Abstract
Background We have developed a multimodal technique for the control of pain following knee and hip surgery, called "local infiltration analgesia" (LIA). It is based on systematic infiltration of a mixture of ropiva-caine, ketorolac, and adrenaline into the tissues around the surgical field to achieve satisfactory pain control with little physiological disturbance. The technique allows virtually immediate mobilization and earlier discharge from hospital.
Patients and methods In this open, nonrandomized case series, we used LIA to manage postoperative pain in all 325 patients presenting to our service from Jan 1, 2005 to Dec 31,2006 for elective hip resurfacing (HRA), primary total hip replacement (THR), or primary total knee replacement arthroplasty (TKR). We recorded pain scores, mobilization times, and morphine usage for the entire group.
Results Pain control was generally satisfactory (numerical rating scale pain score range 0–3). No morphine was required for postoperative pain control in two-thirds of the patients. Most patients were able to walk with assistance between 5 and 6 h after surgery and independent mobility was achieved 13–22 h after surgery. Orthostatic hypotension, nausea, and vomiting were occasionally associated with standing for the first time, but other side effects were unremarkable. 230 (71%) of the 325 patients were discharged directly home after a single overnight stay in hospital.
Interpretation Local infiltration analgesia is simple, practical, safe, and effective for pain management after knee and hip surgery.
Hip and knee arthroplasty may result in severe postoperative pain, usually requiring hospitalization from 5 to 10 days to provide effective analgesia. This prolonged hospital stay and relative immobilization of the patient in bed may invite nosocomial infection and deep vein thrombosis (DVT).
We describe a multimodal technique for control of acute postoperative pain following lower limb joint replacement surgery. The technique is based on systematic infiltration of a mixture of ropiva-caine, ketorolac, and adrenaline (RKA) around all structures subject to surgical trauma, followed by top-ups—and finally, extensive re-injection by hand through a fine catheter after approximately 20 h. The technique, known as local infiltration analgesia (LIA), was developed specifically to avoid sedation and facilitate rapid physiological recovery after lower limb arthroplasty in order to enable early mobilization and discharge. In contrast to conventional acute pain management, opioid drugs are used only sparingly or not at all.
Three reports by others (Reilly et al. Citation2005, Busch et al. Citation2006, Vendittoli et al. Citation2006) involving 64, 44, and 41 patients, respectively, detailing limited but successful trials based on this technique have already been published—but without details of the technique, thereby precluding its evaluation. Three further randomized studies (Andersen et al. Citation2007a, Citationb, Toftdahl et al. Citation2007) comparing outcomes obtained using this technique with continous femoral nerve block or placebo saline injection, respectively, have also reported positive findings and were subject to editorial comment (Rostlund and Kehlet Citation2007). We now describe the technique fully as we have developed it for hip and knee replacement surgery, and document its efficacy as reflected by pain scores and mobilization times.
Patients and methods
All 325 patients presenting to our service during the years 2005 and 2006 for elective hip resurfacing (HRA), primary total hip replacement (THR), or primary total knee replacement arthroplasty (TKR) were included in this open, nonrandomized case series (). Revision procedures resulting in the implantation of a primary total joint prosthesis (e.g. revision of an osteotomy or unicompartmental knee to a primary total knee) were included, but all other revision procedures were excluded.
Table 1. Arthroplasty patients managed by local infiltration analgesia in the period 2005–2006
All surgery was conducted by a single surgeon (LK) and all anesthesia and pain management was conducted by a single physician anesthetist (DK). Written informed consent was obtained before surgery. The study was approved by the Human Research Ethics Committee of the South Eastern Sydney Area Health Service, Sydney, NSW, Australia. The study was conducted in accordance with the Helsinki Declaration.
Anesthesia and surgical technique
A subvastus (Matsueda and Gustillo Citation2000) or medial parapatellar surgical approach was used for total knee replacement arthroplasty. A pneumatic thigh tourniquet inflated to a pressure of 300 mm Hg was used in all but 4 cases, in whom the use of a tourniquet was contraindicated on account of critical peripheral vascular disease and previous vascular surgery. In order to limit ischemia time and minimize tourniquet-generated muscle pain, the tourniquet was released immediately after implantation of the femoral and tibial components but before dealing with the patella.
