Abstract
Background and purpose Neurotization of denervated muscles has been shown to improve muscle bulk, but the neuronal regeneration response has not been compared previously in different surgical techniques of neurotization. Thus, using a rat model of experimental skeletal muscle denervation, we studied neuronal regeneration following sensory neurotization by two methods: sensory nerve to motor branch of muscle and direct sensory nerve implantation to muscle.
Material and methods The lateral head of the gas-trocnemius muscle was denervated in 36 rats, of which the first 12 served as denervated controls. In the second group of 12, the sural nerve was anastomozed to the motor branch of the gastrocnemius muscle (sensory-to-motor nerve neurotization) and in the remaining 12 rats the sural nerve was split into 4 fascicles and embedded into 4 quadrants of the muscle (direct sensory nerve-to-muscle neurotization). Immunohistochemistry was used to examine nerve fibers in muscle containing the sensory neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP), and general neuronal marker protein gene product 9.5 (PGP 9.5).
Results Semiquantitative analysis showed that, compared to the control side, the number of nerve fibers on the experimental side was highest (p < 0.01) for group III (direct sensory nerve-to-muscle neurotization) for all 3 markers. The difference was 71%, 298%, and 254% for PGP 9.5, CGRP, and SP, respectively.
Interpretation This method may be a good option for inducing neuronal regeneration in denervated muscles, and has therapeutic implications for prevention of atrophy of denervated muscles and as an adjunct for reconstruction of soft tissue defects.
Neurotization was initially reported at the beginning of the twentieth century, during World War I, when injuries and poliomyelitis resulted in limb paralysis for which no cure was available (Mayer Citation1950). Application of this technique was limited because of a high failure rate. However, interest in this technique was rekindled in the middle of the twentieth century when good results were reported (Guth and Zalewski Citation1963).
In addition to conventional functions such as nociception and vasoregulation, the peripheral nervous system is also implicated in tissue repair and regeneration. Specifically, various neurotransmit-ters such as substance P (SP) and calcitonin gene-related peptide (CGRP) have been implicated in cell proliferation, neovascularisation, and organo-genesis (Haegerstrand et al. Citation1990, Fan et al. Citation1993, Dietz et al. Citation1996). Presumably sensory, motor, and autonomic neurotransmitters also participate in regeneration of soft tissue (nerve, muscle, and skin) following injury.
Clinically, irreparable nerve trauma is not uncommon, e.g. in segmental nerve loss, nerve root avulsion injuries, and trophic ulcers over pressure areas due to peripheral neuropathy. Hypotheti-cally, in such situations, if neuronal input from an alternative, intact spinal segment is provided to the distal portion of the injured nerve or end-organ, re-innervation of the end-organ may occur if appropriate microenvironmental conditions for nerve regeneration are available.
As for skeletal muscle, denervation due to nerve injury or surgical division (e.g. for free muscle transfer) leads to muscle atrophy, as evidenced by a reduction in the number and size of muscle fibers, with preservation of residual connective tissue. In such situations, provision of the nerve supply may prevent muscle atrophy. Notably, neurotrophic factors have been shown to modulate muscle maturation and development (Ecob Citation1983, Kakulas and Adams Citation1985).
In this project, we studied the response of neu-ronal regeneration in denervated skeletal muscles following sensory neurotization of muscle by either sensory nerve to motor branch of muscle, or by direct sensory nerve implantation to muscle.
Material and methods
Animal experiments
36 male Sprague Dawley rats (weight ∼ 300 g) were allocated into three groups of 12 rats each. Animal experiments were performed after institutional approval from the University Research Council, according to guidelines from the Ethics Committee for Research on Animals (ECRA) at Aga Khan University, Karachi. Under anesthesia with Ketamine (30 mg/kg intraperitoneally), the lateral head of the gastrocnemius muscle (GM) in the right distal thigh was exposed through a dorsal incision. The motor branch to the lateral head of the GM (branch of the tibial nerve) and the sural nerve were identified.
