Abstract
Background and purpose Extracorporeal shock wave treatment has been used successfully for the treatment of avascular necrosis of the femoral head. The pathway of biological events by which this is accomplished has not been fully elucidated. BMP-2 is a key mediator of bone development and repair, and is uniquely required for bone formation. We therefore examined the effect of extracorporeal shock waves on induction of BMP-2 in necrotic femoral heads.
Methods Femoral head necrosis was induced in rabbits by treatment with methylprednisolone and lipopolysaccharide. BMP-2 expression was assessed by immunohistochemistry, quantitative real-time PCR, and western blot analysis. Histomorphometric analysis was performed to evaluate the rate of bone formation in femoral heads treated with extracorporeal shock waves.
Results Extracorporeal shock wave treatment was associated with a significant increase in expression of BMP-2 protein and mRNA in the subchondral bone of the necrotic femoral heads. Moreover, the most intensive immunostaining of BMP-2 was observed in the prolifer-ative zone above the necrotic zone. Histomorphometric analysis showed that extracorporeal shock wave treatment significantly increased the rate of bone formation in the subchondral bone.
Interpretation We conclude that extracorporeal shock wave treatment significantly upregulates the expression of BMP-2 in necrotic femoral heads. The process of repair of femoral head necrosis is accelerated by the use of extracorporeal shock waves.
Avascular femoral head necrosis is characterized by death of cells in the cancellous bone of the femoral head. It often leads to collapse of the load-bearing segment of the femoral head, and eventually to severe osteoarthrosis (Schwarz Lausten Citation1999). Different surgical methods have been used to prevent collapse, such as both vascularized and nonvascularized bone grafting procedures, cementation, muscle pedicle grafting, and rotational osteotomy. Most treatment methods have shown limited effects in selected series, and none have shown universal success (Wood et al. Citation2003, Zhang et al. Citation2005). Accordingly, the development of an effective and noninvasive treatment would be of value.
Extracorporeal shock waves are noninvasive acoustic waves. These have recently been shown to promote bone repair (Schaden et al. Citation2001). Applied to bone, shock waves delivered with the appropriate energy and number of pulses can stimulate osteogenesis and angiogenesis (Wang et al. Citation2003, Ma et al. Citation2007). In Europe, shock waves are used to treat nonunions in humans (McClure et al. Citation2004). Recently, it was reported that extracorporeal shock wave treatment appeared to be more effective than core decompression and nonvascularized fibular grafting in patients with early-stage osteonecro-sis of the femoral head, although the mechanism by which shock wave treatment results in clinical improvement remains unknown (Wang et al. Citation2005).
BMP-2 is a key mediator of bone development and repair, and is uniquely required for bone formation (Li et al. Citation2007). Genetic manipulation of transgenic mice has highlighted the importance of controlling the level of BMP-2 signaling during bone formation (Zhao et al. Citation2002). It would be advantageous to be able to stimulate endogenous tissues in a noninvasive way, to produce sufficient quantities of BMP–2 to locally effect or accelerate in situ healing of skeletal deficiencies. One approach to accomplishing this may be the use of extracorporeal shock wave treatment in vivo. We examined the effect of extracorporeal shock wave treatment on induction of BMP–2 expression in necrotic femoral heads.
Material and methods
Experimental design
Our experimental study was approved by the local Animal Care and Use Committee (approval number 2005N000658). Using methylpredniso-lone and lipopolysaccharide, we induced bilateral femoral head necrosis in New Zealand White rabbits (n = 36, average weight 3.4 kg) according to the method of Yamamoto et al. (Citation1995). Shock wave therapy was applied to the femoral head of the left limb 6 weeks after injection of methylprednisolone. The right limb served as control, and received no shock wave therapy. On the left side, the femoral head received 2,000 shock wave impulses at 0.26 mJ/mm2 using Orthospec equipment (MEDISPEC, Germantown, MD). The dosage of shock waves was based on the results of previous studies in animals (Wang et al. Citation2004). The treatment was applied to the femoral head for 20 min. The rabbits were sedated with intramuscular pentobarbital (30 mg/ kg) while receiving the shock waves. The animals were killed with an overdose of pentobarbital at 24 h (n = 6) and 1 (n = 6), 2 (n = 6), 4 (n = 6), 8 (n = 6), and 12 (n = 6) weeks after shock wave therapy. The femoral heads were harvested and freed of soft tissue and cartilage. They were bisected along both the coronal and sagittal plane. Then, the 4 parts of the femoral head were used for histomorphometry, immunohistochemistry, western blot analysis, and quantitative real-time reverse transcriptase PCR. The histological sections were cut in the coronal plane and stained with hematoxylin and eosin.
