, Yoo J U, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: A patient-matched comparison. J Orthop Res 2005; 23: 1383–9
- Hunter W. Of the structure and diseases of articular cartilages. Philosophical Tr. Roy. Soc. London 1743; 9: 267
- Hunziker E B, Driesang I M K, Morris E A. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop 2001, 391: 171–81
- Kim H K W, Moran M E, Salter R B. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg (Am) 1991; 73: 1301–15
- Miyaki K, Murakami K, Segami N, Iizuka T. Histological and immunohistochemical studies on the articular cartilage after experimental discectomy of the temporomandibular joint in rabbits. J Oral Rehabil 1994; 21: 299–310
- Morioka K, Tone S, Mukaida M, Takano-Ohmuro H. The apoptotic and non–apoptotic nature of the terminal differentiation of erythroid cells. Exp Cell Res 1998; 240: 206–17
- Nishimura K, Solchaga L A, Caplan A I, Yoo J U, Gorldberg V M, Johnstone B. Chondroprogenitor cells of synovial tissue. Arthritis Rheum 1999; 42: 2631–7
- Patel T, Gores G J, Kaufmann S H. The role of proteases during apoptosis. FASEB J 1996; 10: 587–97
- Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak R. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–7
- Sato K, Urist M R. Bone morphogenetic protein-induced cartilage development in tissue culture. Clin Orthop 1984, 183: 180–7
- Sellers R S, Zhang R, Glasson S S, Kim H D, Peluso D, D'augusta D A, Beckwith K, Morris E A. Repair of articular cartilage defects one year after treatment with recombunant human bone morphogenetic protein-2 (rhBMP-2). J Bone Joint Surg (Am) 2000; 82: 151–60
- Shapiro F, Koide S, Glimcher M J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg (Am) 1993; 75: 532–53
- Shum L, Nuckolls G. The life cycle of chondrocytes in the developing skeleton. Arthritis Res 2002; 4: 94–106
- Speer D P, Chvapil M, Volz R G, Holmes M D. Enhancement of healing in osteochondral defects by collagen sponge implants. Clin Orthop 1979, 144: 326–35
- Yoo J U, Barthel T S, Nishimura K, Solchaga L, Caplan I, Goldberg V M, Johnstone B. The chondrogenic potential of human bone-marrow-ferived mesenchymal progenitor cells. J Bone Joint Surg (Am) 1998; 80: 1745–57
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho A D. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005; 33: 1402–16
- Wakintani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, Owaki H, Ono K. Repair of rabbit articulr surface with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg (Br) 1989; 71: 74–80
- Wakitani S, Goto T, Pineda S J, Young R G, Mansour J M, Caplan A I, Goldberg V M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg (Am) 1994; 76: 579–92
- Wakitani S, Goto T, Young R G, Mansour J M, Goldberg V M, Caplan A I. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng 1998; 4: 429–44
- Wong B C Y, Zhu G H, Lam S K. Aspirin induced apoptosis in gastric cancer cells. Biomed Pharmacother 1999; 53: 315–8