In group I (the denervation control group), a 7–8-mm segment of the motor branch was excised and the proximal end was ligated.
In group II (sensory-to-motor neurotization), a 7–8-mm segment of the motor branch was excised with ligation of the proximal end. The distal stump of this motor branch to the GM was anastomozed with the proximal stump of the sural nerve using a 10/0 prolene suture under 4.5× loupe magnification.
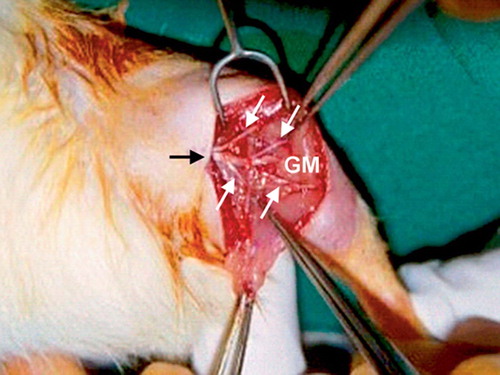
In group III (direct sensory nerve-to-muscle neurotization), a 7–8-mm segment of the motor branch was excised and the proximal end was ligated. The sural nerve was split into 4 fascicles and embedded into 4 arbitrarily divided quadrants of the lateral head of the GM using a 10/0 prolene suture under 4.5× loupe magnification ().
Figure 1. Intraoperative photograph of the right leg of a rat from group III showing the sural nerve (black arrow) split into 4 fascicles (white arrows) and embedded into 4 arbitrarily divided quadrants of the lateral head of the gastrocnemius muscle (GM).
In vivo perfusion-fixation of rats was performed under ketamine anesthesia after 12 weeks. This was done through the ascending aorta using Zamboni's solution (Zamboni and De Martino 1967), following 0.01 M phosphate buffered saline (PBS), pH 7.2. Both the right and left lateral heads of the GM were harvested in all groups. In group II the right lateral head was harvested along with the sural nerve, which was anastomozed to the motor branch of the GM. In group III, the sural nerve fascicles embedded in the GM were harvested along with the gastrocnemius muscles.
The harvested tissues were immersed in Zam-boni's solution for 24 h at 4°C, then transferred to 20% sucrose in 0.1 M Sörenson phosphate buffer, pH 7.2, containing sodium azide, for two days before embedding for immunohistochemical analysis.
Immunohistochemistry
Samples were embedded in Tissue-tek OCT compound (Miles Inc., Elkhart, IN). 12-um sections were cut on a cryostat (CM 3050 S; Leica Microsystems, Nussloch, Beirsheim, Germany), frozen sections were mounted directly on gelatin-coated (Merck, Darmstadt, Germany) glass slides (Super-Frost, Shandon, Germany) and stained by the indirect immunofluorescence technique using the avidin-biotin complex method. Sections were incubated with 10% normal goat serum in PBS, then with primary rabbit antibodies to SP (1:5,000), CGRP (1:5,000) (Peninsula Laboratories Europe Ltd., St. Helens, UK), or to a general neuronal marker of mature nerve fibers, protein gene-product (PGP) 9.5 (1:5,000) (Ultraclone, Cambridge, UK) overnight in a humid atmosphere at 4°C. Sections were then incubated at room temperature for 30 min with biotinylated polyclonal goat anti-rabbit antibodies (1:250) (Vector Laboratories Inc., Burlingham, CA), then with streptavidin-labeled fluorochrome Cy3 (1:5000) (Amersham Pharmacia Biotech Ltd., UK) followed by mounting. To demonstrate specificity, omission of primary and/ or secondary antibodies, and pre-adsorption of the primary antiserum with an excess of homologous antigen were done.
For microscopy, an epifluorescence microscope (Eclipse E800; Nikon, Yokohama, Japan) with a G-2A (EX-510–560) fluorescence filter, 20x objective, and DXM-1200 digital camera with the ACT-1 software supplied by Nikon were used.