A posterior approach was used both for Birmingham hip resurfacing arthroplasty and for total hip replacement (McMinn et al. Citation1996). Minimally invasive techniques were not used.
Almost all knee and hip arthroplasties were performed under combined spinal (3.0 mL bupiva-caine 0.25% at L4–5, 25 gauge pencil point spinal needle) and light general anesthesia (propofol infusion or O2/N2O/sevoflurane).
Local anesthetic: the ropivacaine-ketorolac-adrenaline (RKA) mixture
The injectant mixture consisted of ropivacaine HCl (Naropin; AstraZeneca Pty. Ltd., Sydney, Australia), 2.0 mg/mL mixed with 30 mg ketorolac tro-methamine (Toradol; Roche Products Pty. Ltd., Sydney, Australia) and 10 μg/mL adrenaline, with occasional variations as detailed below. In order to deliver the drug mixture reliably and adequately throughout the surgical field, 150–170 mL was used for TKR and 150–200 mL for HRA and THR. The RKA mixture was diluted with normal saline for volumes in excess of 150 mL, to limit the total dose to a maximum of 300 mg. The total dose of ropivacaine was reduced to 250 mg if the patient was unusually small (< 55 kg), very elderly (> 85 years), infirm (American Society of Anesthesiologists class 3 or 4), or had a history of significant intolerance to analgesics or anesthetic agents. In patients with contraindications to the use of NSAIDs (especially renal failure), ketorolac was omitted from the mixture and other oral or paren-teral analgesics were used. All infiltration was done using 50-mL syringes and 10-cm-long 19-G spinal needles. Injections were done using a "moving needle" technique to avoid depositing large volumes of drug intravascularly, and they were spread over about 1 hour, injecting one layer at a time as the operation progresses, in order to keep the blood levels of local anesthetic to a minimum.
Catheter
We used a 16-G Tuohy needle, an 18-G epidural catheter (Portex, Smiths Medical International Ltd. Hythe, Kent, UK), and a 0.22-μm high-performance antibacterial flat epidural filter (SIMS-Portex Co., Hythe, Kent, UK). Care was taken to ensure that the catheter was not caught in the joint mechanism and that it lay in a position such that the RKA mixture could be delivered to all parts of the joint, tissue planes, and under the wound by injecting as the catheter was withdrawn.
Injection technique and catheter placement
Knee arthroplasty. 150–170 mL of injectant is used for TKR. The injection is made in three stages. The first injection is done after the bone surfaces have been prepared, but before the components have been inserted since access to the posterior capsule is limited once the components are in place. About 30–50 mL is injected through the joint from the front to a depth of 3 mm into the tissues around the posterior joint capsule, using a systematic sequence from one side to the other to ensure uniform delivery to these tissues. The second injection is done after the components have been inserted, but before both wound closure and tourniquet release. About 35–50 mL is injected into the deep tissues around the medial and lateral collateral ligaments and the wound edges. The third injection of 25–50 mL of the same RKA mixture is made into the subcutaneous tissue, carefully avoiding immediate subder-mal injection in order to avoid intense vasocon-striction in the skin. Multiple injections are made in a systematic sequence, approximately every 25 mm around the wound. The needle is inserted each time perpendicular to the wound edge to a depth of about 25 mm and injection is done as the needle is withdrawn.
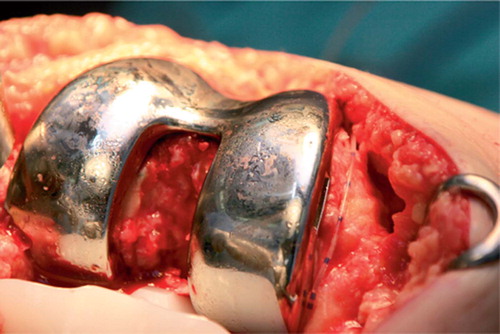
Catheter placement. Immediately before wound closure, a Touhy needle is inserted about 10 cm above the incision through the skin, subcutaneous tissue, and quadriceps muscles. The tip of the catheter is then inserted through the hub of the needle into the surgical field from the outside to the inside. The catheter is then led along the medial femoral condyle (), usually on raw bone, medial to the metal femoral component and adjacent to the medial capsule. Using an artery forceps, the tip is then passed posterior to the medial femoral condyle, so that the tip lies immediately anterior to the posterior capsule. Finally, the needle is removed, the slack is taken up and the catheter is cut to a convenient length such that only about 20 cm protrudes from the skin. The hub and bacterial filter are then connected and about 1–2 mL is injected through the pain catheter to ensure patency. After wound closure, a further 10–15 mL is injected through the catheter to flood the joint with RKA mixture.
