Abstract
Background and purpose Chronic Achilles tendinopathy is a painful condition and there are often unsatisfactory results with conservative treatment. Extracorporal shock-wave therapy (ESWT) has been introduced for the management of various soft tissue conditions. The objective of the study was to compare the effect of supplementing conservative treatment of chronic Achilles tendinopathy with ESWT or placebo.
Patients and methods This was a randomized, double-blind, placebo-controlled trial. Patients assigned to nonoperative treatment of chronic achilles tendinopathy were randomized to receive either active ESWT or sham ESWT over 4 weeks. There were 48 patients (28 men) with a mean age of 47 (19–80) years. American Orthopaedic Foot and Ankle Society (AOFAS) score and pain were assessed before treatment, during the 4-week treatment period, and at 4, 8, and 12 weeks of follow-up.
Results Both groups improved during the treatment and follow-up period. The mean AOFAS score increased from 74 (SD 12) to 81 (16) in the placebo group and from 70 (6.8) to 88 (10) in the intervention group (p = 0.05). Better results were seen in the intervention group at 8 and 12 weeks of follow-up (p = 0.01 and p = 0.04, respectively).
Interpretation Extracorporal shock-wave therapy appears to be a supplement for the treatment of chronic Achilles tendinopathy.
Chronic Achilles tendinopathy is a common disability of the foot during walking and running. Avoidance of painfulactivities, correction of malalignment by arch support, stretching exercises, and eccentric training combined with non-steroidal anti-inflammatory pain killers are the standard treatment regimen (Wilson and Best Citation2005). However, there is little evidence to support any effect of these modalities (McLauchlan and Handoll Citation2001).
Extracorporal shock-wave therapy (ESWT), originally used for treatment of renal stones, has become popular in the last decade for treatment of different soft tissue disorders including calcifying tendinopathy in the rotator cuff, humeral epicondylitis, and plantar fasciitis (Haake et al. Citation2001a, Ogden et al. Citation2001, Chung and Wiley Citation2002, Gerdesmeyer et al. Citation2003, Speed et al. Citation2004). It is now used worldwide and has been FDA-approved for the treatment of plantar fasciitis. The results have, however, been conflicting regarding calcifying tendinopathy in the rotator cuff, humeral epicondylitis, and plantar fasciitis (Rompe et al. Citation1997, Boddeker et al. Citation2001, Gross et al. Citation2002, Hammer et al. Citation2002, Schmitt et al. Citation2002, Haake et al. Citation2003, Pleiner et al. Citation2004, Chung et al. Citation2005). A series of randomized studies on ESWT for soft tissue disorders has been reported (Rompe et al. Citation1997, Krishek et al. Citation1998, Loew et al. Citation1999, Rompe et al. Citation2001, Haake et al. Citation2001a, Citationb, Gross et al. Citation2002, Hammer et al. Citation2002, Schmitt et al. Citation2002, Gerdesmeyer et al. Citation2003, Haake et al. Citation2003, Pleiner et al. Citation2004, Chung et al. Citation2005, Costa et al. Citation2005), with only 4 reporting significant effect of ESWT (Rompe et al. Citation1997, Krishek et al. Citation1998, Loew et al. Citation1999, Gerdesmeyer et al. Citation2003), and there has only been one randomized study for chronic achilles tendinopathy (Costa et al. Citation2005). ESWT was introduced for chronic Achilles tendinopathy in 2 uncontrolled (Perlick et al. Citation2002, Lakshmanan et al. Citation2004) and 1 controlled (Furia Citation2005) series. The results of these studies are strongly conflicting. Some authors included training and others not. Since eccentric training has documented effect (Alfredson and Lorentzon Citation2000), other conservative treatments should be introduced as a supplement.
In this double-blind, randomized trial, we investigated the effects of supplementary ESWT in enhancing recovery of Achilles tendinopathy, i.e. (1) the effect on American Orthopaedic Foot and Ankle Society (AOFAS) score and (2) the effect on pain.
Patients and methods
All patients with chronic symptoms of Achilles tendinopathy for over 3 months—who had full working capacity and who were more than 18 years old and able to comply—were considered candidates for inclusion in the study after giving their informed consent, the aim being to study 48 consecutive patients. The patients were recruited at Aalborg University Hospital. The criteria used for diagnosis af Achilles tendinopathy were as follows: an area of swelling moving with dorsiflexion and platarflexion of the ankle, tenderness in neutral position or slightly plantarflexed, and tenderness exacerberated by dorsiflexion of the ankle.