Rabbit model of avascular necrosis of the femoral head
As atated above, avascular necrosis of the femoral head was induced with methylprednisolone and lipopolysaccharide according to Yamamoto et al. (Citation1995). The rabbits were injected with 10 |ig of lipopolysaccharide per kg, twice intravenously at an interval of 24 h, and after the second injection of lipopolysaccharide they were given 40 mg of methylprednisolone per kg, 3 times intramuscularly at intervals of 24 h. Avascular necrosis of the femoral head occurs 6 weeks after the injection of lipopolysaccharide and methylprednisolone. Histologically, the femoral head was considered necrotic when at least one of the following 3 signs was present: bone marrow cell necrosis, empty osteocyte lacunae, or any evidence of repair—such as the presence of granulation tissue, fibrosis, or appositional bone formation. The necrotic area of the femoral head was examined with the semiautomatic Simple PCI image analyzer (Compix, 2-letter code for US state) and the rate of necrosis (necrotic area/total area examined) was calculated on the coronal section at the maximal width (Yamamoto et al. Citation1995). In this study, subchondral insufficiency fracture of the femoral head was not found in any of the femoral heads. So the femoral head necrosis was in its early stages (stages I, II) and the necrotic rate of the femoral heads was 45% ().
Immunohistochemistry
For immunohistochemistry, the region studied in each specimen was the superficial subchondral bone from the weight-bearing surface, 1–5 mm deep to the cartilage surface. The specimens were fixed in 4% buffered formalin, decalcified using 10% EDTA, embedded in paraffin, and cut into 5-|im-thick sections in the coronal plane. These sections were dewaxed in xylene and rehydrated in a graded series of ethanol. Trypsin digestion was performed by immersing sections in 0.1% trypsin (Sigma) with 0.1% CaCl2 in PBS for 15 min at 37°C, and they were washed with deionized water. Endogenous peroxidase activity was deactivated by immersing sections in 3% hydrogen peroxide in 0.01M phosphate-buffered saline (PBS) for 10 min at 37°C. After incubation in 10% bovine serum for 30 min, sections were incubated with a mouse anti-BMP-2 monoclonal antibody (R&D Systems, Minneapolis, MN) overnight at 4°C. Sections were further incubated with biotinylated anti-mouse secondary antibody and streptavidin peroxidase for 30 min at 37°C. Immunoreactivity was determined by incubating the sections in a chromogen solution containing diaminobenzidine (DAB) and 0.1% hydrogen peroxide in the dark. The sections were counterstained slightly with hematoxylin. Negative-control sections were processed using steps similar to those described above, but they were incubated with PBS only or normal nonimmune mouse IgG.
Total RNA extraction and real-time quantitative polymerase chain reaction (qPCR)
The subchondral bone samples were harvested by dissection clean of surrounding tissue, quick-frozen in liquid nitrogen, and stored at −80°C for later protein and mRNA extraction. Total RNA was extracted using Trizol reagent (Life Technologies, Grand Island, NY) and precipitated in ethanol. Purity and concentration were assessed by agarose gel electrophoresis and UV spectrophotometry.
Reverse transcription followed by quantitative real-time PCR was performed as described previously (Niikura et al. Citation2006). TaqMan primer and probe pairs for the rabbit BMP-2 gene, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control, were based on database sequences (Genbank accession numbers NM001082650.1 and DQ403051.1, respectively) and were designed using PrimerExpress software (Applied Biosystems, Foster City, CA). The probe sequences were as follows. Rabbit BMP-2: CAACTCCGTGAACTCTAAGATTCC-GAAGGCAT; rabbit GAPDH: GGTCGTG-GACCTGACCTG. The primer sequences were as follows. Rabbit BMP-2, forward: CCATGC-CATCGTGCAGACTT; reverse: TGGAGATAG-CACTGAGTTCTGTTG; rabbit GAPDH, forward: AAACTCACTGGCATGGCCTT; reverse: TTAGCAGCTTTCTCCAGGCG. Real-time quantitative PCR was done in triplicate with the ABI 7300 Sequence Detector (Applied Biosystems) following recommended protocols. Results were normalized to GAPDH levels using the formula ΔCt (threshold cycle) = Ct of target gene − Ct of GAPDH. The comparative Ct method was used to investigate the amount of target gene relative to a calibrator. Of the 36 animals, an arbitrary animal was selected as calibrator. The ΔΔCt value was calculated as follows: ΔΔCt of an animal = ΔCt of an animal − ΔCt of the calibrator. The amount of target gene was given by the formula 2−ΔΔCt. The fold change between shock wave-treated femoral head and untreated control was calculated as the value of shock wave-treated femoral head divided by the value of untreated control at each time point.