Figure 1. Pain catheter placement in total knee replacement. The catheter is led along the medial femoral condyle, usually on raw bone, medial to the metal femoral component. Using artery forceps, it is then passed posterior to the medial femoral condyle so that the tip lies in front of the posterior capsule.
Hip arthroplasty. Depending on the size of the surgical incision, 150–200 mL of injectant is injected in stages—in 3 equal 50–70-mL doses. The first injection is made after completion of the acetabular surgery, the second one after insertion of the femoral component, and the final one immediately before the skin is sutured. The first injection is made into the tissues around the rim of the acetabulum, focusing on both the joint capsule if it remains, and around the exposed gluteal and adductor muscles. The injection is done using a systematic sequence around the acetabular rim to ensure uniform delivery to these tissues. The second injection is made into the external rotators, gluteus tendon, and iliotibial band. Multiple injections are done in a systematic sequence every 25 mm or so along the length of the exposure. Care is taken to infiltrate in a fan-wise fashion around the apices of the wound, so that traumatized tissues in these locations are covered. The third injection is made into the subcutaneous tissues under the wound. Multiple injections are performed in a systematic sequence every 25 mm around the wound. The needle is inserted each time perpendicular to the edge of the wound to a depth of about 25 mm and injection is done as the needle is withdrawn.
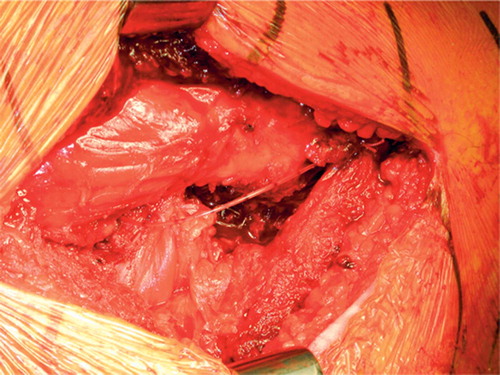
Catheter placement (). Immediately before wound closure, a Touhy needle is inserted about 10 cm below the inferior apex of the incision through the fascial layers and iliotibial band. The tip of the catheter is then inserted through the hub of the needle from the outside into the surgical field, advanced to the superior apex of the wound, and placed with forceps above the piriformis tendon such that its tip lies antero-superior to the joint (within the capsule for HRA). The slack is taken up so that the catheter lies over the long axis of the wound in the plane over the external rotator muscles. The needle is then removed so that the catheter exits through the skin about 10 cm below the distal end of the incision, and the catheter is cut to a convenient length such that only about 20 cm protrudes from the skin. The hub and bacterial filter are then connected and 1–2 mL is injected through the catheter to ensure patency. After wound closure, a further 10–15 mL is injected through the catheter to flood the joint with RKA mixture.
Figure 2. Pain catheter placement in hip resurfacing arthroplasty. The pain catheter is advanced to the superior apex of the wound and placed with forceps above the pyriformis tendon such that its tip lies within the capsule antero-superior to the joint. The slack is then taken up so that the catheter lies over the long axis of the wound, in the plane over the external rotator muscles
Ancillary measures
Measures to restrict drugs to the site of injection. We used a vasoconstrictor (adrenaline 10 mg/mL), compression, cooling, and splinting of the injection site to minimize drug absorption and systemic toxicity (Sutherland and Leonard Citation1995). After knee surgery, a compressive bandage was applied so as to be firm enough to produce venous and lymphatic but not arterial compression. The knee was bandaged with cotton wool (Velband; Smith and Nephew Pty. Ltd.) and a crepe bandage, and finally a self-adherent, non-stretch bandage (Acrylastic bandage, part no. 2699; Smith and Nephew Pty. Ltd.). Ice packs were applied over the incision on the outside of the bandage for the first 4 h postoperatively. In order to limit ischemia time and tourniquet-generated muscle pain, the tourniquet was always released well before wound closure and bandage application.