All patients were given conservative treatment at 4 sessions over 4 weeks and after randomization the patients also received active ESWT or sham ESWT at these 4 sessions. The patients were blinded as to the type of treatment, which was done by a person not involved in the study design. An observer who had been blinded regarding the type of treatment evaluated the outcome.
The conservative treatment and ESWT were given in an outpatient clinic; the former consisted of stretching exercises and eccentric training. Local anesthetics were not used. Following stretching and training, ESWT sham or active treatment was given at 4 sessions once a week. All patients were placed prone, unable to see the machine delivering the ESWT but hearing a ticking sound of therapy. Using Piezoson 100 at each session, 2,000 shots (0.12–0.51 mJ/mm2, 50 Hz) of radial shock waves were delivered to the area of swelling and tenderness. The sham dose was 2,000 shots at 0 mJ/mm2, 50 Hz. Ultrasound guidance was not used. This treatment protocol was based on the results from 3 randomized trials treating plantar fasciitis (Krischenk Citation1998, Haake et al. Citation2001, Hammer et al. Citation2002) where ESWT was applied (100–4,000 shots, 0.08–0.2 mJ/mm2) 3 times with 1 or 2 weeks between each session.
Demographics, AOFAS score and pain were measured before and after treatment. The primary endpoint was AOFAS score, an ankle-hindfoot scale of the American Orthopaedic Foot and Ankle Society measuring function (50 points), pain (40 points), and alignment (10 points) (Kitaoka et al. Citation1994). 100 points represent the best possible result. Additional analyses were done on pain using a visual analog scale (VAS). At each visit during treatment and follow-up, AOFAS score and VAS for pain on walking, walking upstairs, working, and running were measured.
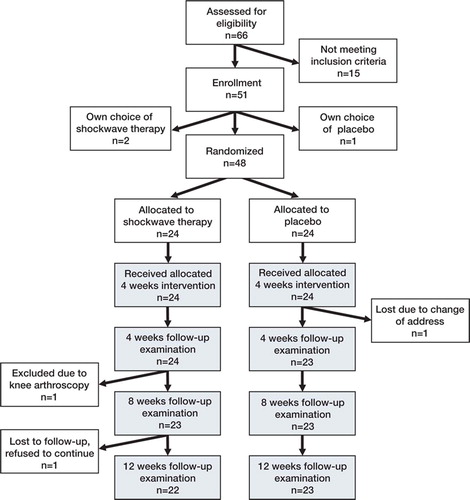
From October 2004 through January 2005, 51 patients with Achilles tendinopathy were evaluated for participation in the study (). Thus, 48 patients (28 men) were included in the study, with an average age of 47 (19–80) years. Patient demographics were similar in the two groups (). All patients completed the treatment period. During the 3-month follow-up, 1 patient was excluded due to a knee arthroscopy and 2 patients did not attend the final 3-month follow-up. For these patients, data until censored were included in the analysis. Follow-up of all patients ended in June 2005.
Table 1. Patient demographics (mean values (SD))
Statistics
The sample size was calculated on the basis of AOFAS score. It was calculated for observations on the interval scale. The trial was powered to detect a difference of 10 in the AOFAS score. Significance was set at 5% and power was set at 90%. The estimated standard deviation of 10 of the AOFAS score was set to be equal to the minimal relevant clinical difference. The number of patients in each group was calculated to be 21. To compensate for non-evaluable patients, we planned to enroll 24 patients in each group. The patients were allocated to the 2 groups according to a computer-generated randomization list concealed in a set of numbered envelopes. After acceptance of the patient, the appropriate numbered envelope was opened; the card inside told if the patient was to be an intervention case or a control case. The inclusion period was expected to last 3–4 months. Results are expressed as mean and standard deviation, and analyzed using repeated-measures analysis of variance test (Matthews et al. Citation1990) with all pairwise multiple comparison procedures (Bonfer-roni t-test). Analysis of change from before treatment to 3-month follow-up was based on analysis of covariance and expressed as mean and 95% confidence intervals (CIs). Values of p < 0.05 were considered statistically significant. The program Stata 9.2 was used.
The study was approved at the local ethics committee in North Denmark Region (VN2004/31) and was carried out in accordance with the Declaration of Helsinki. The study is registered in www.Clini-calTrials.gov as ON-007-01-RAS.