Western blot analysis
For western blot, proteins were extracted from the subchondral bone samples in a radioimmuno-precipitation (RIPA) buffer (8 times the sample volume) containing a protease inhibitor cocktail (Protease Inhibitor Cocktail Set I; Calbiochem, Darmstadt, Germany) at 4°C overnight. The proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 10% gels). A human recombinant BMP-2 (32-kDa; Wyeth, Madison, NJ) and SeeBlue Pre-stained Protein Standard (Invitrogen) were also loaded as positive control and as molecular weight markers, respectively. Proteins were transferred to a PVDF membrane (Invitrogen), blocked with 2% bovine serum albumin (BSA), and exposed to a 1:100 dilution of the mouse anti-BMP-2 monoclonal antibody used for immunohistochemistry (R&D Systems, Minneapolis, MN) in 1% BSA overnight. After washing in 0.1% Tween in PBS, the membrane was incubated in goat anti-mouse IgG horseradish peroxidase-linked antibody (1:5,000) for 1 h at room temperature. Signals were detected by chemoluminescence reaction (ECL kit; Amersham Biosciences, Piscataway, NJ). Western blots to detect β-actin were performed on the same membrane after a stripping procedure. Samples from each animal were repeated twice, and the results were found to be the same.
Deposition of new bone
To evaluate the rate of deposition of new bone, all rabbits were given intramuscular injections of tet-racycline hydrochloride (30 mg per kg; Sigma) on days 7 and 2 before killing. The tetracycline hydro-chloride was used as fluorochrome bone marker for histomorphometry. The specimens were fixed in 70% ethanol solution at 4°C for 2 days, run through the alcohol series for dehydration, embedded in methylmetacrylate, and cut into 15-μm slices parallel to the coronal plane from the center of the specimen. The 15-μm slices were examined under a fluorescent microscope for tetracycline labeling. The mean tetracycline double-labeling interval was determined with a Simple PCI image analyzer (Compix, 2-letter code for US state), the interval was divided by the administration time interval (i.e. 5 days), and the mean value showed the rate of deposition of new bone per day.
Statistics
The values for shock wave-treated femoral head and untreated femoral head were compared at each time point. Statistical differences between multiple groups were determined by ANOVA. Student-Neuman-Keuls test was used to determine significance between 2 groups. A p-value of < 0.05 was considered statistically significant.
Results
Immunohistochemical analysis
In the subchondral bone of the necrotic femoral heads that were not treated with shock waves, a slight immunoreactivity against BMP-2 (BMP-2-IR) was found in the osteoblasts and the fibroblastic cells (). BMP-2-IR was absent when the primary antibody was replaced with PBS or non-immune mouse serum, indicating specific binding of the anti-BMP-2 antibody (.
Figure 1. Photomicrographs of subchondral bone immu-nostained with anti-BMP-2. A. Subchondral bone of the necrotic femoral heads untreated with shock waves: slight BMP-2 immunoreactivity (BMP-2-IR) was found in osteo-blasts and fibroblastic cells. B. 4 weeks after application of shock waves, the osteoblasts and the fibroblastic cells showed an increased level of BMP-2-IR. C. 8 weeks after shock wave application, BMP-2-IR was increased in the fibroblastic cells of the vascular granulation tissue invading the necrotic bone. Bone matrix also showed BMP-2-IR. D. 12 weeks after shock wave application, BMP-2-IR was observed in the trabecular bone and the marrow. Osteoblasts adjacent to the newly formed woven bone showed intensive expression of BMP-2. E. BMP-2-IR was absent when the primary antibody was replaced with PBS or non-immune mouse serum. (All images ×200).