Since hip wounds are difficult to compress with a bandage, a surgical sponge roll was placed along the wound and compressed onto the wound with an elastic binder around the lower part of the pelvis (Dale Abdominal Binder: 4 panel 30 cm white, code 811; Cosmac Surgical Pty. Ltd.). In addition, ice packs were applied on the incision for the first 4h.
Antibiotics. Unless contraindicated, all patients were given a single dose of cephalothin (1 g) at induction. No patient was given aminoglycosides, as urinary catheters were not used and because the combination of aminoglycosides and NS AIDs may increase the risk of renal toxicity (Jaquenod et al. Citation1998).
Wound drains. Wound drains were not used routinely, but on rare occasions when a drain was used it was an important source of pain—and it was considered important to anesthetize the area. Injection was made along the line of the drain and pain catheter by inserting the needle through the wound from inside to outside, under direct vision.
Postoperative management
Recovery room. If the patient had pain in the recovery room, options for treatment included catheter top-up and direct injection of painful spots as well as conventional analgesics. Before leaving the recovery room, a loading dose of ibuprofen (400 mg) or celecoxib (200 mg) was given.
Opioids. All patients were routinely prescribed rescue doses of up to 10 mg of intravenous morphine, to be administered according to our recovery room pain protocol as the need arose (e.g. for pain arising from outside the surgical site, such as tourniquet pain or back pain) and a single rescue dose of IM morphine (10 mg) for use in the ward. The nursing staff were also encouraged to exercise this option overnight if patients were uncomfortable and finding it difficult to sleep. Further doses of morphine were available after consultation with the anesthetist. However, parenteral opioids were avoided during the day where possible because they delay mobilization and may result in nausea and vomiting.
Oral and transdermal medication. Unless con-traindicated, ibuprofen 400 mg was given 4-hourly for 24 h postoperatively, then patients self-medicated as required, with a view to stopping the drug over a 2- to 3-day period. Oral analgesics, usually paracetamol (1 g) combined with either tramadol (50–100 mg) or codeine (32–64 mg), were provided for use not more frequently than 4-hourly as required. After 36 h, residual pain was managed with conventional oral analgesics and/or buprenor-phine skin patches. Patients were instructed to cease tramadol or codeine and to take only paracetamol as soon as the pain had decreased to an acceptable level.
Aspirin (300 mg) was given daily for 6 weeks for thromboprophylaxis. However, when patients could not or would not comply with the aggressive mobilization regimen, or had a history of hereditary or acquired predisposition to thrombosis, or previous thromboembolic events, we used conventional thromboprophylaxis with enoxapa-rin initially followed by warfarin. An H2 blocker such as ranitidine or a proton pump inhibitor such as esomeprazole was also given for the first 24 h to cover the high dose of NSAID, and a psyllium husk preparation such as Metamucil was given to prevent constipation.
Top-up. Occasionally, if we expected the block to recede overnight or to relieve discomfort at any time, we gave a small top-up dose of 10–15 mL of the RKA mixture through the pain catheter to flood the joint with local anesthetic. If 6 h had not elapsed from the time of the last dose, ketorolac was omitted.
Mobilization. All prostheses we used are suitable for immediate weight bearing. We attempted to mobilize all patients on a walking frame 3 to 4 h postoperatively, and thereafter every 2–3 h during the day. We encouraged them to walk a minimum of about 30 meters. An intravenous fluid bolus of 200–300 mL was given immediately before having the patient stand for the first time. As soon as patients could safely walk independently on crutches, they were encouraged to take charge of their own mobilization. Toilet privileges were immediate with assistance, and sitting out of bed was encouraged.
Re-injection. 15 to 20 h postoperatively, the surgical field was re-injected with approximately 50 mL of the RKA mixture by hand through the wound catheter. About 15 mL was injected before the catheter was moved, and the rest spread evenly throughout the wound as the catheter was withdrawn and removed. If a wound drain was used, it was removed before re-injection through the pain catheter in order to prevent drug loss through the drain.
Fluid management. In consideration of the possibility of renal toxicity from NSAIDs (Smith et al. Citation1993), all patients were fully hydrated both during surgery and postoperatively (Jaquenod et al. Citation1998). Our routine approach was to administer 2 L of Hartmann's solution and 500 mL of 4% albumin intraoperatively, and a further 2 L of Hartmann's solution and 0.5 L of 4% albumin over the next 24 h.