Results
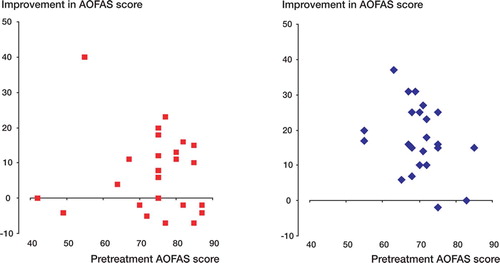
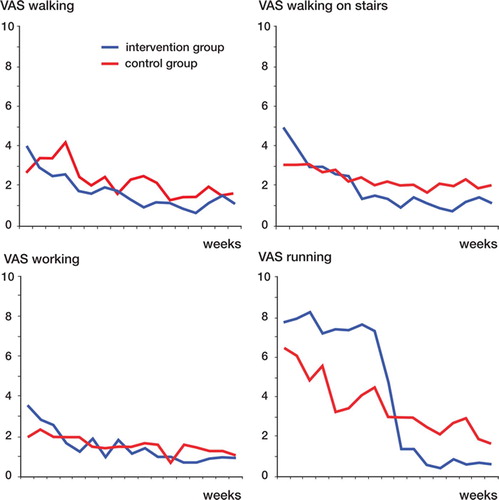
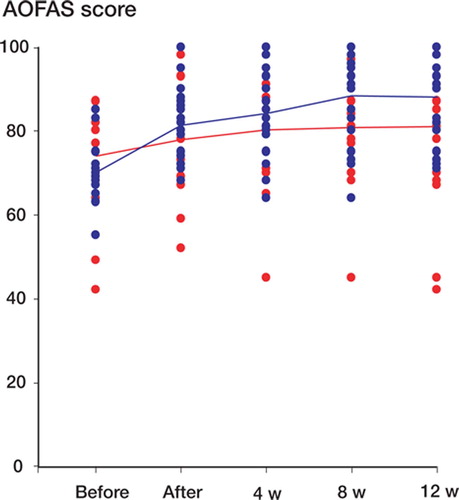
AOFAS score after treatment increased more over time in the intervention group than in the control group (p = 0.05), from 70 (SD 6.8) to 88 (10) in the intervention group and from 74 (12) to 81 (16) in the control group ( and ). Better results were seen in the intervention group at 8 and 12 weeks of follow-up (p = 0.01 and p = 0.04, respectively). The analysis was according to intention to treat and involved all patients who were randomly assigned. Estimated marginal means after treatment were 89 (85–95) in the intervention group and 79 (74–85) in the control group, with a mean difference of 10 (3.2–15.8) (p = 0.006). Pain was reduced in both groups, but there was no statistically significant difference between the groups (). A subgroup analysis, which was not preplanned, showed that women had the best outcome at 3 months of follow-up; the AOFAS score was 91 (10) in the intervention group and 77 (18) in the control group (p = 0.05). In men, the AOFAS score at 3 months of follow-up was similar in the 2 groups. We did not find sex differences regarding pain.
Figure 2. AOFAS score (mean and scatter plot) before and after 4 weeks of treatment and during 12 weeks of follow-up. Blue: intervention group; red: control group.
Discussion
Treatment of Achilles tendinopathy with ESWT led to a clinically relevant effect, with improvement of the AOFAS score. There was no supplementary effect of ESWT on pain. One possible mechanism for and explanation of our findings would be that ESWT improves function and activity. The pain is not reduced since the patients gradually increase activity to a constant, acceptable level of pain. One reason that might explain why we did not find a significant difference regarding pain may have been the difference in sex ratio, as there were more women in the control group who would have had a lower pain threshold and a lower tolerance to painful stimuli (Keogh et al. Citation2005, Ge et al. Citation2006), and thus another level of function and activity. Surprisingly, in our study it was the women who benefited from ESWT. Interaction between the sex of experimenters and subjects is also a possible explanation. One study has shown tolerance of higher pain intensities in subjects tested by female experimenters (Kallai et al. Citation2004). In our study, the experimenters were women.
The AOFAS score may not be appropriate for evaluation of treatment of Achilles tendinopathy. The system includes a score for alignment, which does not change with any treatment of the Achilles tendon. Furthermore, the AOFAS system includes a score for subtalar range of movement and stability of ankle and hindfoot, which may not change. When the primary score is high, the possible gain in score up to 100 is low, thereby reducing the sensitivity of the intervention group to treatment compared to the control group in this study. When there is an upper limit of the score, it may be more realistic to use a ratio of gained increase in relation to possible increase.