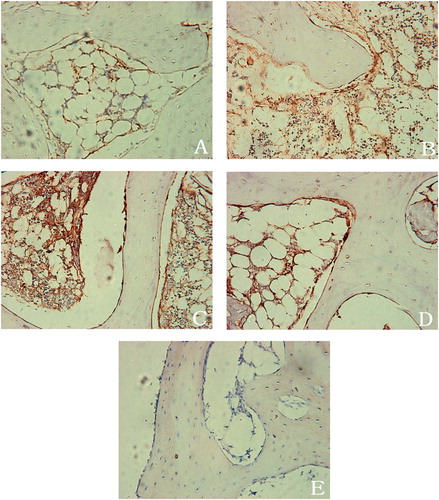
2 and 4 weeks after shock wave therapy, the osteoblasts and the fibroblastic cells showed an increased level of BMP-2-IR (). At 8 weeks, BMP-2-IR was increased in the fibroblastic cells of the vascular granulation tissue invading the necrotic bone. Bone matrix also showed BMP-2-IR (). At 12 weeks, BMP-2-IR was observed in the trabecular bone and the marrow. Osteoblasts adjacent to the newly formed woven bone showed intense expression of BMP-2 ().
BMP-2 mRNA expression
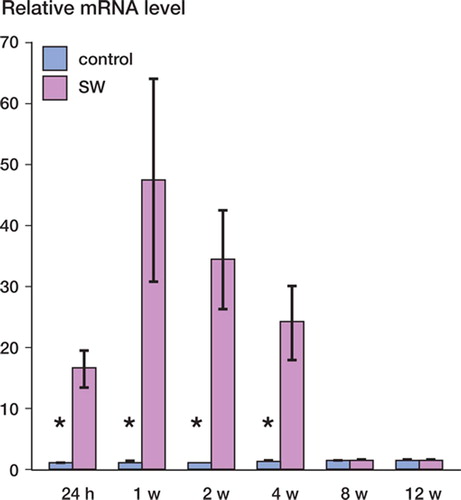
The gene expression of BMP-2 was markedly higher in shock wave-treated femoral heads than in untreated femoral heads throughout the time series. Statistically significant differences were observed at 24 h and at 1, 2, and 4 weeks after shock wave treatment. The maximum fold change was 47 at 1 week after shock wave treatment ().
Figure 2. Gene expression of BMP-2 in shock wave-treated femoral heads (red bars) and in untreated femoral heads (blue bars) at 24 h and 1, 2, 4, 8, and 12 weeks after the application of shockwave treatment, as determined by realtime quantitative RT-PCR.The graph demonstrates the fold change (and range) for each experimental group when the untreated femoral head of 24 h was normalized to 1. The asterisks indicate statistically significant differences between shock wave-treated femoral heads and untreated femoral heads at the indicated time point. SW: shock wave-treated femoral head; control: untreated femoral head.
BMP-2 protein expression
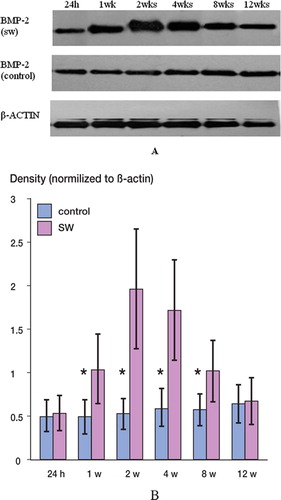
The human recombinant BMP-2 gave a protein band migrating at 32 kDa. The proteins extracted from the subchondral bone of the untreated control and shock wave-treated femoral heads also formed a single BMP-2-IR band migrating between 29 and 34 kDa (). An increase in both density and broadness of the band was consistently observed in the subchondral bone samples obtained from the shock wave-treated femoral heads at 1, 2, 4, and 8 weeks after the shock wave treatment, in comparison with the samples from the untreated femoral heads. In the samples obtained 24 h and 12 weeks after the shock wave treatment, there was no difference between the results from the treated femoral heads and the untreated femoral heads.
Figure 3. A. Western blot analysis of subchondral bone samples obtained from necrotic femoral heads at 24 h and 1, 2, 4, 8, and 12 weeks after the application of shock-wave treatment. (Lanes 24h, 1wk, 2wks, 4wks, 8wks and 12wks represent 24 hours and 1, 2, 4, 8, and 12 weeks, respectively). Western blot analysis consistently showed increased BMP-2 expression at 1, 2, 4, and 8 weeks compared to the samples from the untreated control femoral heads. B. Graph showing densitometric analysis of western blot, normalized to the density of β-actin in each lane. The asterisks indicate statistically significant differences between shock wave-treated femoral heads and untreated femoral heads at each time point. SW: shock wave-treated femoral head. Control: untreated femoral head.