Measurements
Adverse events. All patients were closely attended to by the nursing staff while in hospital, and under close personal supervision by the authors until their follow-up visit 4 weeks after surgery. Patients were encouraged to report any adverse event immediately.
Pain assessment. All patients in the study had their pain scores assessed while at rest and while walking. Pain scores were recorded by the nursing and physiotherapy staff while the patient was in hospital, and by one of the authors (DK) by telephone after discharge. Pain was assessed using a numerical rating scale from 0–10 (with 0 representing no pain and 10 representing the worst possible pain). Zero time was taken from first injection of the RKA mixture for hips, or tourniquet release for knees.
Pain scores were recorded 4 h after zero time, before any supplementary medication was given and before mobilization had been attempted. Later scores were recorded both at rest and while walking, once mobilization had begun.
Mobilization times. Time intervals were recorded from zero time until the first walk (W1) and from zero time until independent mobility, witnessed either by the physiotherapist or by one of the medical staff. Independent mobility was defined as the ability to transit from bed to the upright position, manage the toilet, walk approximately 30 metres, and climb one flight of stairs with minimal assistance. Zero time was taken from first injection for hips, or tourniquet release for knees.
Length of stay (LOS). Discharge dates and times were recorded in the hospital database at the time of discharge. LOS was recorded as the number of nights spent in hospital after surgery. A night in hospital was defined as being in hospital at midnight.
Results
No serious side effects or complications directly attributable to the LIA technique were recorded during the first 10 days postoperatively. In particular, we observed no major toxicity involving cardiac arrest, cardiotoxicity (including widening of the Q-T interval and QRS complex in the ECG) or seizures related to ropivacaine use and no gastrointestinal ulceration), major bleeding, or renal failure related to the use of NSAIDs. There were no inadvertent bolus injections of ropivacaine, either directly or on tourniquet release. We also observed a zero incidence of infections related to the wound catheter. One elderly female patient was re-admitted with bleeding from 2 gastric ulcers 3.5 weeks postoperatively. The ulcers were associated with continued regular use of ibuprofen, against instructions.
Minor adverse events have included transient mild to moderate nausea, vomiting, and dizziness—usually associated with postural hypotension (BHR 18%, TKR 9%, THR 7%) on the first attempt to walk at about 4–5 h postoperatively or with the use of supplementary morphine (BHR 3%, TKR 5%, THR 4%).
Pain scores were recorded 4 h after zero time, after re-injection on the following morning approximately 15–22 h after zero time, and again on the morning of postoperative day 2 approximately 40–48 h after zero time ().
Table 2. Pain scores recorded on postoperative day 0 at 4 h after time zero, postoperative day 1 approximately 15–22 h after time zero, and postoperative day 2 approximately 40–48 h after time zero for each operation category
Pain scores, both at rest and while walking (), were generally in the range 0–3, which we considered to be satisfactory. Worst pain scores were recorded at approximately 4 h postoperatively. By this time, the patients had recovered from spinal anesthesia and pain scores were recorded before any fine tuning or mobilization had been attempted, so as to reflect the effectiveness of LIA alone. Higher pain scores at 4 h were mostly related to incomplete local blockade, but not uncommonly pain originated outside the surgical field and was related to the use of a tourniquet for knee surgery, and thigh adductor stretch for hip surgery; either type of surgery was occasionally associated with lower back pain. Higher pain scores at 4 h were also associated with the longer surgical incisions characteristic of hip resurfacing.
After initial fine tuning at 4 h postperatively, pain scores generally remained in the range 0–3 both at rest and while walking. The higher pain scores were recorded when exercising and if the local blockade had been allowed to recede before top-up or re-injection. Parenteral morphine was used sparingly within the first 24 h (). No patients required morphine after postoperative day 1. Pain scores elicited by phone after discharge were almost universally satisfactory (NRS 0–3) but, surprisingly, some patients preferred pain to medication and were prepared to accept higher levels of pain to avoid taking medication.
Table 3. Morphine usage over the first 48 h postoperatively by patients presenting between Jan 1, 2005 and Dec 31, 2006. Proportion of patients in each category. Dose in parentheses is average total dose in mg over 48 h for each category
Average times to first walk were lowest for HRA patients and highest for TKR patients. ().