Weaknesses of our study may be that the ESWT treatment area was too small compared to the extent of the tendinopathy, that precise location of the lesion was not documented, or that an energy level that was too low was used. Factors that may have influenced the results are too small treatment area, too low energy level and possible up to 1.1 mJ/mm2, ultrasound guidance to localize inflammatory tissue, and improved technology.
The majority of randomized trials comparing ESWT to placebo could not find an effect of ESWT (). Area of treatment, whether or not guidance was used, and energy level may at least partly explain this. One study on calcifying tendinopathy in the rotator cuff found surgery to be superior to ESWT (Rompe et al. Citation2001b), and another randomized study found steroid injection to be superior to ESWT for plantar fasciopathy (Porter and Shad-bolt Citation2005).
Regarding lateral epicondylitis, four randomized trials have been published (Rompe et al. Citation1997, Citation2001, Chung et al. Citation2005, Spacca et al. Citation2005), with only two (Rompe et al. Citation1997, Spacca et al. Citation2005) showing an effect. The trials gave conflicting results and a Cochrane review (McLauchlan and Handoll Citation2001) concluded before the publication of the third and fourth trials (Chung et al. Citation2005, Spacca et al. Citation2005) that further trials were needed to clarify the value of ESWT for lateral elbow pain. The third trial showed no effect. Randomized studies on the effect of ESWT in the treatment of plantar heel pain (Krischek et al. Citation1998, Hammer et al. Citation2002, Haake et al. Citation2003) and calcifying shoulder tendonitis (Rompe et al. Citation1997, Loew et al. Citation1999, Haake et al. Citation2001a, Gross et al. Citation2002, Schmitt et al. Citation2002, Gerdesmeyer et al. Citation2003, Pleiner et al. Citation2004) have also yielded conflicting results.
Table 2. Randomized trials comparing ESWT and placebo
One randomized study (Costa et al. Citation2005), 2 uncontrolled studies (Perlick et al. Citation2002, Lakshmanan et al. Citation2004), and 1 controlled (Furia Citation2005) trial included ESWT in the treatment of Achilles tendinopathy. In the series from Germany, 29 of 40 patients showed satisfactory results (Perlick et al. Citation2002). In the series from the UK, 16 affected tendons in 15 patients improved—as measured by the AOFAS score and the Visa A score systems (Lakshmanan et al. Citation2004). The randomized study (Costa et al. Citation2005), which was published while we were conducting our study, indicated no effect of ESWT. In that study, the treatment group was 10 years younger on average than the placebo group. The data were incomplete, since 9 patients failed to attend the 1-month follow-up and 6 patients failed to attend the 1-year follow-up. The authors did find a reduced pain score in the treatment group, but this was not statistically significant.
Four uncontrolled trials (Lohrer et al. Citation2002, Perlick et al. Citation2002, Lakshmanan et al. Citation2004, Furia Citation2005) have found an effect of ESWT on chronic Achilles tendinopathy.
Traditionally, a chronic painful achilles tendon has been referred to as Achilles tendonitis because of pain, swelling, and increased vascularity. However, recent studies have concluded that little or no inflammation is involved (Alfredson et al. Citation1999, Riley Citation2004). Modern ultrasound-based imaging technology shows that chronic painful Achilles tendons are characterized by increased blood flow and perfusion, called neo-vascularization, intratendinously and peritendinously (Öhberg and Alfredson Citation2004). This new vascularization is most likely an attempt to repair generative lesions on the tendon. Such lesions may be due to tissue hypoxia or repetitive microtrauma, frequently described as overused pathologies. Tissue hypoxia may result from increasing age, vascular degeneration, physical disuse, or trauma (Riley Citation2004). In 60% of patients with Achilles tendinopathy, malalignment has been found, most often limited mobility of the subtalar joint and limited range of motion in the ankle joint. In addition, various deformities of the forefoot and increased foot pronation have been found to be associated with Achilles tendinopathy. Other contributory factors are leg length discrepancy, muscle weakness and imbalance, and training errors (Paavola et al. Citation2002). Several diseases correlate with increased risk of tendinopathy: diabetes mellitus, renal disease, and rheumatological diseases amongst others. Increased body weight and a sedentary lifestyle coupled to the physical demands of certain occupations or sporting activities may account for the increased incidence of tendon rupture in recent times (Riley Citation2004). The traditional nonoperative interventions include relative rest, avoiding painful aggravating activities, non-steroid anti-inflammatory drugs, customized shoes and heel lifts, stretching exercises, and eccentric calf muscle training. The results of these treatments are often unsatisfactory, however. Cor-ticosteroid injection at the site of the lesion has not been recommended because of its possible tendon-weakening effect. The effect on neovascularization has been studied most intensively for eccentric training, which has been demonstrated to reduce revascularizations effectively (Öhberg and Alfredson Citation2004). In pioneer studies, sclerosing with poly-docanol and steroid administered by intratendinous injection and iontophoreasis have been found to be effective (Öhberg and Alfredson Citation2002).