Rate of deposition of new bone
The tetracycline double-labeling interval showed calcium deposition of the femoral heads. Since BMP-2 is known to increase bone formation, the rate of calcification was measured in the subchondral bone of femoral heads treated with shock waves. The rate of calcification was markedly higher in the shock wave-treated femoral heads. Compared to untreated femoral heads through the different time points, statistically significant differences were seen at 2, 4, 8, and 12 weeks after shock wave treatment ().
Figure 4. A. Under epifluorescent light, the tetracy-cline labels in shock wave-treated femoral heads were intense and the distances between two lines were wide (×100). B. Under epifluorescence, the tetracycline labels in untreated femoral head were weak and the distances between two lines were narrow (×100). C. Graph showing the calcification deposition rate of shock wave-treated femoral heads (red bars) and untreated femoral heads (blue bars). The asterisks indicate statistically significant differences between shock wave-treated femoral heads and untreated femoral heads at each time point. SW: shock wave-treated femoral head; control: untreated femoral head.
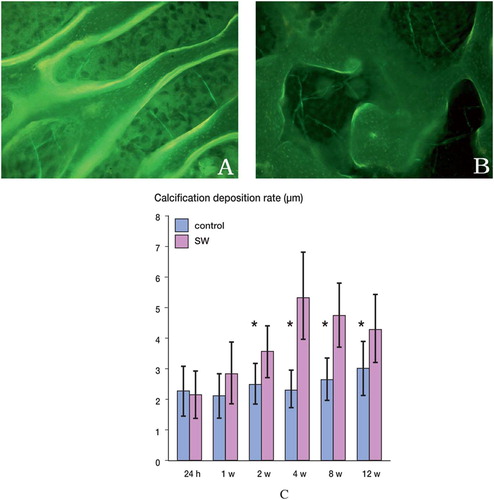
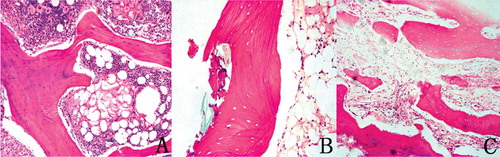
Figure 5. Histological findings in the femoral head following injection of methylprednisolone and lipopolysaccharide in rabbits. Mid-coronal section from rabbit femoral head 6 weeks after the injection, stained with hematoxylin-eosin. A. Marrow necrosis (x100). B. Trabecular necrosis (x200). C. Trabecular necrosis and dense medullary fibrosis (x100).
Discussion
In treating avascular femoral head necrosis, the high reported success rate of shock wave treatment makes it attractive (Wang et al. Citation2005). However, the precise mechanism by which shock wave treatment results in clinical improvement is unknown, and little has been done to define the role of BMP-2 expression in the process of repair of femoral head necrosis. Extracorporeal shock waves are acoustic waves of extremely high pressure and velocity. Shock waves can travel through fluid and soft tissue, and their effects occur at sites where there is a change in impedance, such as the bone-soft tissue interface. When the shock wave meets an interface of different impedance, pressure and shear forces occur (McClure et al. Citation2004). When shock waves are directed at bone, multiple interfaces of cancel-lous bone between soft tissue and bone result in reflection and deposition of shock wave energy. Because the energy is rapidly deposited at the multiple acoustic interfaces, disruption of cancellous bone occurs and a secondary osteoblastic healing response has been reported (Delius et al. Citation1995).
Promotion of new bone formation has been observed in patients with posttraumatic pseudar-throsis after extracorporeal shock wave therapy; this has led some authors to hope that this therapy might also stimulate the formation of new bone in patients with avascular femoral head necrosis (Ludwig et al. Citation2001). Then studies were performed to test the efficacy of shock wave therapy in patients with femoral head necrosis. The results of these studies were encouraging, and significant clinical improvement was achieved (Ludwig et al. Citation2001, Wang et al. Citation2005). In our study, we found that extracorporeal shock wave treatment significantly increased the rate of calcification of the sub-chondral bone, which may result in a cascade of bone regeneration and the repair of avascular femoral head necrosis. Our findings provide further evidence that physical shock wave treatment may be an effective noninvasive method for treatment of avascular necrosis.