Table 4. Mobilization times. Values are time intervals from time zero (first injection of RKA mixture for hips or tourniquet release for knees) to first walk (time W1) and to independent mobility (time IM). Values are mean (SD) [range] hours
In general, patients in all categories were discharged directly home after a single overnight stay in hospital (HRA 89%, THR 41%, TKR 51%) ().
Table 5. Length of stay in hospital
Discussion
Almost all pain after surgery arises as a result of tissue damage at the surgical site. For elective surgery, the cause of the pain, the time of its onset, the site of its generation, and its likely duration are all known in advance. This situation clearly invites pre-emptive action to eliminate generation and transmission of pain impulses at the site from which they arise, and before central processing complicates matters. Local anesthetics and direct-acting anti-inflammatory drugs (ketorolac) injected locally can be used for this purpose.
Control of postoperative pain using local anesthetics, by infiltration around surgical wounds, has met with only limited success in the past—both because there has been no systematic technique available for effective drug delivery to all relevant parts of the surgical site, and because of the short duration and the toxicity of the drugs available (Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine 2005). Our observations show that it is possible to achieve satisfactory control of pain using local infiltration block and that the blockade can be made to last for about 36 h by use of an appropriate local anesthetic drug (ropivacaine), measures to restrict it to the site, and an intraarticular catheter for top-up.
The use of ketorolac in the RKA mixture is seen by some as controversial because of possible renal and gut toxicity, and especially since there is a suggestion from animal studies (not replicated in humans) that NSAIDs may inhibit bony ingrowth and have an adverse effect on endoprosthetic fixation. We encountered 1 case of late gastric ulceration, which was associated with prolonged use of ibuprofen against instructions. There was no clinical or laboratory evidence of renal toxicity over the study period, which might perhaps be explained by very slow systemic uptake of the NSAID as a result of vasoconstriction, cooling, and firm bandaging.
Local infiltration of NSAIDs has not been widely used, and the literature is equivocal as to its efficacy (Ben-David et al. Citation1995, Garcia-Enguita Citation1997, Romsing et al. Citation2000). Nonetheless, sensitization of pain nerves by locally active mediators derived from damaged tissue is believed to be a major mechanism of amplifying and sustaining pain intensity. Synthesis of the prostanoid components of this biological soup can be blocked by NSAIDs, and the infiltration technique that we have described appears to be effective in delivering locally high concentrations of drugs to the appropriate site. If the local anesthetic in the RKA mixture successfully blocks pain nerve conduction, then the NSAID molecules must also be in the immediate vicinity of the nerve endings and in a perfect position to inhibit prostaglandin synthesis and subsequent nerve sensitization. Also, new agents capable of directly blocking peripheral TRP, Nav, and G protein-coupled nociceptors may be developed in the future. The LIA technique could be used to efficiently deliver such drugs, should they eventuate, and they may thus be useful additions to the RKA mixture.
To be useful for local infiltration, a drug must act directly at the site of injection. We chose ketorolac because it was the only directly acting injectable non-selective NSAID available to us. It is worth noting that the COX-2 inhibitor prodrug paracoxib is unsuitable for local infiltration because it is pharmacologically inactive until it is absorbed and activated in the liver.
Infiltration blockade does not result in muscle weakness and is compatible with virtually immediate mobilization. Most patients in this series were capable of mobilizing at 4–6 h postoperatively. Factors that delayed mobilization were hypovolemia, significant co-morbidities, opioid use, and unavailability of staff to initiate and supervise mobilization late in the evening. Orthostatic hypotension and associated nausea also occasionally delayed independent mobility for a few hours. Satisfactory pain control and the rapid achievement of independence in the activities of daily living also engender a positive outlook, and this has been associated with high levels of patient satisfaction.
Surgical pain management can be regarded as falling into four distinct phases, namely preoperative (education, building confidence, and motivation), operative (suitable anesthesia), acute postoperative, and residual periods. Local infiltration analgesia is merely one piece of this jigsaw puzzle of important contributory elements. Specifically, it is the element designed to manage the acute postoperative pain phase lasting about 36 h, and should be regarded as a key enabling technique promoting rapid return to normal activities of daily living and facilitating discharge from hospital. One can only expect surgical outcomes to be improved by this technique, however, if its benefits are exploited by such further measures as reduced invasive interventions (e.g. PCA, urinary catheters), early mobilization, and early discharge—all of which require appropriate attitudes and organization. Improved outcomes also rely on adequate management of the subsequent residual pain phase, which often lasts for a further 1–2 weeks and is an inevitable and essential partner to LIA. Although there are various alternatives, we used buprenorphine skin patches plus supplementation with occasional oral analgesics (mainly paracetamol) to manage this phase.