Semiquantitative image analysis
Manual semiquantitative analysis of nerve fiber density on digital images was performed in a blinded manner; the raters were not aware of the group allocations. Care was taken to confirm examination of nerve fibers in the superficial and deep layers of muscles, avoiding the surrounding loose connective tissue. Mean nerve fiber density for each muscle was calculated by averaging the number of nerve fibers per high-power field (20×) from 3 fields each, in 2 sections from each sample.
Statistics
Median and 95% confidence intervals (CIs) were used as measures of central tendency and variation, respectively. The significance of differences between the experimental and control sides was tested using the Wilcoxon signed rank test, and between groups using the Mann-Whitney U test. A p-value of ≤ 0.05 was considered significant.
Results
Clinical and macroscopic findings
There were no intraoperative deaths. There was no self-mutilation and all wounds healed uneventfully. No signs of discomfort were observed. One rat each in groups I and II died during the 12-week postoperative period.
The muscles that were denervated (group I), appeared atrophic with thin bulk. They were pale and fibrotic, while muscles in group II (sensory to motor neurotization) had preservation of some bulk and color. The muscles with direct sensory nerve-to-muscle neurotization (group III) retained good bulk and appearance as compared to the other two groups.
Immunohistochemistry
By immunohistochemistry, nerve fibers that were immunoreactive to SP, CGRP, and PGP 9.5 were seen in muscle sections of specimens in all 3 groups ().
Figure 2. Fluorescence photomicrographs of sections from gastrocnemius muscles immunohistochemically stained for neuronal markers and labeled with fluorochrome Cy3. CGRP-positive nerve fibers in control (A) and experimental (B) sides in group I; PGP 9.5-positive nerve fibers in experimental side in group II (C), and SP-positive nerve fibers in experimental side in group III (D). Arrows indicate nerve fibers. Bar represents 50 μn.
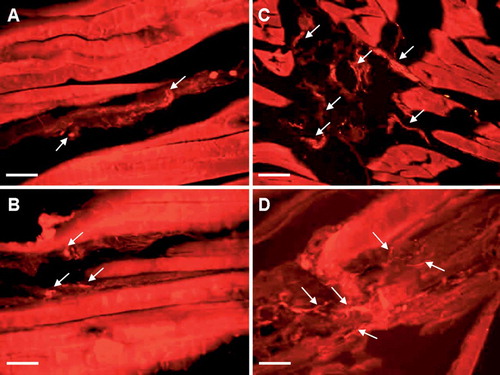
CGRP. These nerve fibers were identified both in superficial and deep layers of the muscle sections in all 3 groups. They were mainly present as thin, varicose nerve terminals in the muscle membranes, which were present around blood vessels in the deeper layers.
PGP 9.5. These were the most abundant nerve fibers of all three groups. They were seen in nerve bundles as well as in free nerve endings. Abundant fibers were seen as networks in the walls of blood vessels.
SP. These nerve fibers occurred mainly as thin, varicose, nonvascular nerve terminals in the muscle sections.
Semiquantitative image analysis of nerve fiber density ()
The number of positive nerve fibers on the experimental side compared to controls was highest for group III (direct sensory nerve-to-muscle neurotization) for all 3 markers (p < 0.01 for all). The difference was 71%, 298%, and 254% for PGP 9.5, CGRP, and SP, respectively.
For group II (where the sural nerve was anasto-mozed to the motor branch of the gastrocnemius muscle), a significant difference between the experimental and control sides was observed-though less marked than for group III.
In group I (denervation), there was no statistically significant difference between the control and experimental sides for SP, though a marginally significant increase was observed for PGP 9.5 and CGRP.
Notably, there was no statistically significant difference in nerve fiber density on the control side for the three groups, irrespective of the nerve marker used. This attests to the validity of the analyses.