ESWT appears to be a clinically relevant supplement to conservative treatment of tendinopathy. Currently, however, there is no convincing evidence for recommendation of ESWT. Further studies should be done to explore the effects of improved technology such as higher energy per area, greater treatment area, and—if possible—one session of treatment. Another important methodological issue is the importance of gender and activity. A scoring system concentrating on handicap, function, and activity may be more relevant than systems that cover pain and alignment. The observations that responsivity may be influenced by the genders of the subject and experimenter (Kallai et al. Citation2004, Ge et al. Citation2006) should be addressed in future trials.
ESWT is being widely used, but it has only been FDA-approved for the treatment of plantar fasci-itis. The worldwide use of ESWT for treatment of muscular-skeletal complaints is still under debate.
No competing interests declared.
Contributions of authors
SR, OS: study concept and design, writing manuscript. MC, IM: acquisition of data. SR: analysis and interpretation of data.
- Alfredson H, Lorentzon R. Chronic Achilles tendinosis: recommendations for treatment and prevention. Sports Med 2000; 29: 135–46
- Alfredson H, Thorsen K, Lorentzon. In situ microdialysis in tendon tissue: high levels of glutamate but not portahlandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc 1999; 7: 378–81
- Boddeker R, Schafer H, Haake M. Extracorporeal shockwave therapy (ESWT) in the treatment of plantar fasciitis-a biometrical review. Clin Rheumatol 2001; 20: 324–30
- Chung B, Wiley J P. Extracorporeal shockwave therapy: a review. Sports Med 2002; 32: 851–65
- Chung B, Wiley J P, Rose M S. Long-term effectiveness of extracorporeal shockwave therapy in the treatment of previously untreated lateral epicondylitis. Clin J Sport Med 2005; 15: 305–12
- Costa M L, Shepstone L, Donell S T, Thomas T L. Shock wave therapy for chronic Achilles tendon pain: a randomized placebo-controlled trial. Clin Orthop 2005, 440: 199–204
- Furia J P. Extracorporeal shockwave therapy in the treatment of chronic insertional Achilles tendinopathy. Orthopade 2005; 34: 571–8
- Ge H Y, Madeleine P, Cairns B E, Arendt-Nielsen L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: a potential experimental model of gender-specific differences. Clin J Pain 2006; 22(1)37–44
- Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Wortler K, Lampe R, Seil R. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA 2003; 290: 2573–80
- Gross M W, Sattler A, Haake M, Schmitt J, Hildebrandt R, Muller H H, Engenhart-Cabillic R. The effectiveness of radiation treatment in comparison with extracorporeal shockwave therapy (ESWT) in supraspinatus tendon syndrome. Strahlenther Onkol 2002; 178: 314–20
- Haake M, Deike B, Thon A, Schmitt J. Value of exact focusing of extracorporeal shock waves (ESWT) in therapy of tendonitis calcarea. A prospective randomized study. Biomed Tech 2001a; 46: 69–74
- Haake M, Sattler A, Gross M W, Schmitt J, Hildebrandt R, Muller H H. Comparison of extracorporeal shockwave therapy (ESWT) with roentgen irradiation in supraspinatus tendon syndrome - a prospective randomized single-blind parallel group comparison. Z Orthop Ihre Grenzgeb 2001b; 139: 397–402
- Haake M, Buch M, Schoellner C, Goebel F, Vogel M, Mueller I, Hausdorf J, Zamzow K, Schade-Brittinger C, Mueller H H. Extracorporeal shock wave therapy for plantar fasciitis: randomised controlled multicentre trial. BMJ 2003; 327: 75–9
- Hammer D S, Rupp S, Kreutz A, Pape D, Kohn D, Seil R. Extracorporeal shockwave therapy (ESWT) in patients with chronic proximal plantar fasciitis. Foot Ankle Int 2002; 23: 309–13
- Kallai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain 2004; 112: 142–7
- Keogh E, Bond F W, Hanmer R, Tilston J. Comparing acceptance- and control-based coping instructions on the cold-pressor pain experiences of healthy men and women. Eur J Pain 2005; 9(5)591–8, Epub 2005 Jan 19.