For the intraoperative phase of pain control, we have used a short-acting spinal anesthetic technique (3.0 mL bupivacaine, 0.25%). This ensures that no pain signals reach the CNS at any time before the infiltration block has been initiated, has had time to spread, and has become fully established. Emergence from spinal blockade is timed to occur at approximately 2 h postoperatively, so as to allow early mobilization. This approach provides a smooth transition from central blockade to infiltration blockade. Secondary considerations for using spinal blockade include facilitation of moderate controlled hypotension and reduction of thrombo-embolic complications (Heit et al. Citation2002, O'Reilly et al. Citation2005).
Extension of the duration of pain control by re-injecting the surgical site is a central feature of this pain management technique. Our standard practice is to hand-inject the wound through the bacterial filter and pain catheter as the initial block wears off, 15–20 h after surgery. Our intention is to again flood the joint capsule and all the tissue planes through which the catheter passes with the RKA mixture. This approach differs conceptually from slow infusion techniques ("painbusters") in that it is designed to extensively flood the tissues throughout the surgical field once only as the initial block recedes.
Currently, continuous wound infiltration with local anesthetics through wound catheters and continuous nerve block techniques may perhaps be considered to be the gold standard for analgesia after knee and hip replacement. A recent systematic review of the efficacy of continuous wound catheter techniques (Liu et al. Citation2006) reported improved analgesia, reduced opioid use and side effects, increased patient satisfaction, and some reduction in length of stay. LIA can be considered to be a similar technique, but with more extensive initial local blockade and an emphasis on intermittent bolus delivery via a wound catheter to flood the entire surgical field rather than continuous low-dose infusion. The outcomes documented in our study are qualitatively similar to those reported by Liu et al., but represent a further improvement.
Considerations of cost and hospital-acquired complications suggest that reducing the length of stay in hospital has considerable merit. Some success has been reported in reducing stays following hip and knee surgery by the use of continuous nerve block techniques (Ilfeld et al. Citation2006a, Citationb, Salinas et al. Citation2006). However, these are difficult, time-consuming procedures with a potentially high failure rate and a complication profile that has not been characterized. LIA is an effective alternative technique. In our patients, satisfactory mobilization was achieved within 5–6 h and discharge directly home after a single overnight stay was the norm. Longer stays were required if patients had significant co-morbidities or inadequate support at home.
There are several limitations to this study, which invite further investigation. Firstly, this study was conducted from a private practice setting. Our patient population was therefore from a more privileged group in the community, with greater access to resources and support than may be found in the general population. In addition, the practice policy of favoring hip and unicompartmental knee resurfacing procedures over total joint replacement implies that our HRA patients would be heavily skewed towards a younger, fitter cohort than those generally presenting for hip replacement. Conversely, our THR and TKR population is older and co-morbidities are more frequent than average because the patients are already selected as being unfit for hip resurfacing or unicompartmental knee resurfacing. Secondly, this is a case series merely documenting outcomes and describing the technique used. No attempt has been made here to directly compare the efficacy of this technique with that of other analgesic techniques, although five randomized trials have been published which are supportive (Busch et al. Citation2006, Vendittoli et al. Citation2006, Andersen et al. Citation2007a, Citationb, Toftdahl et al. Citation2007).
Local infiltration analgesia is simple, practical, safe, and effective for pain management after knee and hip surgery. When combined with efficient residual pain management at home, this technique facilitates early discharge with consequent possible reduction in the incidence of multiresistant nosocomial infection.
This research was funded privately by Orthoplan Research, a private trust fund established by the authors. Financial contributors have included the authors, Smith and Nephew Pty. Ltd., AstraZeneca Pty. Ltd., and Zimmer Pty. Ltd. No private payment was received by the authors for conducting this research. The authors have received no other benefits, consultancies, or licensing arrangements from any entity in association with this work.