Semiquantitative image analysis of digital microscopic images. Numbers indicate median number of positively stained nerve fibers per high-power (20×) field (95% confidence interval)
Discussion
We found that following nerve transection, maximal density of nerve fibers in muscles occurs after direct sensory nerve-to-muscle neurotization. Presumably, establishment of a source of sensory neuropeptides to the muscle is superior with this technique than with sensory nerve to motor nerve neurotization. Such re-innervation—owing to the trophic effects of locally released neuronal mediators—probably underlies the maintenance of muscle mass that we observed. It has been suggested that neurally released acetylcholine or of another yet unidentified neurohormone may act as a neuromuscular trophic agent, reducing muscle atrophy and fibrosis by suppressing muscle collagen biosynthesis and by reduction of lysosomal proteolysis (Zalewski Citation1970, Ip et al. Citation1977, Jakubiec-Puka and Drabikowski Citation1978, Frey Citation1979, Bresolin et al. Citation1984, Tischler et al. Citation1990, Jakubiec-Puka Citation1992, Virtanen et al. Citation1992).
As motor nerves are rarely purely motor, likewise, sensory nerves may not be purely sensory. Histologically, a cutaneous sensory nerve contains sympathetic fibers to sweat glands and efferents to the arrectores pilorum muscles. Moreover, studies have shown that sensory axons can also release acetylcholine (Falempin et al. Citation1989). Fusimotor neurons in extensively denervated muscles have been observed to sprout and innervate extrafusal fibers (Einsiedel et al. Citation1992). Thus, a sensory nerve, with some fibers resembling motor nerves, may be capable of preserving a degree of muscle bulk in a muscle flap with no other physical stimulation.
Generally, it is assumed that the motor nerve is solely responsible for contractility and trophic maintenance of muscle. Zalweski (Citation1970) showed that re-innervation by either sensory or sympathetic neurons could not preserve muscle mass or contractility. On the other hand, using a rat muscle transplant model, Dautel et al. (Citation1992) reported that sensory re-innervation retarded atrophy as significantly as motor re-innervation. Also using a rat model, Zhang and colleagues (Citation1997) showed experimentally that sensory re-innervation in transplanted muscle can preserve as much flap bulk as motor re-innervation. In addition, Chang et al. (Citation1986) reported development of sensation in muscle flaps re-innervated by local sensory nerves.
The rejuvenated interest in sensory re-innervation followed studies of muscle innervation that demonstrated that a motor nerve can contain 30% to 55% sensory fibers (Boyd and Smith Citation1984, Schaafsma et al. Citation1991). These sensory fibers are traditionally thought to innervate mainly the intra-fusal muscle spindles and Golgi tendon organs, and not the motor endplates. However, a previous histological study (Chang et al. Citation1986) on sensory re-innervation following denervation of muscle has shown that such sensory nerve fibers may form many net-like endings and branch in a stellate manner within the muscle, possibly even reaching motor endplates. Clinically, feet reconstructed with skin-grafted free-muscle transfers have been known to recover crude sensation in the flaps when the flap motor nerve has been neurotized with a local cutaneous sensory nerve (Chang et al. Citation1986).
In our experimental study, we simulated the clinical situation of muscle denervation and the scenario of sensory neurotization, both through sensory to motor neurorrhaphy and direct sensory nerve-to-muscle neurotization, and then evaluated nerve regeneration. Studies on the efficacy of this technique are still being carried out; thus, it is not a widely prescribed treatment yet (Keilhoff and Fansa Citation2005).