- Kitaoka H B, Alexander I J, Adelaar S, Nunley J A, Myerson M S, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int 1994; 15: 349–53
- Krischek O, Rompe J D, Herbsthofer B, Nafe B. Symptomatic low-energy shockwave therapy in heel pain and radiologically detected plantar heel spur. Z Orthop Ihre Grenzgeb 1998; 136: 169–74
- Lakshmanan P, O'Doherty D P. Chronic Achilles tendinopathy: treatment with extracorporeal shock waves. Foot Ankle Surg 2004; 10: 125–30
- Loew M, Daecke W, Kusnierczak D, Rahmanzadeh M, Ewerbeck V. Shock-wave therapy is effective for chronic calcifying tendonitis of the shoulder. Shock-wave therapy is effective for chronic calcifying tendonitis of the shoulder. J Bone Joint Surg (Br) 1999; 81: 863–7
- Lohrer H, Scholl J, Arentz S. Achilles tendinopathy and patellar tendinopathy. Results of radial chockwave therapy in patients with unsuccessfully treated tendinosis. Sportverletz Sportschaden 2002; 16: 108–24
- Matthews J N, Altman D G, Campbell M J, Royston P. Analysis of serial measurements in medical research. Br Med J 1990; 300: 230–5
- McLauchlan G J, Handoll H H. Intervention for treating acute and chornic Achilles tendonitis. Cochrane Database Syst Rev, 2, CD000232 2001.
- Ogden J A, Alvarez R, Levitt R, Cross G L, Marlow M. Shock wave therapy for chronic proximal plantar faciitis. Clin Orthop 2001, 387: 47–59
- Paavola M, Kannus P, Järvinen T A H, Khan K, Józsa L, Järvinen M. Current concepts review. Acilles Tendinopathy. J Bone Joint Surg (Am) 2002; 84: 2062–76
- Perlick L, Schiffmann R, Kraft C N, Wallny T, Diedrich O. Extracorporal shock wave treatment of the achilles tendonitis: Experimental and preliminary clinical results. Z Orthop Ihre Grenzgeb 2002; 140: 275–80
- Pleiner J, Crevenna R, Langenberger H, Keilani M, Nuhr M, Kainberger F, Wolzt M, Wiesinger G, Quittan M. Extra-corporeal shockwave treatment is effective in calcific tendonitis of the shoulder. A randomized controlled trial. Wien Klin Wochenschr 2004; 116: 536–41
- Porter M D, Shadbolt B. Intralesional corticosteroid injection versus extracorporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med 2005; 15: 119–24
- Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 2004; 43: 131–42
- Rompe J D, Eysel P, Hopf C, Krischek O, Vogel J, Burger R, Jage J, Heine J. Extracorporeal shockwave therapy in orthopedics. Positive results in tennis elbow and tendinosis calcarea of the shoulder. Fortschr Med 1997; 115: 29–33
- Rompe J D, Riedel C, Betz U, Fink C. Chronic lateral epicondylitis of the elbow: A prospective study of low-energy shockwave therapy and low-energy shockwave therapy plus manual therapy of the cervical spine. Arch Phys Med Rehabil 2001a; 82: 578–82
- Rompe J D, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendonitis of the shoulder. Clin Orthop 2001b, 387: 72–82
- Schmitt J, Tosch A, Hunerkopf M, Haake M. Extracorporeal shockwave therapy (ESWT) as therapeutic option in supraspinatus tendon syndrome? One year results of a placebo controlled study. Orthopade 2002; 31: 652–7
- Spacca G, Necozione S, Cacchio A. Radial shockwave therapy for lateral epicondylitis: a prospective randomised controlled study. Eur Med Phys 2005; 41: 17–25
- Speed C A, Richards C, Nichols D, Burnet S, Wies J T, Humphreys H, Hazleman B L. Extracorporeal shock-wave therapy for tendonitis of the rotator cuff. A double-blind, randomised, controlled trial. J Bone Joint Surg (Br) 2002; 84: 509–12
- Wilson J J, Best T M. Common overuse tendon problems: A review and recommendations for treatment. Am Fam Physician 2005; 72: 811–8
- Öhberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med 2002; 36: 173–5
- Öhberg L, Alfredson H. Effects of neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis?. Knee Surg Sports Tarumatol Arthrosc 2004; 12: 465–70