BMP-2 expression has been detected in normal bone and in human osteosarcoma (Celeste et al. Citation1990, Yoshikawa et al. Citation1994). The significance of BMP-2 lies in its capacity to mobilize osteopro-genitor cells, thereby promoting osteoblastic differentiation processes and resulting in bone formation (Harris et al. Citation1994). We found that expression of BMP-2 mRNA was upregulated as early as 24 h after shock wave treatment of necrotic femoral heads, and that the upregulation was maintained 2 and 4 weeks after treatment. In concert with the increase in mRNA, levels of BMP-2 protein showed a significant increase at 1, 2,4, and 8 weeks after shock wave treatment. Immunohistochemical analysis showed intensive BMP-2 expression in fibroblastic cells and osteoblasts adjacent to the newly formed bone during the first 2 and 4 weeks after treatment. It is well established that BMP-2 has a central role in initiating and regulating bone formation (Zhao et al. Citation2002). We found a significant increase in the rate of calcification in the sub-chondral bone of the treated femoral heads. It is possible that BMP-2 acts as a key regulator that promotes bone formation and repair of necrotic subchondral bone.
Our findings suggest that noninvasive physical shock wave treatment can cause local upregulation of BMP-2 expression. Since shock wave treatment is safe, noninvasive, and repeatable, it provides a useful alternative to surgery for treatment of avas-cular femoral head necrosis.
This work was supported by a grant from the National Natural Science Foundation of China (30571898).
No competing interests declared.
Contributions of authors
BFZ, HZM, and XLL designed the study. HZM, BFZ, and YMC performed the experiments and collected and analyzed the data. HZM wrote the draft manuscript. BFZ and XLL revised the manuscript.
- Celeste A J, Iannazzi J A, Taylor R C, Hewick R M, Rosen V, Wang E A, Wozney J M. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA 1990; 87(24)9843–7
- Delius M, Draenert K, Al Diek Y, Draenert Y. Biological effects of shock waves: in vivo effect of high energy pulses on rabbit bone. Ultrasound Med Biol 1995; 21(9)1219–25
- Harris S E, Sabatini M, Harris M A, Feng J Q, Wozney J, Mundy G R. Expression of bone morphogenetic protein messenger RNA in prolonged cultures of fetal rat calvar-ial cells. J Bone Miner Res 1994; 9(3)389–94
- Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun 2007; 356(4)836–42
- Ludwig J, Lauber S, Lauber H J, Dreisilker U, Raedel R, Hotzinger H. High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop 2001, 387: 119–26
- Ma H Z, Zeng B F, Li X L. Upregulation of VEGF in sub-chondral bone of necrotic femoral heads in rabbits with use of extracorporeal shock waves. Calcif Tissue Int 2007; 81(2)124–31
- McClure S R, Van Sickle D, White M R. Effects of extra-corporeal shock wave therapy on bone. Vet Surg 2004; 33(1)40–8
- Niikura T, Hak D J, Reddi A H. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res 2006; 24(7)1463–71
- Schaden W, Fischer A, Sailler A. Extracorporeal shock wave therapy of nonunion or delayed osseous union. Clin Orthop 2001, 387: 90–4
- Schwarz Lausten G. Non-traumatic necrosis of the femoral head. Int Orthop 1999; 23(4)193–7
- Wang F S, Yang K D, Kuo Y R, Wang C J, Sheen-Chen S M, Huang H C, Chen Y J. Temporal and spatial expression of bone morphogenetic proteins in extracorporeal shock wave-promoted healing of segmental defect. Bone 2003; 32(4)387–96
- Wang C J, Yang K D, Wang F S, Hsu C C, Chen H H. Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone 2004; 34(1)225–30
- Wang C J, Wang F S, Huang C C, Yang K D, Weng L H, Huang H Y. Treatment for osteonecrosis of the femoral head: comparison of extracorporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg (Am) 2005; 87(11)2380–7
- Wood M L, McDowell C M, Kelley S S. Cementation for femoral head osteonecrosis: a preliminary clinic study. Clin Orthop 2003, 412: 94–102
- Yamamoto T, Hirano K, Tsutsui H, Sugioka Y, Sueishi K. Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Shwartzman reaction. Clin Orthop 1995; 316: 235–43
- Yoshikawa H, Rettig W J, Takaoka K, Alderman E, Rup B, Rosen V, Wozney J M, Lane J M, Huvos A G, Garin-Chesa P. Expression of bone morphogenetic proteins in human osteosarcoma. Immunohistochemical detection with monoclonal antibody. Cancer 1994; 73(1)85–91
- Zhang C, Zeng B, Xu Z, Song W, Shao L, Jing D, Sui S. Treatment of femoral head necrosis with free vascularized fibula grafting: a preliminary report. Microsurgery 2005; 25(4)305–9
- Zhao M, Harris S E, Horn D, Geng Z, Nishimura R, Mundy G R, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol 2002; 157(6)1049–60