Brunelli and Monini (Citation1985) and Brunelli and Brunelli (Citation1998) addressed the question of whether new motor end-plates are generated following the ingrowth of axons into a denervated muscle. In rabbits, they grafted the motor branch of the pero-neal nerve into the "aneural" zone of the lateral head of the gastrocnemius muscle, where, under physiological conditions, no motor end-plates are detectable. 4 weeks postoperatively, when motor function had recovered, motor end-plates were found in histological sections (Brunelli and Monini Citation1985). Subsequently, they applied this technique to clinical cases with good results (Brunelli and Brunelli Citation1998). Earlier studies have reported return of function after neurotization of denervated muscles as well as histological evidence of new motor end-plate formation in aneural zones of the muscle (Brunelli Citation1989, Chiu et al. Citation1991). By implanting tibial nerve into the soleus muscle of rats proximally and distally, Payne and Brushart (Citation1997) showed that new motor end-plates can form in the region of axonal ingrowth into a denervated muscle. There has been experimental evidence showing that new end-plates do indeed form in the vicinity of growing axons (Korneliussen and Sommerschild Citation1976, Payne and Brushart Citation1997, Brunelli and Brunelli Citation1998). The increased sensitivity of denervated muscle to cholinesterase is one possible contributory factor to this phenomenon (Guth and Zalewski Citation1963). Zhang et al. (Citation1997) experimentally compared muscle weight following conventional nerve-to-nerve grafting in free muscle flaps and found no significant improvement in muscle weight. However, after direct nerve-to-muscle re-innervation (motor and sensory), there was a significant improvement in muscle weight. No functional or histochemical evaluation was performed (Zhang et al. Citation1997).
When the surgical technique of direct muscular neurotization was modified to ensure widespread distribution of motor axons throughout the denervated muscle, good motor function was restored in 10 clinical cases (Brunelli and Monini Citation1985, Brunelli Citation1989, Brunelli and Brunelli Citation1998). Sakel-larides et al. (Citation1972) achieved a highly consistent level of 60–75% of original muscle function in a dog by dividing the donor nerve into 2 or 3 fascicles prior to implantation. Becker et al. (Citation2002) reported on a series of 10 patients in whom the supplying motor nerve had been lost at the level of the neuromuscular junction, as a result of trauma or tumor resection. They used sural nerve grafts with a modified technique. The distal end of each segment was microsurgically dissected to separate the fascicles in a proximal direction, thus constituting additional sources of neurotization. After coaptation of the nerve grafts to the proximal nerve stump, the distal branches of the grafts were evenly distributed throughout the muscle tissue. Tunnels were created by blunt dissection along the axis of the muscle fibers, and the grafts were inserted far enough into these tunnels that all nerve stumps were located intramuscularly. This was done to simulate terminal branching of motor nerves entering skeletal muscle. The authors reported a mean motor recovery to M4 after a period of 1–2 years. This corroborates our findings, where higher levels of PGP 9.5, SP, and CGRP were seen in group III.
In clinical situations, where no distal nerve stump is available for neural coaptation and there is loss of the distal stump of a motor nerve or the nerve is avulsed from the muscle belly, intramuscular neurotization provides a good option for reconstruction. In certain cases, the nerve may still be present in the vicinity of the neuromuscular junction, although having undergone fibrotic degeneration as a result of partial avulsion. Reconstruction requires the ingrowth of axons into denervated muscle and formation of new neuromuscular junctions. Insertion of peripheral nerves into a skeletal muscle results in the re-establishment of a certain number of neuromuscular junctions, as demonstrated by several other authors (Saito and Zacks Citation1969, Brunelli and Monini Citation1985, McNamara et al. Citation1987, Mackinnon et al. Citation1993, Brunelli and Brunelli Citation1998, Park et al. Citation2000). In view of the anatomical structure of the neuromuscular junction with extensive branching of the nerve endings, our aim in group III was to establish a maximum number of neuromatous stumps within the muscle tissue. Thus, the sural nerve was divided into 4 fascicles and each was embedded into 1 of 4 quadrants of the GM. The achievement of the highest levels of the neuronal markers in this group thus validates the procedure. On the other hand, presence of the neuronal markers in the denervated group (group I), albeit at the lowest levels, indicates that collateral sprouting may play a role in muscle re-innervation in that group.
In addition to surgical technique, the final outcome in terms of function depends on a number of other factors, including the quality of the donor nerve, the age of the patient, the size of the nerve defect (regenerative distance), the quality and quantity of remaining muscle mass, and the time interval between trauma and reconstruction (Millesi Citation1987). Furthermore, because Schwann cell transplantation increases the number of neuromuscular junctions and thereby improves functional recovery of muscle, these cells may have potential as a cell therapy in addition to other nerve growth factors, to promote nerve regeneration and re-innervation of muscle (Fukuda et al. Citation2005).