- Andersen K V, Peiffer - Jensen M, Haraldsted V, Søballe K. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty. A randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop 2007a; 78(2)180–6
- Andersen L J, Poulsen T, Krogh B, Nielsen T. Postoperative analgesia in total hip arthroplasty. A randomized double-blinded, placebo-controlled study on preoperative and postoperative ropivacaine, ketorolac and adrenaline wound infiltration. Acta Orthop 2007b; 78(2)187–92
- Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute Pain Management: Scientific Evidence. In: 2nd ed: 2005: 110–4
- Ben-David B, Katz E, Gaitini L, Goldik Z. Comparison of IM and local infiltration of ketorolac with and without local anaesthetic. Br J Anaesth 1995; 75: 409–12
- Busch C, Shore B, Bhandari R, Ganapathy S, MacDonald S, Bourne R, et al. Efficacy of periarticular multimodal Drug injection in total knee arthroplasty. A randomised trial. J Bone Joint Surg (Am) 2006; 88: 959–63
- Garcia-Enguita M A, Ortega-Lahuerta J P, Arauzo-Perez P, Laglera-Trebol S, Giron-Mombiela J A, Lopez-Sicilia S, et al. The utility of digital infiltration of mepivacaine and ketorolac in postoperative analgesia of the unilateral hallux valgus. Rev Esp Anestesiol Reanim 1997; 44(9)345–8
- Heit J A, O'Fallon W M, Petterson T M, Lohse C M, Silverstein M D, Mohr D N, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med 2002; 162: 1245–8
- Ilfeld B, Gearen P, Enneking F, Berry L, Spadoni P, George S, et al. Total hip arthroplasty as an overnight-stay procedure using an ambulatory continuous psoas compartment nerve block: A prospective feasibility study. Re Anaesth Pain Med 2006a; 31(2)113–8
- Ilfeld B, Gearen P, Ennekink F, Berry L, Spadoni E, George S, et al. Total knee artroplasty as an overnight-stay procedure using continuous femoral nerve blocks at home: A prospective study. Anesth Analg 2006b; 102(1)87–90
- Jaquenod M, Ronnhedh C, Cousins M J, Eckstein R P, Jordan V, Mather L E, et al. Factors influencing ketorolac-associated perioperative renal dysfunction. Anesth Analg 1998; 86: 1090–7
- Liu S S, Richman J M, Thirlby R C, Wu C L. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: A quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 2006; 203(6)914–32
- Matsueda M, Gustillo R B. Subvastus and medial parapa-tellar approaches in total knee arthroplasty. Clin Orthop 2000, 371: 161–8
- McMinn D, Treacy R, Lin K, Pynsent P. Metal on metal surface replacement of the hip. Clin Orthop 1996, 329S: S89–S98
- O'Reilly R F, Burgess A, Zicat B. The prevalence of venous thromboembolism after hip and knee replacement surgery. Med J Aust 2005; 182: 154–9
- Reilly K A, Beard D J, Barker K L, Dodd C A, Price A J, Murray D W. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty - a ran-domosed controlled trial. Knee 2005; 12(5)351–7
- Romsing J, Moiniche S, Ostergaard D, Dahl J B. Local Infiltration with NSAIDs for postoperative analgesia: Evidence for a peripheral analgesic action. Acta Anaesthesiol Scand 2000; 44/6: 672–83
- Rostlund T, Kehlet H. High-dose local infiltration analgesia after hip and knee replacementqqqqqmdashssssswhat is it, why does it work. and what are the future challenges?. Acta Orthop 2007; 78(2)159–61
- Salinas F, Liu S, Mulroy M. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long term functional recovery within an established clinical pathway. Anesth Analg 2006; 102(4)1234–9
- Smith K, Halliwell R M T, Lawrence S, Klineberg P L, O'Connell P. Acute renal failure associated with intramuscular ketorolac. Anaesth Intens Care 1993; 21: 700–3
- Sutherland S K, Leonard R L. Snakebite deaths in Australia 1992–1994 and a management update. Med J Aust 1995; 163: 616–8
- Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tønnesen E K, Søballe K. Comparison of peri- and intraar-ticular analgesia with femoral nerve block after total knee arthroplasty. A randomized clinical trial. Acta Orthop 2007; 78(2)172–9
- Vendittoli P, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin M. A multimodal analgesia protocol for total knee arthroplasty. A randomized controlled study. J Bone Joint Surg (Am) 2006; 88(2)282–9