In summary, direct insertion of nerves into muscle appears to be a reliable reconstructive option for denervated muscles. For optimal outcome, certain preconditions need to be met, including short regeneration distance (permitting direct implantation of the donor nerve into muscle), short period of denervation, healthy donor nerves, and a surgical technique that ensures a wide distribution of fibers in the muscle.
This study was supported by a grant from the University Research Council, Aga Khan University, Karachi (Project ID: 022F432XP). The authors wish to thank Mr Ayaz A. Qureshi for assistance with the animal experiments and initial immunohistochemistry set-up.
Contributions of authors
SN: study design, animal surgery, data analysis, interpretation of results, manuscript preparation. MA: study design, animal anesthesia and surgery, microscopic analysis, interpretation of results, manuscript preparation. RR: care of animals, tissue processing, microscopic analysis, compilation of results. TA: study design, data analysis, manuscript preparation. PH: study design, animal surgery, interpretation of results, manuscript preparation.
- Becker M, Lassner F, Fansa H, Mawrin C, Pallua N. Refinements in nerve to muscle neurotization. Muslce Nerve 2002; 26(3)362–6
- Boyd I A, Smith R S. The muscle spindle. Peripheral neuropathy2. Saunders, Philadelphia 1984; 171
- Bresolin N, Freddo L, Tegazzin V, Bet L, Armani M, Angelini C. Carnitine and acyltransferase in experimental neu-rogenic atrophies: changes with treatment. J Neurol 1984; 231: 170–5
- Brunelli G. Direct muscular neurotization. Ann Chir Main 1989; 8: 324–8
- Brunelli G, Monini L. Direct muscular neurotization. J Hand Surg (Am) 1985; 10: 993–7
- Brunelli G A, Brunelli G R. Direct muscle neurotization. Management of peripheral nerve problems, A L van Beek. Saunders, Philadelphia 1998; 393–7
- Chang K N, DeArmond S J, Buncke H J, Jr. Sensory reinner-vation in microsurgical reconstruction of the heel. Plast Reconstr Surg 1986; 78: 652–64
- Chiu D T, Chen L, Spielholtz N, Beasley R W. A comparative electrophysiological study on neurotisation in rats. J Hand Surg (Br) 1991; 16: 505–10
- Datuel G, Lineaweaver W C, Campagne-Pinto D, Buncke H J. Preservation of transplanted muscle mass by motor and sensory reinnervation. Transactions of the third Vienna muscle symposium, G Freilinger, M Deutinger. Blackwell-MZV, Vienna 1992; 96
- Dietz F R, Mukhopadhyay B, Becker G, Daniels K, Solursh M. Peripheral nerve extract effects on mesenchymal cells. Iowa Orthop J 1996; 16: 46–57
- Ecob M S. The application of organotypic nerve cultures to problems in neurology with special reference to their potential use in research into neuromuscular diseases. J Neurol Sci 1983; 58: 1–15
- Einsiedel L, Luff A R, Proske U. Sprouting of fusimotor neurones after partial denervation of the cat soleus muscle. Exp Brain Res 1992; 90: 369–74
- Falempin M, Ternaux J P, Palouzier B, Chamoin M C. Presence of cholinergic neurons in the vagal afferent system: involvement in a heterogenous reinnervation. J Auton Nerv Syst 1989; 28: 243–50
- Fan T P, Hu D E, Guard S, Gresham G A, Watling K J. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK1 or interleukin-1 receptor antagonists. Br J Pharmacol 1993; 110: 43–9
- Frey M. Trophic significance of sensory innervation of the skeletal muscles--an experimental study on the m. sterno-mastoideus of the rat. Handchirurgie 1979; 11: 181–4
- Fukuda A, Hirata H, Akeda K, et al. Enhanced reinnervation after neurotization with Schwann cell transplantation. Muscle Nerve 2005; 31: 229–34
- Guth L, Zalewski A A. Disposition of cholinesterase following implantation of nerve into innervated and denervated muscle. Exp Neurol 1963; 7: 316–26
- Haegerstrand A, Dalsgaard C J, Jonzon B, Larsson O, Nilsson J. Calcitonin gene-related peptide stimulates proliferation of human endothelial cells. Proc Natl Acad Sci U S A 1990; 87: 3299–303
- Ip M C, Vrbova G, Westbury D R. The sensory reinnervation of hind limb muscles of the cat following denervation and de-efferentation. Neuroscience 1977; 2: 423–34
- Jakubiec-Puka A. Changes in myosin and actin filaments in fast skeletal muscle after denervation and self-reinnerva-tion. Comp Biochem Physiol Comp Physiol 1992; 102: 93–8
- Jakubiec-Puka A, Drabikowski W. Influence of denervation and reinnervation on autolytic activity and on protein composition of skeletal muscle in rat. Enzyme 1978; 23: 10–21
- Kakulas B A, Adams R D. Diseases of muscle. Harper & Row, Philadelphia 1985
- Keilhoff G, Fansa H. Successful intramuscular neurotization is dependent on the denervation period. A histomorphological study of the gracilis muscle in rats. Muscle Nerve 2005; 31: 221–8
- Korneliussen H, Sommerschild H. Ultrastructure of the new neuromuscular junctions formed during reinnervation of rat soleus muscle by a “foreign” nerve. Cell Tissue Res 1976; 167: 439–52
- Mackinnon S E, McLean J A, Hunter G A. Direct muscle neurotization recovers gastrocnemius muscle function. J Reconstr Microsurg 1993; 9: 77–80
- Mayer L. Orthopedic surgery in the United States of America. J Bone Joint Surg (Br) 1950; 32: 461–569
- McNamara M J, Garrett W E, Jr, Seaber A V, Goldner J L. Neurorrhaphy, nerve grafting, and neurotization: a functional comparison of nerve reconstruction techniques. J Hand Surg (Am) 1987; 12: 354–60
- Millesi H. Brachial plexus injuries: management and results. Microreconstruction of nerve injuries, J K Terzis. Saunders, Philadelphia 1987; 355–8
- Park D M, Shon S K, Kim Y J. Direct muscle neurotization in rat soleus muscle. J Reconstr Microsurg 2000; 16: 135–9, discussion 140
- Payne S H, Jr, Brushart T M. Neurotization of the rat soleus muscle: a quantitative analysis of reinnervation. J Hand Surg (Am) 1997; 22: 640–3
- Saito A, Zacks S I. Fine structure of neuromuscular junctions after nerve section and implantation of nerve in denervated muscle. Exp Mol Pathol 1969; 10: 256–73
- Sakellarides H T, Sorbie C, James L. Reinnervation of denervated muscles by nerve transplantation. Clin Orthop 1972, 83: 195–201
- Schaafsma A, Otten E, Van Willigen J D. A muscle spindle model for primary afferent firing based on a simulation of intrafusal mechanical events. J Neurophysiol 1991; 65: 1297–312
- Tischler M E, Rosenberg S, Satarug S, et al. Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metabolism 1990; 39: 756–63
- Virtanen P , Tolonen U, Savolainen J, Takala T E, . Effect of reinnervation on collagen synthesis in rat skeletal muscle. J Appl Physiol 1992; 72: 2069–74
- Zalewski A A. Effects of reinnervation on denervated skeletal muscle by axons of motor, sensory, and sympathetic neurons. Am J Physiol 1970; 219: 1675–9
- Zamboni L, De Martino C. Buffered picric acid-formaldehyde: A new, rapid fixative for electron microscopy. J Cell Biol 1967; 35: 148
- Zhang F, Lineaweaver W C, Ustuner T, et al. Comparison of muscle mass preservation in denervated muscle and transplanted muscle flaps after motor and sensory reinnervation and neurotization. Plast Reconstr Surg 1997; 99: 803–14
